Abstract
Bleeding time is a screening test for the evaluation of primary haemostasis. As there is currently limited information on the reference interval (RI) and repeatability of the test in the cat compared with the dog, the purpose of the study was to establish the RI of buccal mucosa bleeding time (BMBT) in healthy cats and to investigate the intra-observer repeatability of the test. Fifty-six cats were prospectively enrolled in the study. The animals were deemed to be healthy based on history, physical examination, complete blood count, serum biochemistry, and negative serological testing for feline leukaemia and immunodeficiency viruses. All cats were sedated with ketamine, dexmedetomidine and morphine, and the BMBT was sequentially measured in the left and right exposed buccal mucosa following a standardised incision made by a commercially available, disposable, bleeding time device. The mean BMBT was 58.6 s and the RIs ranged from 34 to 105 s (Bootstrap estimation). The intra-observer repeatability was up to 87 s (Bland–Altman plot). The results of this study imply that the combination of ketamine, dexmedetomidine and morphine is a safe and useful sedative protocol allowing for the reliable measurement of BMBT in the cat. The RI of feline BMBT may range from 34 to 105 s and the BMBT may differ by up to 87 s for any two consecutive readings for an individual cat.
Introduction
Bleeding time (BT) is a point-of-care test for the in vivo evaluation of primary haemostasis in the dog and cat.1–3 Provided that platelet count is adequate (>70,000–100,000/μl), BT is expected to be prolonged in inherited or acquired platelet defects and vascular impairment.4–7 Unlike dogs, in which certain BT techniques [ie, buccal mucosal bleeding time (BMBT), toe capillary bleeding time] have been sufficiently standardised and evaluated for their diagnostic utility in healthy or in dogs with a range of haemostatic defects,1,3,8–13 limited information is currently available regarding the performance characteristics of BT techniques in the cat, including properly defined reference intervals (RIs), repeatability and clinical application. BMBT measurements in the cat have mostly been reported in studies investigating the potential interference of analgesic medications with platelet function,14,15 or in those assessing the efficacy of antiplatelet drugs in cats at risk of cardiogenic thromboembolism.16–18 Moreover, the BMBT was shown to demonstrate a reasonable diagnostic sensitivity and specificity when evaluated among three groups of cats, including healthy individuals, and cats with inherited platelet (Chediak–Higashi syndrome) or coagulation (Hageman factor deficiency) defects, respectively. 19 With the exception of the study by Carroll et al, 14 in which BMBT was measured in 138 cats evaluated for the efficacy and side effects of pre-operative analgesia, the remaining studies included small numbers of cats or described BT techniques not widely used in the clinical setting; notably, no study has evaluated the repeatability of the applied techniques. Therefore, the purpose of this study was to establish the RI of the BMBT in a population of clinically healthy sedated cats and to investigate the intra-observer repeatability of the test by means of a commercially-available, disposable, single-blade device.
Materials and methods
Fifty-six healthy cats were prospectively enrolled in the study. They included domestic shorthair (52/56, 92.9%) and domestic longhair (4/56, 7.1%) cats of which 20 (35.7%) were males and 36 (64.3%) females with an age range of 6 to 72 months (median 27 months). The animals were admitted to the Companion Animal Clinic, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki (FVM-AUTh) for neutering. Informed consent was obtained from the owners for participation in the study and the latter was approved by the Research and Ethical Committee of FVM-AUTh. The cats were deemed healthy based on history, physical examination, a normal complete blood count performed in an automated analyser (PocH-100iV Diff; Sysmex) and a normal serum biochemistry profile, including total protein, creatinine and urea nitrogen concentrations, and alanine aminotransferase and alkaline phosphatase activities. Additionally, all the cats tested negative for feline leukaemia virus antigens and feline immunodeficiency virus antibodies (Witness FeLV-FIV; Synbiotics). For those cats in which the haematology analyser generated a spuriously low platelet count [n = 19 (platelet counts <180,000/μl, RIs 180,000–550,000/μl)], the adequacy of platelet counts (ie, >150,000/μl) (and eventually enrollment in the study) was based on a blood smear reviewed for clumping and platelet count estimation. All the cats were readmitted 7–10 days after the operation for suture removal. At that time they all remained healthy based on clinical examination.
Prior to BMBT measurement, the cats were sedated with an intramuscular injection of ketamine (Imalgene 1000, 5 mg/kg; Merial), dexmedetomidine (Dexdomitor, 30 μg/kg; Pfizer) and morphine (Demo SA, 0.2 mg/kg; Pharmaceutical Industry) and placed in left lateral recumbency. The right upper lip was everted and secured with a gauze strip which was tightly tied through the mouth and around the cranium rostral to the ipsilateral and caudal to the contralateral ear. A commercially-available, disposable, single-blade, spring-loaded BT device (Surgicutt ‘adult’; International Technidyne Corporation) was used from a clinician (DGA) familiar with the BMBT measurement procedure as previously described in the cat. 19 The device was placed parallel to the gum line on a grossly non-vascular and non-lesional mucosal area of the lip, and the trigger was depressed to cause a standardised laceration (5 mm × 1 mm). Filter paper was used to blot the blood 1–2 mm away from the incision, avoiding disturbing the incision site. When fibrin films occluded the blood flow from the wound, the filter paper was used to gently remove the fibrin without disturbing the incision site. A stopwatch was used to measure the BMBT, defined as the time between the start and cessation (no more blood absorbed on the filter paper) of bleeding. After the BMBT measurement was completed on the right side, the procedure was repeated at the opposite side of the lip mucosa.
The BMBT RI was calculated by bootstrap estimation 20 that adjusted for the within-cat correlation of the BMBT measurements. Thus, 56 observations were randomly selected, with replacement, from the original 112 BMBT observations with equal probability of selection between the BMBT on the left (BMBTL) and right (BMBTR) lip mucosa. This was repeated 50,000 times so as to obtain estimates of the mean, the 1st, 2.5th, 5th, 50th (median), 95th, 97.5th and 99th percentiles. Bootstrap estimation was carried out in R (http://www.r-project.org/) and the corresponding code is freely available by one of the authors (PK) upon request.
For the evaluation of the intra-observer repeatability the Bland–Altman plot21,22 was initially used to graphically assess the differences (d’s) between the BMBTL and the BMBTR, plotted against the averages of BMBTL and BMBTR. Subsequently, we calculated the repeatability coefficient rw=2.77* sw, where sw is the SD of the d’s. Eventually, the degree of intra-observer repeatability, which is the range over which the 95% of the d’s lie was calculated as . These measures of the intra-observer repeatability are based on the assumption of normality for the d’s. Despite the fact that the Shapiro–Wilk test (SW) revealed deviation from normality for the BMBTL and BMBTR (SW = 0.79, P <0.001 and SW = 0.91, P <0.001, respectively), their d’s were expected to, and did, follow a normal distribution (SW = 0.99, P = 0.99). This is owing to the fact that a lot of the variation between subjects is removed when calculating the d’s.
Results
In all cats, the combination of ketamine, dexmedetomidine and morphine induced a rapid-onset sedation and anaesthesia, and recovery was uneventful. The disposable spring-loaded blades consistently produced a standardised incision in the oral mucosa, which bled sufficiently to permit the BMBT evaluation. In a small number of cats (n = 4), no bleeding was observed despite the fact that the mucosa had been lacerated. In these animals, a new laceration was made close to the original, and the procedure was completed successfully.
The frequency distribution of the bootstrap sample is shown in Figure 1. The estimated mean and percentiles are given in Table 1. The mean BMBT was 58.6 s, while the RI was 34–105 s. The Bland–Altman plot is shown in Figure 2. The rw was 61.22. The degree of the intra-observer repeatability ranged from −34.59 to +52.05, which means that BMBTL may be approximately 35 s below or 52 s above BMBTR. Consequently, for any two sequential readings within a cat, the BMBT may differ by up to 87 s.
Figure 1.
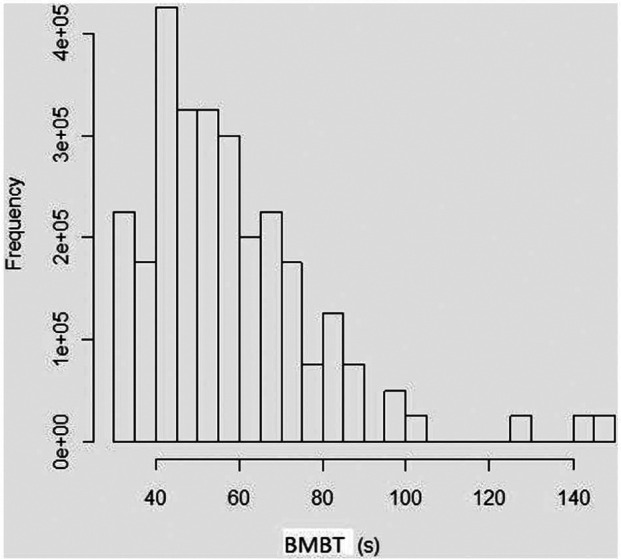
Frequency distribution of the estimated bleeding time (in seconds) of the buccal mucosa bleeding time (BMBT). The distribution is based on 50,000 samples obtained through bootstrap estimation method that assigned equal weights to BMBT observations in the left and right lip mucosa
Table 1.
Mean and percentiles (in seconds) for the buccal mucosa bleeding time (BMBT) in 56 healthy cats sedated with ketamine, dexmedetomidine and morphine. Estimates are based on 50,000 samples obtained through bootstrap estimation method that assigned equal weights to the left (BMBTL) and right (BMBTR) lip mucosa observations
| Mean | 1st | 2.5th | 5th | 50th | 95th | 97.5th | 99th |
|---|---|---|---|---|---|---|---|
| 58.63 | 32.39 | 33.66 | 35.17 | 55.62 | 91.11 | 104.56 | 118.02 |
Figure 2.
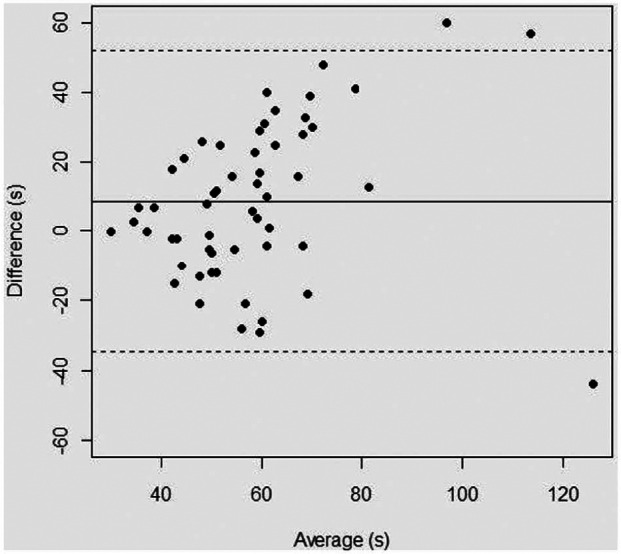
Bland–Altman plot of the differences (in seconds) between the buccal mucosa bleeding time (BMBT) of the left and right lip mucosa of 56 cats plotted against the corresponding average of the left and right side BMBT for each cat. The continuous central horizontal line corresponds to the mean difference, and the dotted lines at either side indicate the range over which the 95% of differences lie (ie, the degree of intra-observer repeatability)
Discussion
The RI of the BMBT established in this study were consistently below 2 mins (34–105 s) and compared favourably with those reported in the majority of the studies measuring the BMBT with a single-blade device, totaling 159 sedated cats.14,16–18 In another study of 30 sedated cats, the BMBT interval measured with the same device ranged up to 3 mins. 19 To the best of our knowledge, there has been only one report of 10 cats in which the BMBT interval ranged up to 4 mins. 23 In the latter, the cats had received only light sedation induced by ketamine hydrochloride (3–5 mg/kg), which is in contrast to the other studies14,16–19 in which heavy sedation or general anaesthesia was induced by combining multiple sedatives and/or anaesthetics, possibly implying that the difference in the plane of sedation may partially affect the BMBT. Overall, the results of this and previous studies indicate that for the clinical decision-making, a BMBT above 3 mins should be considered abnormal in the vast majority of sedated cats. In dogs, a BMBT less than 4 mins is considered normal. This interspecies difference, along with the observation that a few cats in this study did not bleed until a second laceration was effected, probably underscores the robustness of the feline primary haemostatic system. Feline platelets are more responsive to aggregation inductors (eg, collagen, adenosine diphosphate or thrombin) than those of dogs. In addition, the dense granules of cats were shown to contain three or 1.5 times the amount of serotonin compared with dense granules of humans or dogs, respectively. Serotonin is a potent vasoconstrictor and induces primary aggregation of platelets. In addition, serotonin will potentiate the aggregation effect of other agonists. 24
To our knowledge, this is the first study evaluating the intra-observer repeatability of the BMBT in the cat, contributing to the improvement of the performance profile of the test. The results indicated that for any two sequential measurements within a cat, performed by the same observer, the BMBT may differ by up to 87 s, presumably owing to technical variables, such as differences in the density of capillary web at the incision site, the degree of venostasis, the vertical or horizontal orientation of the laceration, and, possibly, the plane of sedation.25,26 This means that before a treatment can be interpreted to have affected the BMBT in a cat, the pre- and the post-treatment BMBT would need to differ by at least 87 s. Of comparative interest, the repeatability of the BMBT is 2 mins in the dog. 12 The Bland–Altman plot may also indicate that the difference between two consecutive BMBT measurements is higher for higher BMBT values because there seems to be a wider scattering of the differences for higher BMBT averages (Figure 2). It is unclear if this tendency is of clinical relevance.
Similar to dogs, the BMBT appears to be the most commonly used technique for the BT measurement in the cat. However, chemical restraint is invariably required for a reliable BMBT measurement.8,14,19 Various multidrug protocols have been used in previous studies, including ketamine, atropine and acetylpromazine,16,17,19 acetylpromazine, glycopyrrolate, propofol and isoflurane, 14 and ketamine and diazepam. 18 To our knowledge, the combination of ketamine, dexmedetomidine and morphine used in this study is reported for the first time in cats subject to BMBT measurements and apparently does not affect platelet function. Opioids, α2-adrenergic agonists and ketamine are frequently used for short-term anaesthesia in cats. This combination does not cause hypotension, it produces excellent analgesia and attains a great reduction in anaesthetic requirements if a deeper plane of anaesthesia is required, thus is less likely that hypotension will occur. 27 The in vitro effect of α2-adrenergic agonists and opioids in the platelet function is well known. 13 However, its clinical relevance, at least in the dog, was recently reported to be negligible. 13 An alternative technique has been used in a single study in which BT was measured by puncturing the external surface of the ear with a scalpel in 40 conscious cats. 15 The BT ranges in the latter (overall, below 2 mins) were quite similar to the BMBT intervals of the present and other studies,14,16–18 indicating that this technique may be of value in alert, cooperative cats or in cats in which chemical restraint is contraindicated or declined by the owner.
In this study, the BMBT RI was calculated by bootstrap estimation, which is a general, distribution-free method used to estimate parameters of interest by drawing samples from the original data with replacement. 20 Sound estimation of reference intervals should take into proper consideration the presence of correlated observations, while, at the same time, making efficient use of all available data. 28 Specifically, if we picked at random a single observation from each subject or used only the first measurement to calculate the normal range, this would clearly not make efficient use of all of the available data. Further, it would have been wrong to take the average of the values for each subject and then calculate the percentiles because this would have inappropriately removed the within-subject variability and led to narrower, incorrect estimates. Finally, ignoring the fact that we have paired measurements and treating them as independent may have worked in our case as we have equal repetitions for each cat, but is generally wrong if different numbers of repeated measurements are obtained for different individuals. 28 Thus, we developed and applied a bootstrap estimation algorithm in R that adjusted for the within-cat correlation of the BMBT observations. In our case, equal weights were assigned to BMBTL and BMBTR measurements as we had paired observations within each cat, but our algorithm can be easily modified for its application in studies of uneven number of repeated measurements per subject.
Conclusions
The results of this study suggest that in the clinical setting, the combination of ketamine, dexmedetomidine and morphine is a useful sedative protocol allowing for the measurement of BMBT in the cat. Under sedation, the RI of feline BMBT was 34–105 s, while the intra-observer repeatability may be up to 87 s.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 28 July 2013
References
- 1. Brooks M, Catalfamo J. Buccal mucosa bleeding time is prolonged in canine models of primary hemostatic disorders. Thromb Haemostas 1993; 70: 777–780. [PubMed] [Google Scholar]
- 2. Marks SL. The buccal mucosal bleeding time. J Am Anim Hosp Assoc 2000; 36: 289–290. [DOI] [PubMed] [Google Scholar]
- 3. Mischke R. Haemostasis: diagnostic techniques. In: Day MJ, Kohn B. (eds). Manual of canine and feline haematology and transfusion medicine. 2nd ed. Gloucester: BSAVA, 2012, pp 189–200. [Google Scholar]
- 4. Sakai M, Watari T, Miura T, et al. Effects of DDAVP administered subcutaneously in dogs with aspirin-induced platelet dysfunction and hemostatic impairment due to chronic liver disease. J Vet Med Sci 2003; 65: 83–86. [DOI] [PubMed] [Google Scholar]
- 5. Smith JW, Day TK, Mackin A. Diagnosing bleeding disorders. Comp Cont Educ Pract Vet 2005; 27: 828–843. [Google Scholar]
- 6. Bromel K. Buccal mucosal bleeding time. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, MO: Saunders, 2010, pp 364–365. [Google Scholar]
- 7. Tarnow I, Ktistensen AT. Evaluation of platelet function. In: Weiss DJ, Wardrop KJ. (eds) Schalm’s veterinary hematology. 6th ed. Ames, IA: Wiley-Blackwell, 2010, pp 1123–1132. [Google Scholar]
- 8. Jergens AE, Turrentine MA, Kraus KH, et al. Buccal mucosa bleeding times of healthy dogs and of dogs in various pathologic states including thrombocytopenia, uremia, and von Willebrand’s disease. Am J Vet Res 1987; 48: 1337–1342. [PubMed] [Google Scholar]
- 9. Brassard JA, Meyers KM. Evaluation of the buccal bleeding time and platelet glass retention as assays of hemostasis in the dog: the effects of acetylsalicylic acid, warfarin and von Willebrand factor deficiency. Thromb Haemostas 1991; 65: 191–195. [PubMed] [Google Scholar]
- 10. Brassard JA, Meyers KM, Person M, et al. Experimentally induced renal failure in the dog as an animal model of uremic bleeding. J Lab Clin Med 1994; 124: 48–54. [PubMed] [Google Scholar]
- 11. Nolte I, Niemann C, Bowry SK, et al. A method for measuring capillary bleeding time in non-anesthetized dogs: prolongation of the bleeding time by acetylsalicylic acid. J Vet Med 1997; 44: 625–628. [DOI] [PubMed] [Google Scholar]
- 12. Sato I, Anderson GA, Parry BW. An interobserver and intraobserver study of buccal mucosal bleeding time in Greyhounds. Res Vet Sci 2000; 68: 41–45. [DOI] [PubMed] [Google Scholar]
- 13. Mylonakis ME, Kazakos GM, Pardali D, et al. Bleeding time in healthy dogs sedated with morphine and medetomidine. Vet Rec 2011; 169: 470. [DOI] [PubMed] [Google Scholar]
- 14. Carroll GL, Howe LB, Peterson KD. Analgesic efficacy of preoperative administration of meloxicam or butorphanol in onychectomized cats. J Am Vet Med Assoc 2005; 226: 913–919. [DOI] [PubMed] [Google Scholar]
- 15. Brondani JT, Luna SPL, Marcello GCG, et al. Perioperative administration of vedaprofen, tramadol or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg 2009; 11: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogan DF, Andrews DA, Green HW, et al. Antiplatelet effects and pharmacodynamics of clopidogrel in cats. J Am Vet Med Assoc 2004; 225: 1406–1411. [DOI] [PubMed] [Google Scholar]
- 17. Hogan DF, Andrews DA, Talbott KK, et al. Evaluation of antiplatelet effects of ticlopidine in cats. Am J Vet Res 2004; 65: 327–332. [DOI] [PubMed] [Google Scholar]
- 18. Cathcart CJ, Brainard BM, Reynolds LR, et al. Lack of inhibitory effect of acetylsalicylic acid and meloxicam on whole blood platelet aggregation in cats. J Vet Emerg Crit Care 2012; 22: 99–106. [DOI] [PubMed] [Google Scholar]
- 19. Parker MT, Collier LL, Kier AB, et al. Oral mucosal bleeding times of normal cats and cats with Chediak-Higashi syndrome or Hageman trait (factor XII deficiency). Vet Clin Pathol 1988; 17: 9–12. [DOI] [PubMed] [Google Scholar]
- 20. Efron B, Tibshirani R. An introduction to the bootstrap. Monographs on statistics and applied probability 57. 1st ed. New York: Chapman and Hall, 1993. [Google Scholar]
- 21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–160. [DOI] [PubMed] [Google Scholar]
- 23. Bright JM, Sullivan PS, Melton SL, et al. The effects of n-3 fatty acid supplementation on bleeding time, fatty acid composition, and in vitro platelet aggregation in cats. J Vet Intern Med 1994; 8: 247–252. [DOI] [PubMed] [Google Scholar]
- 24. Wondratschek C, Weingart C, Kohn B. Primary immune-mediated thrombocytopenia in cats. J Am Anim Hosp Assoc 2010; 46: 12–19. [DOI] [PubMed] [Google Scholar]
- 25. Mielke CH., Jr. Aspirin prolongation of the template bleeding time: influence of venostasis and direction of incision. Blood 1982; 60: 1139–42. [PubMed] [Google Scholar]
- 26. Buchanan GR, Holtkamp CA. A comparative study of variables affecting the bleeding time using two disposable devices. Am J Clin Pathol 1989; 91: 45–51. [DOI] [PubMed] [Google Scholar]
- 27. Wiese AJ, Muir WW. Anaesthetic and cardiopulmonary effects of intramuscular morphine, medetomidine and ketamine administered to telemetered cats. J Feline Med Surg 2007; 9: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor JMG, Cumberland WG, Meng X, et al. Normal range estimation for repeated immunologic measures. Clin Diagn Lab Immunol 1996; 3: 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]


