Abstract
The objective of this study was to evaluate the prevalence of regional and distant metastasis in cats with advanced oral squamous cell carcinoma (SCC) in a retrospective case series. Forty-nine cats with cytologically- or histopathologically-confirmed oral SCC presented to the Matthew J Ryan Veterinary Hospital of the University of Pennsylvania. History, clinical and laboratory results, diagnostic imaging findings and survival times were obtained from the medical records of patients who received diagnostic evaluation for metastasis. The prevalence of metastasis was assessed by means of mandibular lymph node cytology and three-view thoracic radiography. The prevalence of mandibular lymph node metastasis was 31% (15/49). Evidence of possible thoracic metastasis was seen in 10% (5/49) of cases. Of the patients with mandibular lymph node metastasis, 53% (8/15) were maxillary, 27% mandibular (4/15), 13% sublingual (2/15) and 7% caudal pharyngeal (1/15). Pulmonary metastasis was seen in three mandibular, one maxillary and one sublingual mass. Forty-one patients died or were euthanased owing to progression of local disease, and seven patients were lost to follow-up. The prevalence of regional metastasis in this study was more common than most previously reported studies, while the rate of pulmonary metastasis was higher than has previously been published. Although significant conclusions cannot be drawn, control of the primary tumor, regardless of tumor size at diagnosis, appears to be an important factor in improving survival time, and therefore treatment of metastasis may be important in those cases where long-term control of the primary tumor is possible.
Introduction
Squamous cell carcinoma (SCC) is the most common oral tumor in domestic cats, 1 with the sublingual/lingual region being the most commonly affected site,2–4 although in a recent report the mandibular gingiva was most often involved. 5 Metastasis to mandibular lymph nodes in these cases, as evaluated by fine-needle aspiration cytology, has been previously documented to range from 0% to 36%. However, all previous studies have evaluated small numbers of patients.2,3,6 Pulmonary metastasis, evaluated by three-view thoracic radiography or necropsy, has been documented to be far less common than regional metastasis, but these data are also based on small numbers of patients.1–3,6
Despite its historically low metastatic potential, feline oral SCC is generally considered to carry a poor prognosis. Outcome has been largely dependent on the degree of primary tumor invasion at diagnosis.2,7 Most feline patients with oral SCC succumb to effects of progressive local disease and local treatment failure.2,8,9
The prognostic effect of completeness of surgical margins is unclear. Tumor-free surgical margins in many cases does not consistently spare patients from progression or recurrence of their disease.9,10 Conversely, lack of recurrence in incompletely excised tumors has also been demonstrated. 9 Studies evaluating multimodal treatment have shown improved survival times compared to monotherapy. A median survival time of 14 months was reported for cats with SCC treated with mandibulectomy and adjunctive radiation therapy. 8 Similarly, a study evaluating the efficacy of a radiation therapy protocol consisting of 14 fractions of 3.5 Gy within a 9-day period and simultaneous chemotherapy with carboplatin showed individual survival times ranging from 53 to 770 days, and a median survival of 163 days. 11 A median survival time for cats with complete responses in a similar study without simultaneous chemotherapy was 298 ± 187 days. 12 A study evaluating multimodal therapy for unresectable tumors found survival times with complete remission of up to 759 days. 13 Also, 21 cats treated with 10 once-daily fractions of 4.8 Gy showed a median overall survival of 174 days. 14
Factors other than treatment method may also influence survival time, but few studies have evaluated their true effect. Anatomic location within the mouth has been correlated with survival times. In the aforementioned study evaluating the effectiveness of adding carboplatin to a radiation therapy protocol consisting of 14 fractions of 3.5 Gy within a 9-day period, cats with tonsillar and cheek SCC had a longer mean survival time (724 days) than cats with SCC arising from the tongue, mandible or maxilla (141 days). 11 In a study evaluating the use of a liposomal cisplatin analogue, survival times of cats with oral SCC did not differ significantly when patients were grouped according to age, sex, tumor location or clinical stage. 15 Although tumor volume may play an important role in the selection of treatment options, these variables have not been significantly associated with survival time. Size of the primary tumor has been found to be a predictor of tumor spread in dogs with oral malignant melanoma, but a similar relationship in cats with oral SCC has not been determined. 16
The purpose of the present study was to evaluate the prevalence of metastasis in cats with oral SCC when assessed by means of ipsilateral or bilateral mandibular lymph node cytology and thoracic radiography. Survival times were evaluated to assess the prognostic implications of regional and distant metastasis, tumor location and tumor volume, irrespective of treatment provided. Additionally, correlations of tumor location and tumor volume to lymph node status and pulmonary metastasis were evaluated.
Materials and methods
Criteria for selection of cases
Medical records from cats diagnosed with advanced oral SCC between January 2005 and August 2011 at Matthew J Ryan Veterinary Hospital of the University of Pennsylvania were reviewed. Cats were included in the study if they met the following criteria: (1) confirmed oral SCC based on cytological or histopathological examination of tissue samples; (2) diagnostic cytological tissue samples present from at least one ipsilateral mandibular lymph node; and (3) diagnostic quality thoracic radiographs available for review.
Medical records review
Data reviewed for each cat included results of physical examination (including oral examination and presence or absence of mandibular lymphadenomegaly), complete blood count, serum biochemical panel, cytological mandibular lymph node examination by board-certified clinical pathologists, three-view thoracic radiographic examination by board-certified radiologists, computed tomography (CT) of the head when available, treatment type, treatment duration and outcome. Survival time was defined as the time elapsed between diagnosis of SCC and euthanasia. Information consent obtained from clinical studies was from patients enrolled in studies approved by an Institutional Animal Care and Use Committee.
Tumor evaluation
Tumor location was classified as one of the following: maxillary, mandibular, lingual/sublingual, pharyngeal/tonsillar and labial. Tumor volume was calculated for those patients in whom rostrocaudal, mediolateral and dorsoventral measurements were recorded as part of the routine anesthetized oral examination. When CT measurements were available, these values were used to calculate gross tumor volume.
Statistical analysis
Descriptive statistics were calculated. The type of distribution was determined with the Kurtosis test. Continuous data were expressed as means and SD, unless not normally distributed, in which case median values and ranges were reported along with 95% confidence interval (CI). The Kaplan–Meier product limit method was used to determine median survival time and 95% CI. Categorical data were expressed as frequencies. Fisher’s exact test was used to evaluate the association of tumor location with the presence of lymph node metastasis and the association of tumor location with pulmonary metastasis. Because of non-normality of the data, the Mann–Whitney test was used to evaluate the association between tumor volume and the presence of lymph node metastasis and the association of tumor volume with possible metastasis on thoracic radiographs. All tests were two-tailed and P <0.05 was considered to be statistically significant. Differences in survival time according to prognostic variables (tumor location, presence of regional metastasis, presence of distant metastasis) were assessed by the log rank test. Statistical significance was defined as P <0.05. Cox multivariable survival methods were employed to determine which factors were associated with survival time following diagnosis of oral SCC. Factors investigated included tumor location (mandibular, maxillary, caudal pharyngeal, lingual/sublingual), tumor volume, presence of lymphadenomegaly, presence of regional and/or distant metastatic disease, and the use of anti-tumor therapies or other therapies. Continuous variables were centered prior to analysis. Two-way interactions among the main effects were investigated. An interaction term would be retained based on a P <0.05. If not effect modifiers, any factors identified with a P-value <0.20 on univariate analysis were tested for significance in the multivariate model. Factors with P <0.05 in the multivariate model were retained. The proportional hazards assumption was tested using Schoenfeld residuals. All analyses were performed in Stata version 10 (StataCorp). Survivor functions controlling for significant variables were plotted.
Results
Population demographics and tumor locations are listed in Table 1. Mandibular lymph node cytology was evaluated in all patients. Thirty-five patients had both right and left mandibular lymph nodes sampled. Five lymph nodes from patients with bilateral aspirates were non-diagnostic. Three of the non-diagnostic samples were in the contralateral lymph node to the mass and the other two non-diagnostic lymph node samples were in centralized masses. Thirty-one percent of cats (15/49) had metastasis to the mandibular lymph node. Only one cat showed contralateral spread. Mandibular lymphadenomegaly was noted in 88% of cats. Thirty-five percent (15/43) of palpably enlarged mandibular lymph nodes had metastatic disease. All mandibular lymph nodes with metastasis palpated enlarged. Thoracic radiographs were compatible with pulmonary metastasis in 10% (5/49) of patients. Three of these patients had metastasis to the mandibular lymph nodes.
Table 1.
Study population demographics of 49 cats with oral squamous cell carcinoma
| n | % | |
|---|---|---|
| Sex | ||
| Male castrated | 26 | 53.0 |
| Female spayed | 23 | 46.9 |
| Age (years; mean ± SD) | 13.1 ± 2.9 | |
| Breed | ||
| Domestic shorthair | 37 | 75.5 |
| Domestic longhair | 4 | 8.2 |
| Himalayan, Maine Coon, Persian, Siamese | 8 | 16.3 |
| Median tumor volume in cm3 (range) | 15.4 (2.0–74.5) | |
| Tumor location | ||
| Mandibular | 12 | 24.5 |
| Maxillary | 19 | 38.8 |
| Caudal pharyngeal/tonsillar | 2 | 4.1 |
| Sublingual or lingual | 16 | 32.6 |
| Right | 10 | 20.4 |
| Left | 7 | 14.3 |
| Centralized | 32 | 65.3 |
Median survival time was 91 days (14–251 days; 95% CI 40–112 days). Twenty-three patients were euthanased owing to progression of local disease with one patient also showing dyspnea that could be attributed not only to a sublingual mass, but also to the presence of thoracic metastasis. Four patients were dead on arrival 14, 36, 49 and 229 days after diagnosis and staging. One patient arrested shortly after arrival, 97 days after diagnosis and staging. Seven patients were lost to follow-up and the cause of death was not determined for the remainder of the patients. No significant association was found between tumor location and the presence of regional or distant metastasis. Additionally, there was no significant difference in tumor volume between cats with and without mandibular lymph node metastasis and cats with and without thoracic metastasis. A log rank test showed a significant association between maxillary and caudal pharyngeal tumor location and survival time when evaluating these variables individually. Cats with maxillary tumors had an increased survival time when compared with other sites (P = 0.0146; Figure 1). Cats with tumors in the caudal pharyngeal region showed a decreased survival time (P = 0.0338; Figure 2); however, there were only two cats with tumors in this region. Tumor volume was measured in 67% of patients (33/49) and no significant association was seen with survival. Survival time was not significantly affected by the presence of mandibular lymph node metastasis (median 75 days, 95% CI 39–106 days without regional metastasis versus median 91 days, 95% CI 30–173 days with regional metastasis) or pulmonary metastasis (median 91 days, 95% CI 40–121 days without pulmonary metastasis versus median 68 days, 95% CI 30, undetermined owing to population size with pulmonary metastasis). Of the 49 patients, 76% received novel chemotherapy as part of clinical trials, 8% received palliative radiation therapy (with one also being treated with chemotherapy), 2% underwent surgery for tumor excision and 8% received supportive care, including antibiotics, anti-inflammatory medication, pain medication or a combination of these.
Figure 1.
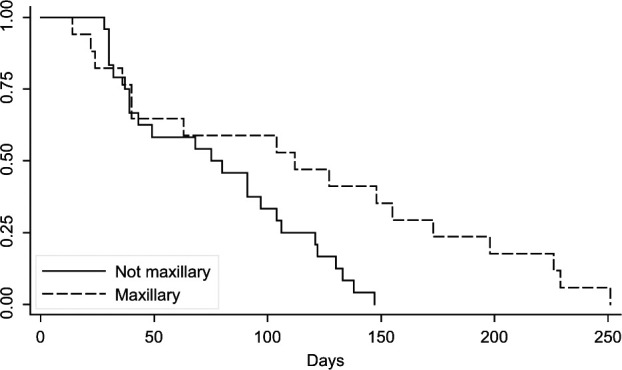
Kaplan–Meier survival curve for cats with maxillary tumors versus cats with non-maxillary tumors
Figure 2.
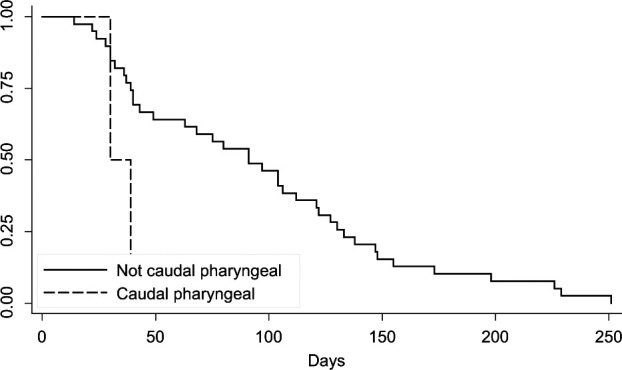
Kaplan–Meier survival curve for cats with caudal pharyngeal tumors versus cats with non-caudal pharyngeal tumors
Discussion
In humans with supraglottic cancers, cervical lymph node metastasis reduces 3-year survival time by approximately 40%, irrespective of tumor stage. 17 A significant association between mandibular lymph node metastasis and survival time was not found in the present study. Pulmonary metastasis was also not a significant negative prognostic factor in the present study. Both lymph node and pulmonary metastasis occur in feline oral SCC with greater frequency than reported in many previous studies. However, the presence of metastatic disease was not associated with decreased chance of survival or survival time. This is likely owing to the devastating effects of primary tumor progression and a reflection of frequent inability to effectively treat the primary tumor. Additionally, this may be a reflection of the advanced stage at which these cases are usually detected and of those seen in third referral facilities. More than half of the deceased patients were euthanased or died because of local disease, with only one possibly having complications from metastatic disease.
In the present study, the age of cats at diagnosis was similar to what has been reported in the literature.1–4,6 No apparent breed or sex predilections were seen. The maxilla was the most common tumor location in the present study. This differs from previous reports, which described the lingual/sublingual area and the mandibular gingiva as the most common sites.2–5
Previous reports involving canine oral malignant melanoma, 18 cats and dogs with solid tumors, 19 and feline oral SCC2,3,20 showed lymph node enlargement on palpation to be an insensitive predictor of metastasis. In the present study, lymph node enlargement was instead specific, as all affected nodes were enlarged and no evidence of metastasis was found in normal-sized nodes. Studies evaluating regional metastasis in dogs and cats with solid tumors have shown a sensitivity of 90–100% for cytological evaluation of lymph nodes by fine needle aspiration.19,20 Consequently, this method was used as a less invasive means of staging compared to biopsy. The prevalence of regional metastasis was higher than that of many previous reports2,3 but lower than one recent report. 6 The previously reported low incidence of metastasis for this tumor was thought to be partly owing to the limited amount of lymphatic drainage of the gingiva. 1 As only the mandibular lymph nodes were cytologically evaluated in the present study, the true prevalence of regional metastasis may actually be higher than 31%. Evaluating only the mandibular lymph nodes in cases of oral and maxillofacial tumors may result in an underestimation of prevalence of metastasis. One study found that of the 35.5% of oral and maxillofacial tumors in dogs and cats that had regional metastasis only 54.5% of metastasis was diagnosed in the mandibular lymph nodes. 20 Additionally, a study that evaluated the lymph pathways of the medial retropharyngeal lymph node in dogs showed that lymph can flow from the mandibular lymph nodes along anastomotic lymphatic connections to the contralateral medial retropharyngeal lymph node, in addition to the ipsilateral medial retropharyngeal node. 21 The higher rate of mandibular lymph node metastasis can also be attributed to the stage of disease, with advance cases being overrepresented in this study, which is also true for the general population of cats with oral SCC. 13 Perineural spread is considered a negative prognostic indicator in humans with oral SCC, and this may also represent an important pathway for tumor spread in cats; however, this was not evaluated in this study.8,22–24
Pulmonary metastasis was more prevalent in the present study than in previous reports, where prevalence was reported at 0–6.25%.1–3,6 Three-view thoracic radiography may have overestimated the prevalence of distant metastasis as other pulmonary diseases or primary lung tumors may mimic pulmonary metastasis. Thoracic CT has been shown to be a more sensitive and reliable tool in the detection of soft tissue pulmonary nodules in dogs than three-view thoracic radiography; however, this, too, can overestimate the prevalence of pulmonary metastasis.25,26 Possible explanations for these higher prevalence include referral bias and advanced stage of disease.
Interestingly, tumor volume appeared not to be a predictor for regional and distant metastasis, as no significant association was seen between tumor size and metastasis. A study of canine oral malignant melanoma showed that patients with tumors smaller than 8 cm3 had a significantly longer remission and survival time. 17 Tumor volume, however, was not significantly associated with survival time in the present study.
On the one hand, caudal pharyngeal tumors showed a significantly decreased survival time in the present study; however, this represented only 4% of the study population. Maxillary tumors, on the other hand, were shown to have a significantly longer survival time when compared with non-maxillary tumors. Maxillary tumors in cats represent a challenge when attempting to obtain tumor-free surgical margins owing to the close proximity of other vital structures and the difficulty in obtaining wide margins in this location; therefore, a better prognosis for these tumors was not expected. However, when patients with inoperable maxillary masses are compared with patients with inoperable tumors arising from other sites, it is reasonable to expect that maxillary masses may allow for maintenance of function better than similarly sized sublingual, lingual and mandibular tumors, which may more rapidly affect the patient’s quality of life. 9 Tumor location also did not significantly influence the likelihood of regional or distant metastasis in the present study. This lack of significance may be owing to relatively small numbers of patients representing each tumor location. A significant association may be seen with a larger cohort or if nodes of all regional lymph centers are evaluated.
Previous studies evaluating response to treatment in cats with oral SCC have reported a wide range of median survival times (45–420 days). Median survival time in the present study was comparable with previous reports of both accepted and novel treatment regimens, despite having a higher metastatic rate.2,3,7,27 The presence of lymph node and pulmonary metastasis did not show a significant effect on survival time, though these results may have differed in a patient population where primary tumor control was achieved. Effect of primary tumor control on survival time could not be evaluated as only one patient had a resectable mass and received surgery with a curative intent. Only one cat in the study had a feeding tube placed. Although, this patient survived for only 37 days after diagnosis, which is less than the median survival time for this study, feeding tube placement may increase the survival time of patients.
This study was limited by the fact that only one lymphocentrum was evaluated.
Conclusions
Neither regional nor distant metastasis affected survival time of patients in the present study, where the majority of patients (98%) received palliative treatment. Primary tumor control appears to be an important factor for improving survival times. In cases where primary tumor control is possible, metastatic status may play an important role in patient survival.
Footnotes
Funding: The research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 4 August 2013
References
- 1. Stebbins KE, Morse CC, Goldschmidt MH. Feline oral neoplasia: a ten-year survey. Vet Pathol 1989; 26: 121–128. [DOI] [PubMed] [Google Scholar]
- 2. Postorino Reeves NC, Turrel JM, Withrow SJ. Oral squamous cell carcinoma in the cat. J Am Anim Hosp Assoc 1993; 29: 438–441. [Google Scholar]
- 3. Hayes AM, Adams VJ, Scasey TJ, Murphy S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J Small Anim Pract 2007; 48: 394–399. [DOI] [PubMed] [Google Scholar]
- 4. Cotter S. Oral pharyngeal neoplasms in the cat. J Am Anim Hosp Assoc 1981; 17: 917–920. [Google Scholar]
- 5. Martin CK, Tannehill-Gregg SH, Wolfe TD, Rosol TJ. Bone-invasive oral squamous cell carcinoma in cats. Vet Pathol 1984; 48: 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gendler A, Lewis JR, Reetz JA, Schwarz T. Computed tomographic features of oral squamous cell carcinoma in cats. J Am Vet Med Assoc 2010; 236: 319–325. [DOI] [PubMed] [Google Scholar]
- 7. Bostock D. Prognosis in cats bearing squamous-cell carcinoma. J Small Anim Pract 1972; 13: 119–125. [DOI] [PubMed] [Google Scholar]
- 8. Hutson C, Willauer C, Walder E, Stone J, Klein M. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy - 7 cases (1987–1989). J Am Vet Med Assoc 1992; 201: 777–781. [PubMed] [Google Scholar]
- 9. Northrup NC, Selting KA, Rassnick KM, et al. Outcomes of cats with oral tumors treated with mandibulectomy: 42 Cases. J Am Anim Hosp Assoc 2006; 42: 350–360. [DOI] [PubMed] [Google Scholar]
- 10. Bradley RL, MacEwen EG, Loar AS. Mandibular resection for removal of oral tumors in 30 dogs and 6 cats. J Am Vet Med Assoc 1984; 184: 460–463. [PubMed] [Google Scholar]
- 11. Fidel J, Lyons J, Tripp C, et al. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J Vet Intern Med 2011; 25: 504–510. [DOI] [PubMed] [Google Scholar]
- 12. Fidel JL, Sellon RK, Houston RK, Wheeler BA. A nine-day accelerated radiation protocol for feline squamous cell carcinoma. Vet Radiol Ultrasound 2007; 48: 482–485. [DOI] [PubMed] [Google Scholar]
- 13. Marconato L, Buchholz J, Keller M, et al. Multimodal therapeutic approach and interdisciplinary challenge for the treatment of unresectable head and neck squamous cell carcinoma in six cats: a pilot study. Vet Comp Oncol 2013; 11: 101–112. [DOI] [PubMed] [Google Scholar]
- 14. Poirier VJ, Kaser-Hotz B, Vail DM, Straw RC. Efficacy and toxicity of an accelerated hypofractionated radiation therapy protocol in cats with oral squamous cell carcinoma. Vet Radiol Ultrasound 2013; 54: 81–88. [DOI] [PubMed] [Google Scholar]
- 15. Fox LE, Rosenthal RC, King RR, et al. Use of cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum (II), a liposomal cisplatin analogue, in cats with oral squamous cell carcinoma. Am J Vet Res 2000; 61: 791–795. [DOI] [PubMed] [Google Scholar]
- 16. Harvey H, Mac Ewen E, Braun D, et al. Prognostic criteria for dogs with oral melanoma. J Am Vet Med Assoc 1981; 178: 580–582. [PubMed] [Google Scholar]
- 17. Hahn S, Spaulding C, Kim J, Constable W. The prognostic significance of lymph node involvement in pyriform sinus and supraglottic cancers. Int J Radiat Oncol Biol Phys 1987; 13: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 18. Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987–2001). J Am Vet Med Assoc 2003; 222: 1234–1236. [DOI] [PubMed] [Google Scholar]
- 19. Langenbach A, McManus P, Hendrick M, et al. Sensitivity and specificity of methods of assessing the regional lymph nodes for evidence of metastasis in dogs and cats with solid tumors. J Am Vet Med Assoc 2001; 218: 1424–1428. [DOI] [PubMed] [Google Scholar]
- 20. Herring E, Smith M, Robertson J. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. J Vet Dent 2002; 19: 122–126. [DOI] [PubMed] [Google Scholar]
- 21. Belz GT, Heath TJ. Lymph pathways of the medial retropharyngeal lymph node in dogs. J Anat 1995; 186: 517–526. [PMC free article] [PubMed] [Google Scholar]
- 22. Tannehill-Gregg SH, Levine AL, Rosol TJ. Feline head and neck squamous cell carcinoma: a natural model for the human disease and development of a mouse model. Vet Comp Oncol 2006; 4: 84–97. [DOI] [PubMed] [Google Scholar]
- 23. Gardner DG. Spontaneous squamous cell carcinomas of the oral region in domestic animals: a review and consideration of their relevance to human research. Oral Dis 1996; 2: 148–154. [DOI] [PubMed] [Google Scholar]
- 24. Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature. Cancer 2009; 115: 3379–3391. [DOI] [PubMed] [Google Scholar]
- 25. Nemanic S, London CA, Wisner ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med 2006; 20: 508–515. [DOI] [PubMed] [Google Scholar]
- 26. Armbrust LJ, Biller DS, Bamford A, et al. Comparison of three-view thoracic radiography and computed tomography for detection of pulmonary nodules in dogs with neoplasia. J Am Vet Med Assoc 2012; 240: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 27. Bond E, Dorfman HD. Squamous cell carcinoma of the tongue in cats. J Am Vet Med Assoc 1969; 154: 786–789. [PubMed] [Google Scholar]


