Abstract
A 4-year-old female neutered domestic shorthair cat was presented for evaluation of gradual onset of lethargy and anorexia. Physical examination revealed moderate abdominal distension. Investigations performed included complete blood count, serum biochemistry, urinalysis, pyelocentesis, abdominal fluid analysis, abdominal ultrasonography and exploratory celiotomy. Nephrectomy was performed on the hydronephrotic kidney and a sample of the omentum was also taken, as it was grossly abnormal. No other abnormalities were found in the remainder of the abdominal organs. Findings were consistent with unilateral hydronephrosis and squamous cell carcinoma of the renal pelvis with abdominal carcinomatosis. The patient was given supportive treatment while the results of the biopsies from the renal tissue and the omentum were pending. The patient deteriorated a short time after surgical intervention and was euthanased. This is the first report of a squamous cell carcinoma arising from the renal pelvis in a cat. A comparison with the disease presentation in humans is also discussed.
Case Report
A 4-year-old rescued female neutered domestic shorthair cat presented with a 5-day history of anorexia, lethargy and weight loss, and a 2-day history of evident abdominal distension. The patient had been in the owner’s possession for 3 years; the age of the cat was estimated as being 9 months when it was acquired. According to the owner, the patient had no previous medical conditions. The patient did not receive any treatment prior to presentation.
Upon physical examination, the patient was lethargic, but responsive, and slightly underweight. Cardiopulmonary auscultation was unremarkable, and abdominal palpation revealed marked abdominal distension with a fluid thrill. Peripheral lymph nodes and rectal temperature were normal.
Complete blood count revealed a moderate regenerative anaemia [haematocrit 0.18 l/l, reference interval (RI) 0.26–0.46; reticulocyte count 102 × 109/l, RI 0–40 (102 × 103/μl, RI 0–40)] and marked neutrophilia without a left shift [40.58 × 109/l, RI 2.5–12.5 [40.58 × 103/μl, RI 2.5–12.5)]. Serum biochemistry showed mild hypoalbuminaemia [23 g/l, RI 25–40 (2.3 g/dl, RI 2.5–4.0)] and hyponatraemia [133 mmol/l, RI 138–155 (13 mEq/l, RI 138–155)] with hypochloridaemia [99 mmol/l, RI 112–129 (99 mEq/l, RI 112–129)], but no other abnormalities. The abdominal ultrasound revealed a large volume of echogenic fluid and significant abnormalities on both kidneys. The right kidney had a medullary rim sign with echogenic foci throughout the parenchyma (without distal shadow), but good vascularisation and normal size (4.64 cm in the longitudinal direction). The left kidney had a markedly distended renal pelvis containing echogenic fluid, but had a normal size (4.58 cm in the longitudinal direction). The renal pelvis distension was compressing the parenchyma, leaving a non-vascularised rim of parenchyma visible (Figure 1). The left ureter was markedly distended (up to 0.75 cm proximally and 0.7 cm distally), which could be followed to the level of the trifurcation of the aorta, but not further. No cause for the ureteral distension was identified ultrasonographically. The urinary bladder contained some urinary sediment, but had a normal appearance and it was poorly distended.
Figure 1.
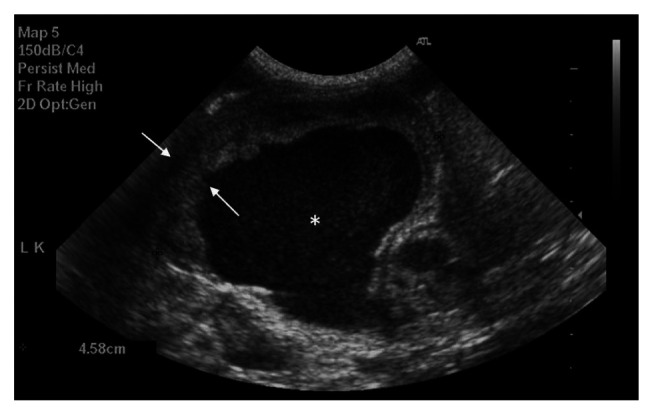
Hydronephrosis in the left kidney at abdominal ultrasound. Rim of renal parenchyma (arrows) and echogenic intrapelvic fluid (*)
Abdominocentesis was performed. The cytology of the effusion revealed occasional red cells without signs of erythrophagocytosis and small numbers of mesothelial cells. The analysis of the effusion was consistent with a transudate [nucleated cell count 0.37 × 109/l (0.37 × 103/μl), fluid protein 23 g/l (2.3g/dl), fluid red cell count 0.11 × 1012/l (0.11 × 103/μl), packed cell volume (PCV) of the fluid 1%]. A sample of urine was taken by pyelocentesis and its analysis revealed a fluid with similar cytological characteristics to the abdominal effusion with no atypical cells identified. However, this fluid contained a high protein content [42.7 g/l (4.27 g/dl)] and there were occasional haematoidin crystals.
An exploratory laparotomy was performed in order to find the cause of the ureteral distension and consider unilateral nephrectomy if indicated. On laparotomy approximately 250 ml of abdominal fluid were drained. The left kidney was located and its ureter followed distally to the bladder, but no cause for the distension was identified. The omentum had multiple colourless micronodules on its surface, giving it an irregular appearance (Figure 2) and suggesting carcinomatosis, but the rest of the abdominal organs, including the right kidney, had a normal gross appearance. A unilateral nephrectomy was performed and a sample of omentum was taken. Histopathology on both the kidney and the omentum was requested. The kidney was dissected in two portions, which gave it the appearance of an ‘egg shell’ (Figure 3). The patient recovered uneventfully from the anaesthesia and was hospitalised while the results of the histopathology were pending. The patient received intravenous crystalloids (0.9% sodium chloride at 4 ml/kg/h) from before the surgery, intravenous antibiotics [amoxicillin at 30 mg/kg (15 mg/lb), intravenously q8h] and analgesia [methadone 0.2 mg/kg (0.1 mg/lb), intramuscularly q4h for the first 12 h and then buprenorphine at 0.02 mg/kg (0.01 mg/lb), intramuscularly q8h for the next 24 h], and the patient was pain scored afterwards using the Glasgow pain score scale.
Figure 2.
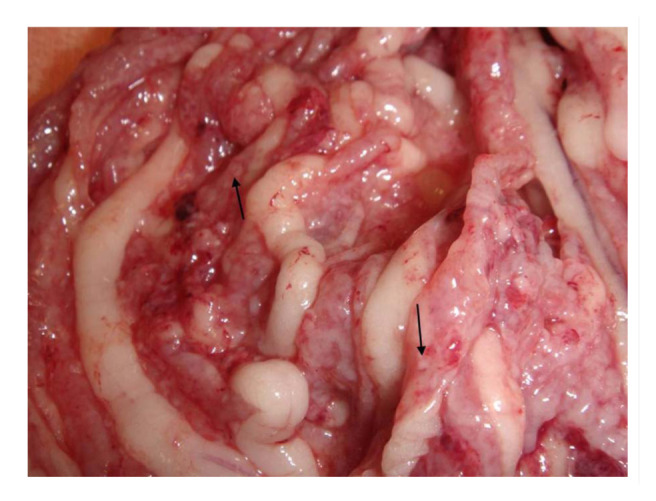
Macroscopic appearance of the omentum during exploratory laparotomy. Multiple micronodules on the omental surface (arrows)
Figure 3.
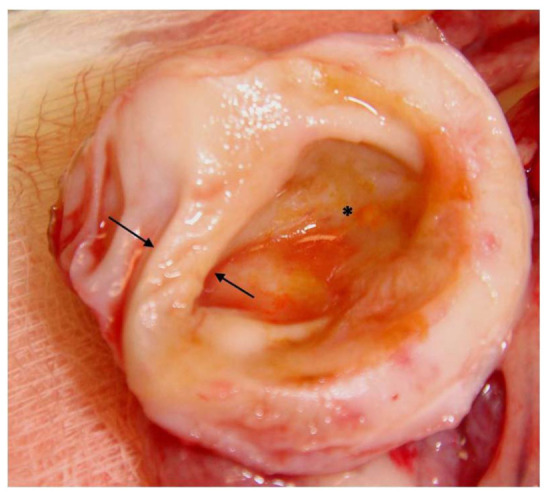
Macroscopic appearance of the hydronephrotic kidney after transverse section. Rim of renal parenchyma similar to Figure 1 (arrows) and dilated renal pelvis (*)
During the first 12 h the patient’s demeanour improved slightly, but its appetite remained irregular during the subsequent days of hospitalisation. A urinary catheter was not placed as the patient was urinating frequently and the urine output was estimated to be normal. Through the laparotomy wound there was a constant clear discharge, caused by the abdominal effusion leakage, but there was no evidence of a septic process developing.
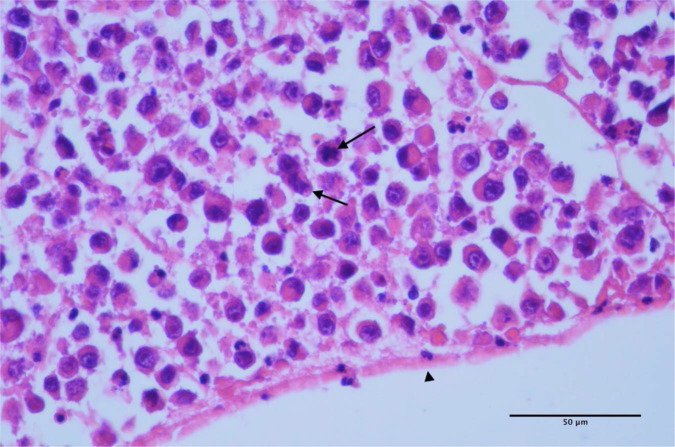
Histopathology revealed a squamous cell carcinoma of the renal pelvis that lead to severe hydronephrosis in the kidney with metastasis and carcinomatosis of the omentum (Figures 4–8). The tumour appeared to have developed from the pelvic transitional epithelium (Figure 4), resulting in infiltration along and around existing medullary tubules throughout the pelvis, with very extensive sclerosis and necrosis of the surrounding renal parenchyma. The neoplastic cells exhibited individual cell keratinisation and ill-defined keratin pearl formation (Figure 5), and distinct intercellular eosinophilic fibrillar adhesions (Figure 6). Immunohistochemical staining for cytokeratin AE1/AE3 revealed diffuse intense cytoplasmic staining of the neoplastic cells (Figure 7). The neoplastic cells extending within the omentum were very poorly differentiated, often individualised and exhibited frequent mitoses (Figure 8). These histological features are consistent with those described for squamous cell carcinoma of the urinary bladder in the most recent World Health Organization histological classification system for urinary system tumours. 1
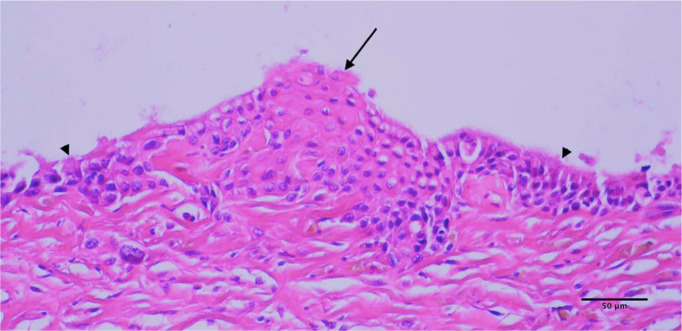
Figure 4.
In some areas of the pelvis, foci of squamous cell carcinoma formation (arrow) can be seen developing from areas of the pre-existing transitional epithelium lining the pelvis (arrow-heads)
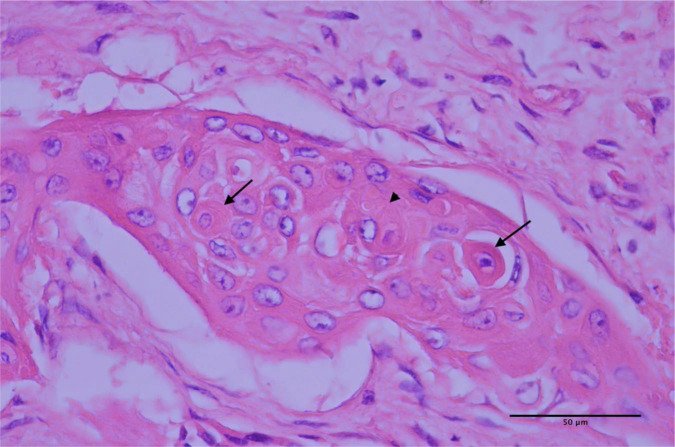
Figure 5.
Small clusters of neoplastic cells are infiltrating within the supporting connective tissue of the kidney. The neoplastic cells exhibit squamous differentiation, and exhibit individual keratinisation (arrows) and occasional ill-defined keratin-pearl formation (arrow-head)
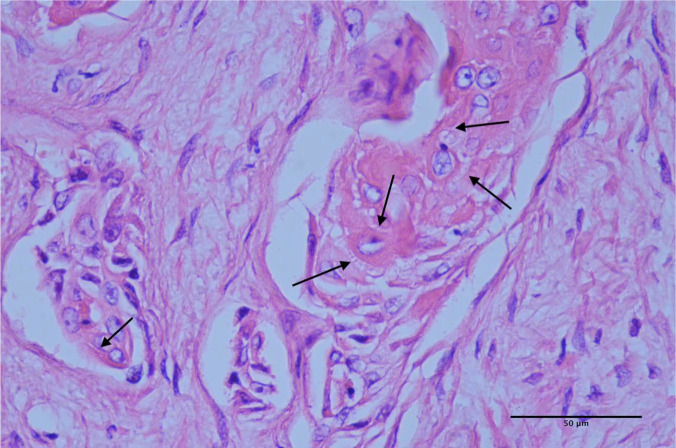
Figure 6.
In some areas, neoplastic cells exhibit distinct intercellular eosinophilic fibrillar adhesions (‘prickle cells’) (arrows)
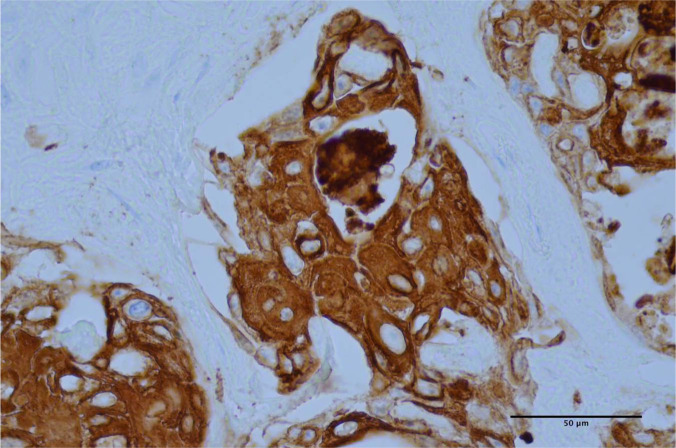
Figure 7.
Neoplastic cells exhibit diffuse intense cytoplasmic immunohistochemical staining for cytokeratin AE1/AE3
Figure 8.
Within the omentum, the neoplastic cells are very poorly differentiated, with frequent mitoses (arrows) forming dense sheets and extend to the serosal surface (arrow-head)
Considering the poor prognosis for metastatic carcinoma and the lack of improvement after the nephrectomy, the owner declined further treatment and the patient was euthanased. The owner declined a post-mortem examination.
Renal neoplasias are uncommon in cats — lymphoma being the most common renal neoplasia. Other renal neoplasias in cats include adenoma, adenocarcinoma, transitional cell carcinoma and nephroblastoma. 2 Hydronephrosis in cats is commonly caused by ureteral or urethral blockage due to urinary tract calculi, chronic inflammation, ureteral or urethral neoplasia, or neurogenic functional disorders, 3 but less common causes such as obstruction caused by blood clots in the renal pelvis or ureters after renal biopsies 4 , ectopic ureters5–7 and retroperitoneal fibrosis after renal transplantation 8 have been reported.
Squamous cell carcinoma of the renal pelvis has been reported as a rare renal neoplasia in older-aged people and commonly presents with hydronephrosis9,10 owing to obliteration of the proximal ureter with the tissue of the tumour. In humans, a link with previous history of nephroliths, 11 previous radiation therapy, 12 long-term treatment with cyclophosphamide 13 and horseshoe kidney 14 has been reported. There is only one case report of squamous cell carcinoma of the renal pelvis in one dog 15 that presented with bilateral multiple nephroliths and a unilateral mass in the renal pelvis that had metastasised to the small intestine and lungs. To our knowledge, squamous cell carcinoma of the renal pelvis has not been reported in cats.
This is the first case reported of squamous cell carcinoma of the renal pelvis in a cat. Chronic inflammation of the urothelium could cause hyperplasia of the transitional epithelium, with accumulations of mutations resulting in squamous metaplasia and subsequent malignant transformation through carcinoma in situ to invasive carcinoma, 16 but also from transformation of transitional cell carcinomas. 17 In humans, this type of cancer has been reported in elderly people, often following chronic nephrolithiasis. Nephrotomy for nephrolith removal has been recommended in humans to decrease the risk of development of this type of neoplasia. In comparison with transitional cell carcinomas, squamous cell carcinomas have a worse prognosis in humans owing to the presence of an advanced stage of the tumour at the time of diagnosis. However, when both tumours are compared at similar stages this difference does not exist. 18
The cat in this case report was young, and there was no nephrolith visible in the renal pelvis, furthermore, the cause of the hydronephrosis was not established, either ultrasonographically, or at the exploratory surgery. There was no visible mass within the renal pelvis that could have caused urine outflow obstruction or an intra- or extraluminal cause for the distal ipsilateral ureteral distension. The patient did not have any known history of urinary tract disease prior to presentation to our hospital for the 3 years that it had been with its current owners.
This cat presented with concurrent moderate regenerative anaemia. The amount of blood seen in the abdominal effusion was not enough to explain the degree of anaemia. However, the analysis of the fluid in the renal pelvis of the left kidney revealed the presence of haematoidin crystals that would suggest previous haemorrhage despite a current low PCV (1%) and a low red cell count [0.11 × 1012/l (0.11 × 103/μl)] of the fluid present in the hydronephrotic kidney. There was no evidence of haemolysis based on the history and clinico-pathological findings. The marked neutrophilia seemed to be caused by a combination of increased inflammatory demand to the neoplasia and a paraneoplastic neutrophilia, as it has been described previously in a dog with a renal tubular carcinoma. 19 The presence of hyponatraemia and hypochloridaemia were suspected to be due to third space loss (ie, abdominal effusion).
In humans, squamous cell carcinoma of the renal pelvis can occasionally invade into the inferior caudal vena cava20,21 and in the dog of the only available case report there was concurrent intestinal and pulmonary metastasis. The cat in this case report presented with extensive abdominal carcinomatosis, but no other metastases were found on ultrasonography or exploratory surgery. Thoracic radiographs were, however, not taken so pulmonary metastasis cannot not be fully excluded. The poorly differentiated omental tumour was assumed to be derived from the renal pelvis squamous cell carcinoma because no other primary tumour was found and the omental lesions were positive for immunohistochemical staining for cytokeratin AE1/AE3.
The prognosis for the squamous cell carcinoma is considered poor in humans and survival times between 4 11 and 16 months 12 have been reported. Surgical resection, chemotherapy and radiotherapy have shown disappointing results 11 because by the time the diagnosis is made, there is significant renal damage and often extra-renal metastasis.
Conclusion
To our knowledge, this is the first report of a squamous cell carcinoma arising from the renal pelvis in a cat.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Accepted: 9 June 2013
References
- 1. Meuten DJ, Everitt J, Inskeep W, et al. Urinary bladder tumors. In: Meuten DJ, Everitt J, Inskeep W, et al. (eds). World Health Organization International Histological Classification of Tumors of Domestic Animals, Histological Classification of Tumors of the Urinary System of Domestic Animals, Second Series, Vol XI. Washington, DC: The Armed Forces Institute of Pathology, 2004, pp 26–37. [Google Scholar]
- 2. Henry CJ, Turnquist SE, Smith A, et al. Primary renal tumours in cats: 19 cases (1992–1998). J Feline Med Surg 1999; 1: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newman SJ, Confer AW, Panciera RJ. Urinary system. In: McGavin MD, Zachary JF. (eds). Pathologic basis of veterinary disease. 4th ed. St Louis, MO: Mosby Elsevier, 2007, pp 667–668. [Google Scholar]
- 4. Vaden SL, Levine JF, Lees GE, et al. Renal biopsy: a retrospective study of methods and complications in 283 dogs and 65 cats. J Vet Intern Med 2005; 19: 794–801. [DOI] [PubMed] [Google Scholar]
- 5. Holt PE, Gibbs C. Congenital urinary incontinence in cats: a review of 19 cases. Vet Rec 1992; 130: 437–442. [DOI] [PubMed] [Google Scholar]
- 6. Steffey MA, Brockman DJ. Congenital ectopic ureters in a continent male dog and cat. J Am Vet Med Assoc 2004; 224: 1607–1610. [DOI] [PubMed] [Google Scholar]
- 7. D’Ippolito P, Nicoli S, Zatelli A. Proximal ureteral ectopia causing hydronephrosis in a kitten. J Feline Med Surg 2006; 8: 420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aronson LR. Retroperitoneal fibrosis in four cats following renal transplantation. J Am Vet Med Assoc 2002; 221: 984–989. [DOI] [PubMed] [Google Scholar]
- 9. Silverstone M. Squamous-cell carcinoma of the renal pelvis: Report of a case. Br J Surg 1935; 23: 332–336. [Google Scholar]
- 10. Shimasaki N, Inoue K, Nishigawa H, et al. Combined small cell carcinoma and sarcomatoid squamous cell carcinoma in the renal pelvis. Int J Urol 2005; 12: 686–689. [DOI] [PubMed] [Google Scholar]
- 11. Kayaselçuk F, Bal N, Guvel S, et al. Carcinosarcoma and squamous cell carcinoma of the renal pelvis associated with nephrolithiasis: a case report of each tumor type. Pathol Res Pract 2003; 199: 489–492. [DOI] [PubMed] [Google Scholar]
- 12. O’Daly BJ, O’Brien MF, Dowling CM, et al. Squamous cell carcinoma of the renal pelvis after curative retroperitoneal radiotherapy for seminoma. Urology 2007; 70: 812.e3–812.e6. [DOI] [PubMed] [Google Scholar]
- 13. McDougal WS, Cramer SF, Miller R. Invasive carcinoma of the renal pelvis following cyclophosphamide therapy for nonmalignant disease. Cancer 1981; 48: 691–695. [DOI] [PubMed] [Google Scholar]
- 14. Mizusawa H, Komiyama I, Ueno Y, et al. Squamous cell carcinoma in the renal pelvis of a horseshoe kidney. Int J Urol 2004; 11: 782–784. [DOI] [PubMed] [Google Scholar]
- 15. Dagli ML, Calderaro FF, Silva MT, et al. Squamous cell carcinoma of the renal pelvis with metastasis in a dog. J Comp Pathol 1997; 116: 397–402. [DOI] [PubMed] [Google Scholar]
- 16. Tsutsumi M, Tsai YC, Gonzalgo ML, et al. Early acquisition of homozygous deletions of p16/p19 during squamous cell carcinogenesis and genetic mosaicism in bladder cancer. Oncogene 1998; 17: 3021–3027. [DOI] [PubMed] [Google Scholar]
- 17. Kunze E. Histogenesis of non-urothelialcarcinomas in the human and rat urinary bladder. Exp Toxicol Pathol 1998; 50: 341–355. [DOI] [PubMed] [Google Scholar]
- 18. Berz D, Rizack T, Weitzen S. Survival of patients with squamouscell malignancies of the upper urinary tract. Clin Med Insights Oncol 2012; 6: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lappin MR, Latimer KS. Hematuria and extreme neutrophilic leukocytosis in a dog with renal tubular carcinoma. J Am Vet Med Assoc 1988; 192: 1289–1292. [PubMed] [Google Scholar]
- 20. Oh SJ, Lim DJ, Cho JY, et al. Squamous cell carcinoma of the renal pelvis with invasion of the infradiaphragmatic inferior vena cava. Br J Urol 1998; 82: 918–919. [DOI] [PubMed] [Google Scholar]
- 21. Kimura T, Kiyota H, Asano K, et al. Squamous cell carcinoma of the renal pelvis with inferior vena caval extension. Int J Urol 2000; 7: 316–319. [DOI] [PubMed] [Google Scholar]