Abstract
A prospective clinical trial to compare the effects of age and reproductive status on postoperative pain was conducted in 145 female cats undergoing ovariohysterectomy using injectable anaesthesia. The cats were grouped appropriately: 60 kittens <4 months old (K), 85 adults >4 months old (A) and, within the adult group, 57 normal adults (nA) and 28 adults who were either pregnant or in oestrus (rA). Pain was assessed using a simple descriptive scale (SDS; 0–3), a dynamic and interactive visual scale (DIVAS; 0–100 mm) and mechanical nociceptive thresholds (MNT; N, 2 mm diameter probe) pre-operatively and at 4 and 24 h postoperatively. Kittens had lower DIVAS areas under the time curve and SDS than adults (P <0.05), but similar MNT (K: 3.3 ± 2.6, A: 4.3 ± 2.5 N at 4 h, P >0.05). Data from nA and rA were not different (P >0.05). Kittens had similar wound tenderness, but less affective pain than adults, and reproductive status had no effect.
Introduction
Although pain management in cats has improved considerably in recent years there is still room for further refinement to establish the most appropriate protocols for the range of surgical conditions, traumatic injury and diagnostic procedures that may be encountered. Intrinsic features of the procedure may also affect the degree of postoperative pain. For example, the optimal surgical approach for ovariohysterectomy was evaluated by Grint et al, 1 who concluded that a midline incision caused less wound tenderness than the flank approach. It seems likely that the degree of trauma caused by the surgery will affect the degree of postoperative pain, and it might be expected that reproductive status would affect postoperative pain management when an enlarged or friable uterus makes the surgery more difficult. The stage of the oestrus cycle may also affect the perception of pain, a recognised effect in women. 2 A further consideration is the current debate about the relative merits of neutering very young kittens, particularly in rescue centres, where there may be only one chance for surgery as the cat is unlikely to be presented again. 3
Polson et al 4 reported previously that the ‘quad’ anaesthetic protocol using midazolam, medetomidine, ketamine and either buprenorphine or butorphanol, with one of the non-steroidal anti-inflammatory drugs (NSAID) meloxicam or carprofen, provided excellent analgesia for routine ovariohysterectomy in cats. 4 The study also suggested that age and reproductive status may influence postoperative pain and recovery. However, there were insufficient young kittens or cats with abnormal reproductive status for sufficient statistical power to allow robust comparison between groups.
The present study aimed to evaluate further the suggestion that age and reproductive status affect postoperative pain in cats. The primary aim was to evaluate whether kittens experience more or less pain than adult cats after ovariohysterectomy. The second aim was to investigate whether reproductive status affects postoperative pain.
Materials and methods
The project was approved by the Animal Health Trust Clinical Research Ethics Committee, (project number AHT 15 2010) and signed owner consent was given in all cases.
Animals and anaesthesia
One hundred and forty-five female cats admitted to the RSPCA Greater Manchester Animal Hospital for neutering were studied. All were domestic shorthair cats aged between 8 weeks and 6 years. On the grounds of age, each cat was assigned to group K (kittens younger than 4 months) or group A (cats older than 4 months). All group A cats were assigned to a subgroup according to their reproductive status, which was ascertained during surgery: group nA (normal status) or group rA (in oestrus: presence of ovarian follicles with increased uterine oedema and vascularity; or pregnant: presence of uterine fetal swellings). Food was withheld for 6 h before surgery for adult cats and 3 h before surgery for kittens, but all had access to drinking water until the induction of anaesthesia. All cats were housed individually in kennels prior to and for 24 h after surgery. Free access to food and water and a litter tray was provided from 2 h after surgery. Prior to surgery, cats underwent a physical examination to confirm normal health, record heart and respiratory rates, check for pregnancy and to award pain scores. Five to 10 mins before the start of surgery all animals were anaesthetised using a single intramuscular (IM) injection containing midazolam (Hypnovel; Roche) 3 mg/m2 (~0.25 mg/kg), medetomidine (Sedator; Dechra) 600 μg/m2 (~50 µg/kg), ketamine (Ketaset; Pfizer Animal Health) 60 mg/m2 (~5 mg/kg) and buprenorphine (Vetergesic Multidose; Alstoe Animal Health) 180 µg/m2 (~15 µg/kg). At the same time, all cats received meloxicam 0.3 mg/kg (Metacam; Boehringer Ingelheim) subcutaneously.
During anaesthesia oxygen from an oxygen generator was provided via a face mask. Routine ovariohysterectomy was carried out by one experienced veterinary surgeon (DY) using a ventral midline approach. Surgical conditions were scored by the surgeon (good, adequate, poor) and reproductive status (normal, pregnant or oestrus) was recorded. Environmental conditions were kept constant throughout the surgery: the operating table was heated to 37oC and the temperature of the operating theatre stayed between 21 and 22oC. The duration of surgery was recorded from the first incision until placement of the last suture. Following surgery the cats were placed under close observation in individual recovery cages maintained at 37oC. Atipamezole (Atipam; Dechra) at half the volume of medetomidine given (125 µg/ m2, ~15 µg/kg) was injected IM 40 mins after the anaesthetic injection. Recovery time was recorded from the atipamezole injection to first achieving a sternal position.
Pain assessment
All assessments were made by a single observer (SP). Three methods of pain assessment were used pre- and postoperatively at 4 and 24 h after the anaesthetic injection: 4
A simple descriptive scale (SDS) (0–3 scale) based on a general observation of the cats’ appearance (Table 1)
A dynamic and interactive visual scale (DIVAS) using a mark on line from 0–100 mm, with 0 as no pain and 100 as the worst pain imaginable 5
Wound sensitivity by measuring the mechanical nociceptive threshold (MNT) (force, N) at a site approximately 1 cm from the surgical wound, using a pneumatic device with a hemispherical probe tip of 2 mm diameter (Prod; Topcat Metrology). As pressure = force/area, at 10 N a 2 mm diameter probe tip exerts a pressure of approximately 32 kgf/cm2.
Table 1.
Simple descriptive scale (SDS) used to score pain
| Score | Behaviour |
|---|---|
| 0 | No pain; cat is in normal posture, no response to wound palpation |
| 1 | Mild pain; cat looks normal, but responds to firm wound pressure |
| 2 | Moderate pain; cat appears abnormal, eg, slightly hunched posture, and responds to gentle wound pressure |
| 3 | Severe pain; cat looks miserable — hunched, half-closed eyes, coat-staring — and cannot bear wound to be touched, growls and hisses |
Each cat was first observed in the cage, without disturbance, and note was taken of posture and behaviour. The cat was then stroked, offered food and the wound site gently touched to determine the SDS and DIVAS scores. The cat was then calmly held by an experienced assistant and MNT measured by applying the tip of the Prod 1 cm from the wound edge. Force was increased at 2N/s, controlled by traffic lights (green = go faster, red = slow down) on the unit. When the cat reacted to the pressure the stimulus was stopped immediately, thereby holding the force reading on the display; the cat’s response and the MNT were recorded. This was repeated three times at 30 s intervals and the mean taken as the MNT for that time point.
Buprenorphine 20 µg/kg was to be used as rescue analgesia in any cat deemed to be in unacceptable pain postoperatively (DIVAS >40 mm and SDS >2).
Statistical analyses
Prospective power calculations to estimate the group sizes required to detect clinically relevant differences (DIVAS: 10 mm; SDS: 1; MNT: 2.5 N) were made using data from Polson et al. 4 These data indicated that 25 cats per group would give 90% power to detect relevant DIVAS differences, and 40 cats per group would give 80% power to detect relevant SDS and MNT differences. Post-study power calculations showed smaller SDs and larger group numbers giving the study 99% power to detect all relevant differences between cats and kittens, and 90% power to detect differences between cats with normal and abnormal reproductive status.
The Kolmogorov–Smirnov test indicated that most of the data were not normally distributed, so non-parametric tests were employed. Single-factor data from groups K and A, and nA and rA were compared using the Mann–Whitney U-test (body weight, time from injection to start of surgery, duration of surgery, recovery time from atipamezole injection to sternal position, pre-operative body temperature, heart and respiratory rates). The DIVAS and MNT data from these group pairs were compared using the area under the time curve (AUC) as a summary measure; the AUCs were compared with a Student’s t-test. The proportions of animals in each group receiving each SDS score were compared using the χ2 test. Within-group changes with time (DIVAS, SDS, MNT) were compared using the Friedman test followed by Dunn’s test when appropriate. Statistical significance was set at P <0.05. Data are shown as mean ± SD for clarity.
Results
Sixty kittens aged 52–103 days, mean 77 days (group K, <4 months) and 85 cats aged 116–1153 days, mean 443 days (group A, >4 months) were studied. Group A was subdivided into 57 nA and 28 rA. Single-factor data from each group are shown in Table 2. Kittens weighed less than adults (P <0.001), and both surgical procedure and recovery time were shorter (P <0.001). Group nA cats were lower body weight than rA and the recovery time was shorter (P <0.05). There were no other statistical differences between the comparable groups. Surgery was uneventful in all cases, and all cats recovered satisfactorily and were discharged according to plan on the following day. No cats required rescue analgesia.
Table 2.
Mean (± SD) weight, time from anaesthetic injection to start of surgery, surgery duration, recovery time from atipamezole injection to sternal recumbency, pre-operative temperature, heart rate and respiratory rate in 165 cats
| Group K (<4 m) | Group A (>4 m) |
MWU | Group nA | Group rA | MWU | |
|---|---|---|---|---|---|---|
| n | 60 | 85 | 57 | 28 | ||
| Weight (kg) | 1.2 ± 0.5 | 2.5 ± 0.7 | *** | 2.3 ± 0.6 | 3.0 ± 0.6 | *** |
| Injection to start surgery (min) | 7.7 ± 1.8 | 8.8 ± 2.2 | *** | 8.8 ± 1.9 | 8.8 ± 2.8 | NS |
| Duration of surgery (min) | 8.3 ± 1.1 | 9.4 ± 2.3 | *** | 9.1 ± 1.1 | 9.9 ± 3.8 | NS |
| Recovery (min) | 10.7 ± 6.7 | 19.9 ± 11.4 | *** | 18.3 ± 11.1 | 23.2 ± 11.6 | * |
| Pre-operative temperature (0C) | 38.8 ± 0.4 | 38.7 ± 0.4 | NS | 38.7 ± 4.0 | 38.7 ± 4.0 | NS |
| Pre-operative HR | 184 ± 18 | 182 ± 16 | NS | 183 ± 16 | 181 ± 16 | NS |
| Pre-operative RR | 20 ± 3 | 20 ± 4 | NS | 20 ± 4 | 21 ± 4 | NS |
nA = normal adults; rA = pregnant adults or adults in oestrus; MWU = Mann–Whitney U-test; HR = heart rate; RR = respiratory rate; NS = no significant difference
P <0.05, **P <0.01, ***P <0.001
Pre-operatively there were no statistical differences in measures of pain between any of the comparable groups. DIVAS and SDS scores were all zero. MNT was 5.5 ± 4.0 N in group K, 6.3 ± 3.8 N in A, 6.0 ± 3.7 N in nA and 6.2 ± 3.6 N in rA.
All postoperative measures of pain were low in all groups, and none exceeded the criteria for rescue analgesia. The highest DIVAS value recorded in two group A cats was 23 mm at 4 h; most were <20 mm. All SDS scores were <3 and fewer than 10 MNT values <1 N were recorded at the 4 h time point only.
In group K DIVAS increased above pre-operative baseline at 4 h (P <0.05), but returned to the pre-operative range by 24 h (Figure 1). There were significantly more higher SDS scores at 4 h than at baseline and 24 h (P <0.01) (Table 3). MNT decreased from baseline (5.5 ± 4.0 N) to 3.3 ± 2.6 N at 4 h (P <0.01), but returned to pre-operative values (4.3 ± 2.4 N) at 24 h (Figure 2).
Figure 1.
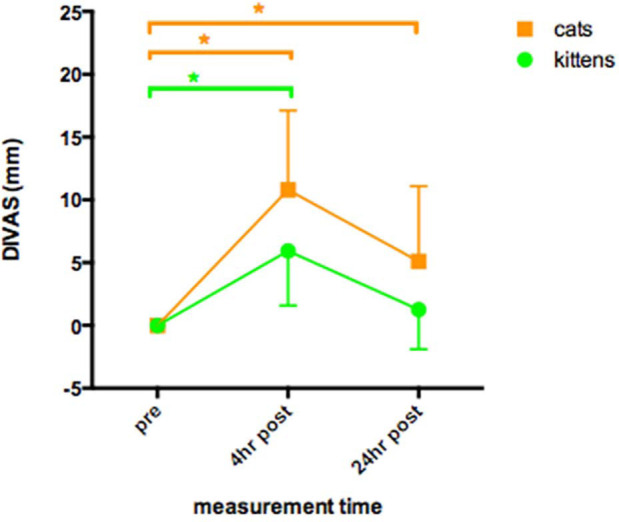
Dynamic interactive visual analogue scores (DIVAS, 0–100 mm) (mean ± SD) of 60 kittens and 85 cats undergoing ovariohysterectomy. Measurements taken pre-operatively, and postoperatively at 4 and 24 h after induction of anaesthesia. *Indicates a statistical difference between the identified points (P <0.05) (kittens: green; cats: orange). The DIVAS time area under the time curve (AUC) in kittens was significantly lower than in cats (P <0.05)
Table 3.
Simple descriptive scale (SDS) scores in group K (kittens) and A (cats >4 months old). Table shows percentage of group with SDS scores of 0, 1 and 2 pre-operatively, and 4 and 24 h postoperatively. Friedman and Dunn’s test for within group changes, χ2 test for comparison between groups
| SDS score (%) | 0 (%) | 1 (%) | 2 (%) | Difference from pre-operation | Difference K vs A |
|
|---|---|---|---|---|---|---|
| K | Pre-operative | 100 | 0 | 0 | NS | |
| 4 h postoperative | 20 | 70 | 10 | P <0.01 | P <0.01 | |
| 24 h postoperative | 87 | 12 | 2 | NS | P <0.01 | |
| A | Pre-operative | 100 | 0 | 0 | ||
| 4 h postoperative | 11 | 51 | 38 | P <0.001 | ||
| 24 h postoperative | 54 | 29 | 15 | P <0.01 | ||
NS = not significant
Figure 2.
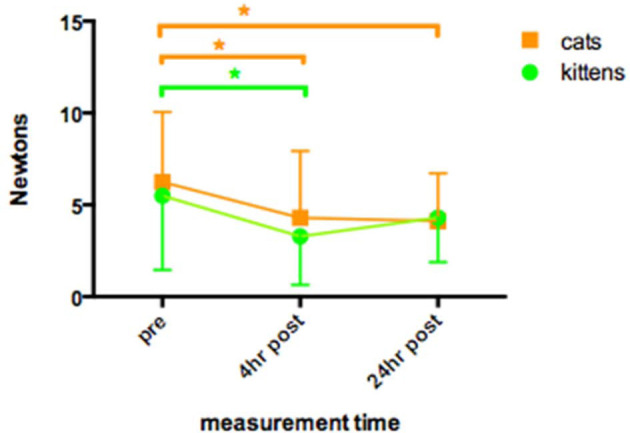
Mechanical nociceptive thresholds (MNT) (N, mean ± SD) recorded in 60 kittens and 85 cats undergoing ovariohysterectomy. Measurements taken pre-operatively, and postoperatively at 4 and 24 h after induction of anaesthesia. *Indicates a statistical difference between the identified points (P <0.05) (kittens: green; cats: orange). The MNT-time area under the time curve in kittens and cats was not significantly different
In group A, DIVAS increased above pre-operative baseline at 4 h and 24 h (P <0.001) (Figure 1). There were significantly more higher SDS scores at 4 h and 24 h than at baseline (P <0.001) (Table 3). MNT decreased from baseline (6.3 ± 3.8 N) to 4.3 ± 3.2 N at 4 h and 4.2 ± 2.5 N at 24 h (P <0.01) (Figure 2).
Group K DIVAS AUC was significantly lower than group A AUC (P <0.001) (Figure 1). Group K also had a higher proportion of low SDS scores than group A at both 4 h and 24 h postoperatively (P <0.01) (Figure 3; Table 3). There were no significant differences in MNT between groups A and K (Figure 2).
Figure 3.
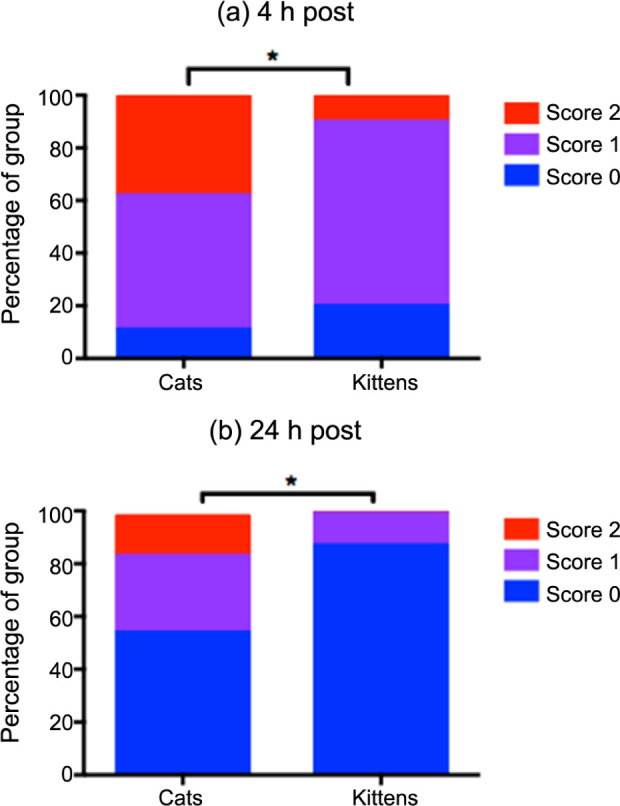
Simple descriptive scale (SDS, 0–3) scores awarded postoperatively to 60 kittens and 85 cats undergoing ovariohysterectomy. (a) At 4 h and (b) at 24 h after induction of anaesthesia. All pre-operative scores = 0. Graphs show the proportion (as percentage) of animals in each group awarded scores 0–2. No animals scored 3. *Indicates a significantly different distribution of scores between cats and kittens (more low scores in kittens)
There were no differences in any of the pain measures (DIVAS, SDS, MNT) between the subgroups nA and rA (P >0.05). Both followed the same pattern in all three measurements as the main group A (Figure 4; Table 4).
Figure 4.
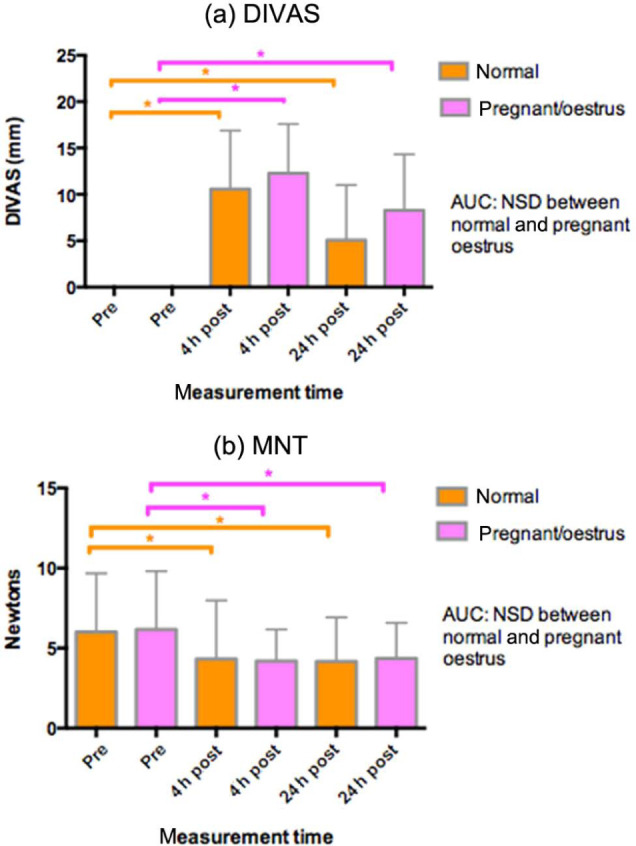
(a) Dynamic interactive visual analogue scores (DIVAS, 0–100 mm) (mean ± SD) and (b) mechanical nociceptive thresholds (MNT) (N, mean ± SD) recorded in 85 cats (>4 months): 57 with normal reproductive status, and 28 who were either pregnant or in oestrus. Measurements taken pre-operatively, and postoperatively at 4 and 24 h after induction of anaesthesia. *Indicates a statistical difference between the identified points (P <0.05) (pregnant/oestrus: pink; normal: orange). No significant differences between the normal and pregnant/oestrus groups. AUC = area under the time curve; NSD = no significant difference
Table 4.
Simple descriptive scale (SDS) scores in group nA (normal reproductive status) and rA (pregnant or in oestrus). Table shows percentage of group with SDS scores of 0, 1 and 2 pre-operatively, and 4 and 24 h postoperatively. Friedman and Dunn’s test for within-group changes, χ2 test for comparison between groups
| SDS score (%) | 0 (%) | 1 (%) | 2 (%) | Difference from pre-operation | Difference K vs A |
|
|---|---|---|---|---|---|---|
| nA | Pre-operative | 100 | 0 | 0 | NS | |
| 4 h postoperative | 9 | 58 | 33 | P <0.01 | NS | |
| 24 h postoperative | 63 | 26 | 11 | P <0.05 | NS | |
| rA | Pre-operative | 100 | 0 | 0 | ||
| 4 h postoperative | 15 | 35 | 50 | P <0.001 | ||
| 24 h postoperative | 38 | 38 | 24 | P <0.01 | ||
NS = not significant
Discussion
The anaesthetic and analgesic protocol employed in this study broadly provided good analgesia for both adult cats and kittens. None of the criteria for rescue analgesia were met, and, although all indicators of pain increased after surgery (higher DIVAS and SDS, and lower mechanical threshold indicating wound-site sensitivity), particularly at 4 h, all behavioural (DIVAS and SDS) values remained low, suggesting that pain was mild. By 24 h pain behaviour in all animals was diminishing, and had ceased completely in kittens.
A number of systems have been used to assess postoperative pain in cats, although there is not as yet a validated system, such as the Glasgow composite measure pain scale now available for dogs. 6 A multidimensional composite scale has recently been refined and partially validated, 7 and although the current study did not follow the same system, the behaviours used during assessment were similar. The need for rescue analgesia is a valuable additional measure of the degree of postoperative pain, although the point at which it is given is not always linked to a pain-scoring system. 8 Brondani et al 9 reported remarkable consistency between 10 culturally and lingually distinct anaesthesiologists in describing the point at which rescue should be given. Specificity of at least 96% and sensitivity of at least 91% was shown for rescue at >28 for VAS (0–100), >1 for SDS (0–3) and >3 for numerical rating scale (0–10). In the present study, rescue was to be given to any cat deemed to be in unacceptable pain postoperatively, thought to be equivalent to DIVAS >40 mm and SDS >2. These values are higher than in the study by Brondani et al 9 and may have missed cats requiring rescue analgesia. However, the highest DIVAS score recorded was 23, well below the 28 cut-off reported by Brondani et al. 9 Some SDS scores of 2 were, however, recorded for at least one time point in about one third of the cats, which would have triggered rescue under the Brondani et al 9 system. This suggests a discrepancy between our SDS and DIVAS scoring. However, as the prime decision for rescue analgesia was a subjective decision that a cat was ‘in unacceptable pain’ it is likely that the SDS was operating artificially, but consistently, high owing to the absence of seriously painful cats.
It is of note that behavioural scoring in kittens indicated less pain and a more rapid return to normal than the older animals. In contrast, wound sensitivity, measured using MNT at the wound site, was not different between the age groups. It is likely that behavioural pain scoring most closely measures the affective component of pain (how it makes you feel) and that wound sensitivity follows the sensory component (what you feel). 10 If this interpretation is correct it indicates that kittens are less affected by pain than older cats in spite of experiencing a similar degree of physical discomfort. This suggests that not only do kittens readily tolerate surgery, but also that they cope with it better than adults — a clear argument for early neutering on the grounds of welfare, quite apart from the other benefits. 3
In a previous study investigating postoperative analgesia after ovariohysterectomy in cats, 4 kittens younger than 5 months also had lower DIVAS and SDS scores than older animals. However, using an algometer with a larger probe tip (2.5 mm diameter vs 2.0 mm in the present study), kittens recorded lower MNT than adults. Although this suggested that they experienced more sensory pain, an alternate methodological explanation is more likely. Mechanical pain is produced by the pressure distorting nociceptors 11 and, as pressure = force/area, the larger the probe tip, the greater the force required to produce that pressure. Forces of around 10 N were required at threshold in the adult cats at 4–24 hours after surgery in a previous study. 4 These forces would be sufficient to move a whole kitten weighing <1 kg and might easily be erroneously misinterpreted as the MNT. In the present study a smaller probe tip was used, considerably reducing the required forces (see Table 5). Hence, the MNTs recorded in this study are more likely to be true thresholds.
Table 5.
Effect of changing algometer tip diameter on forces required to reach mechanical nociceptive threshold (MNT)
| • Pressure = force/area • Relationship of diameter to area is not linear: Area = Πr2 • Relationship between MNT and pressure or force is not linear 22 but, as force increases, pressure also increases | |
|
Tip diameter 2.5 mm4
Area: 4.9 mm2 Reported MNT at 4 h: ~10 N, pressure = 20 kgf/cm2 | |
|
Tip diameter 2.0 mm (current study) Area: 3.1 mm2 At 10 N pressure = 32 kgf/cm2 |
At 5 N pressure = 16 kgf/cm2
Reported MNT at 4 h ~5 N (see Figure 2), pressure = 16 kgf/cm2 |
Surgery in kittens was also of slightly shorter duration than in adults. Although this was only a minute it still represents a reduction of 10%. Probably of greater importance, the recovery time was much reduced, taking little over half the time of adults. This is in keeping with data from other studies reporting quicker surgery and more rapid recovery in kittens than adults, 12 again supporting the concept that it is better to neuter kittens when they are young, rather than waiting for them to mature to adulthood. Our data complement a number of other studies that also provide evidence in favour of neutering kittens. Early neutering is an asset to shelter medicine, as it helps to prevent an exponential growth in the cat population, which already places a huge burden on overstretched animal shelters. 13 Pre-pubertal neuters can be performed prior to rehoming, which eliminates the risk of poor owner compliance with post-adoption neutering. 12 Other benefits include shorter surgery times, 14 faster recovery and a lower rate of peri-operative complications. 15 Smaller organ size necessitates smaller incisions and, consequently, causes less tissue trauma. 13
‘Abnormal reproductive status’ (group rA) included all cats who were either pregnant or in oestrus. It might be expected that the more invasive surgery required for an enlarged, vascular and friable uterus might cause more pain than after straightforward ovariohysterectomy. However, none of the assessments detected any differences in postoperative pain between the normal and rA cats. This suggests that, under the circumstances reported here, using ketamine, midazolam, medetomidine, buprenorphine and an NSAID, with surgery performed by a very experienced surgeon, the additional surgical insult was minimal and did not exacerbate postoperative pain. Small group size might obscure a true, but small, difference. However, post-study power calculations indicate that the numbers were sufficient to detect a meaningful clinical difference, so this explanation appears unlikely.
It is conceivable that the stage of the oestrus cycle may affect the experience of pain. Jarvis et al 16 reported that women had heightened sensitivity to noxious stimuli during the luteal phase of the cycle. If this was also the case in cats, the lower sensitivity during the follicular stage might counteract any effects of increased surgical trauma. Hypoalgesia occurs in late pregnancy in a number of species,16–18 and might have reduced the postoperative pain in the rA group. These effects are probably mediated by oestrogen, oxytocin and progesterone; prolactin may also contribute. 19
There has been little previous research in either the medical or veterinary literature on the effects of age on postoperative pain, 20 and the results are contradictory, 21 probably as methods and procedures are not entirely comparable. Lautenbacher et al 21 reported that humans become increasingly sensitive to pressure stimuli with age, but also become less sensitive to thermal stimuli. This may result from changes occurring in either or both peripheral nociceptor function and endogenous pain inhibitory systems. 21
The present study enabled some evaluation of the effect of the α2 adrenoceptor antagonist atipamezole on recovery and postoperative pain. Recovery times in a previous study were prolonged, with the majority still heavily sedated 2 h after surgery. 4 No direct comparisons could be made as all cats in the present study received atipamezole. However, as pain relief was generally very good, use of atipamezole did not appear to affect postoperative pain relief adversely. It also led to apparently much shorter recovery times: 170 mins was reported by Polson et al 4 using the same protocol without atipamezole compared with a maximum of around 25 mins in the present study. Rapid recovery is generally regarded as advantageous; close monitoring is required for a shorter period, and the likelihood of postoperative hypoglycaemia and hypothermia is reduced, which is of particular importance in kittens. 20
Conclusions
This investigation indicates that the anaesthetic and analgesic protocol, including atipamezole antagonism of the medetomidine, provided generally good analgesia, that kittens appear to be less severely affected by ovariohysterectomy than adults and that, in the hands of an experienced surgeon, the reproductive status did not affect postoperative pain relief.
Acknowledgments
Thanks are due to Ben Faulkner (cat neutering coordinator at the RSPCA Greater Manchester Animal Hospital) for his role in recruiting cases and peri-operative monitoring, and to International Cat Care (formerly known as the Feline Advisory Bureau) for providing funds for the purchase of the Prod algometer.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Dr PM Taylor is a director of Topcat Metrology Ltd.
Accepted: 7 August 2013
References
- 1. Grint NJ, Murison PJ, Coe RJ, Waterman Pearson AE. Assessment of the influence of surgical technique on postoperative pain and wound tenderness in cats following ovariohysterectomy. J Feline Med Surg 2006; 8: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rezaii T, Hirschberg AL, Carlstrom K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain 2012; 13: 646–655. [DOI] [PubMed] [Google Scholar]
- 3. Yates D, Yeates J, Roberts M. Optimum age for neutering cats. Vet Rec 2013; 172: 53–54. [DOI] [PubMed] [Google Scholar]
- 4. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steagall PV, Taylor PM, Rodrigues LC, et al. Analgesia for cats after ovariohysterectomy with either buprenorphine or carprofen alone or in combination. Vet Rec 2009; 164: 359–363. [DOI] [PubMed] [Google Scholar]
- 6. Reid J, Nolan AM, Hughes JML, et al. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Animal Welfare 2007; 16: 97–104. [Google Scholar]
- 7. Brondani JT, Luna SP, Padovani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res. 2011; 72: 174–183. [DOI] [PubMed] [Google Scholar]
- 8. Taylor PM, Kirby JJ, Robinson C, et al. A prospective multi-centre clinical trial to compare buprenorphine and butorphanol for postoperative analgesia in cats. J Feline Med Surg 2010; 12: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brondani J, Luna SP, Mama KR, et al. Cut-off point for rescue analgesia of unidimensional scales used to assess post operative pain in cats. In: Association of Veterinary Anaesthetists spring conference, London, 11–12 April 2013, p 78. Association of Veterinary Anaesthetists. [Google Scholar]
- 10. Reid J, Scott M, Nolan A, Wiseman-Orr L. Pain assessment in animals. In Pract 2013; 35: 51–56. [Google Scholar]
- 11. Guyton AC, Hall JE. Sensory receptors; Neuronal circuits for processing information. In: Guyton AC, Hall JE. (eds). Textbook of medical physiology. 9th ed. Philadelphia, PA: WB Saunders, 1996, pp 583–588. [Google Scholar]
- 12. Bushby PA, Griffin B. An overview of pediatric spay and neuter benefits and techniques. Vet Med 2011; 106: 283–290. [Google Scholar]
- 13. Joyce A, Yates D. Help stop teenage pregnancy! Early-age neutering in cats. J Feline Med Surg 2011; 13: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howe LM. Short-term results and complications of prepubertal gonadectomy in cats and dogs. J Am Vet Med Assoc 1997; 211: 57–62. [PubMed] [Google Scholar]
- 15. Spain CV, Scarlett JM, Houpt KA. Long-term risks and benefits of early-age gonadectomy in cats. J Am Vet Med Assoc 2004; 224: 372–379. [DOI] [PubMed] [Google Scholar]
- 16. Jarvis S, McLean KA, Chirnside J, et al. Opioid-mediated changes in nociceptive threshold during pregnancy and parturition in the sow. Pain 1997; 72: 153–159. [DOI] [PubMed] [Google Scholar]
- 17. Cook CJ. Steroidal hormones determine sex-related differences in opioid-induced elevation of nociceptive threshold in sheep (Ovis aries). N Z Vet J 1998; 46: 68–71. [DOI] [PubMed] [Google Scholar]
- 18. Whipple B, Josimovich JB, Komisaruk BR. Sensory thresholds during the antepartum, intrapartum and postpartum periods. Int J Nurs Stud 1990; 27: 213–221. [DOI] [PubMed] [Google Scholar]
- 19. Cruz Y, Martinez-Gomez M, Manzo J, et al. Changes in pain threshold during the reproductive cycle of the female rat. Physiol Behav 1996; 59: 543–547. [DOI] [PubMed] [Google Scholar]
- 20. Mathews KA. Pain management for the pregnant, lactating, and neonatal to pediatric cat and dog. Vet Clin North Am Small Anim Pract 2008; 38: 1291–1308. [DOI] [PubMed] [Google Scholar]
- 21. Lautenbacher S, Kunz M, Strate P, et al. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 2005; 115: 410–418. [DOI] [PubMed] [Google Scholar]
- 22. Dixon MJ, Taylor PM. The relationship between probe tip diameter and mechanical nociceptive threshold. In: 9th world congress of veterinary anaesthesia, Capetown, South Africa, 24-28 September 2012. http://www.topcatmetrology.com/bibliography/ [Google Scholar]


