Abstract
The purpose of this study was to describe changes in calcium, phosphorus, magnesium, parathyroid hormone, calcitriol and calcidiol in cats from 3 to 15 months of age. Fourteen European shorthair healthy cats of both sexes (seven males, seven females) belonging to a research colony were studied from 3 to 15 months of age. Plasma concentrations of total calcium, ionised calcium, albumin, phosphorus, magnesium, intact parathyroid hormone (I-PTH), whole parathyroid hormone (W-PTH), calcidiol and calcitriol were measured at 3, 6, 9, 12 and 15 months of age. From 3 months of age to adulthood cats showed a decrease in calcium (both total and ionised), phosphorus and magnesium. No major changes in PTH were evident, although the ratio of W-PTH:I-PTH decreased significantly with age. A reciprocal change in vitamin D metabolites (decrease in calcitriol and increase in calcidiol) was identified during the growing process. Our results, showing changes in most parameters of mineral metabolism during growth, reinforce the need to use adequate age-related reference values for diagnostic purposes.
Mineral metabolism in growing animals is subjected to changes derived from the different rates of bone growth along time. These changes are reflected in the blood concentrations of minerals (calcium, phosphorus and magnesium) and hormones that participate in their homeostasis [parathyroid hormone (PTH) and vitamin D]. Thus, blood parameters of mineral metabolism tend to be different in the young growing animal and in the adult. 1
Plasma calcium and phosphorus have been reported to be higher in young animals along growth phases because of increased bone turnover.2–4 Calcium concentrations are greatest in puppies younger than 8 weeks of age and then decrease to adult levels at about 1 year of age. 3 Kittens have also been reported to have higher calcium concentrations before 8 weeks of age. 4 In puppies, phosphorus concentrations are increased above adult levels throughout the growth process and adult concentrations are reached at approximately 1 year of age. 3 The age effect is supposed to be less pronounced in cats, but immature cats also tend to have higher serum phosphorus concentrations. 4 Age variations have not been described for serum magnesium concentrations in puppies or kittens. 2
In contrast with the minerals, changes in calciotropic hormones during growth have not been studied with detail in domestic carnivores. Two major hormones, PTH and 1,25-dihydroxyvitamin D (calcitriol) are involved in bone remodelling during growth. In humans, several studies have reported an increase of serum PTH concentrations throughout childhood and adolescence.5,6 Calcitriol concentration is also elevated significantly in infants and adolescents when compared with adult standards. 7 However, serum concentrations of the precursor of calcitriol, 25-hydroxyvitamin D (calcidiol) are almost identical in infants, children, adolescents and adults. 8 The elevated calcitriol concentration during infancy and adolescence correlates with increased growth rates and, presumably, functions to enhance intestinal mineral absorption for rapid growth. 9
In cats, knowledge about blood parameters related to mineral metabolism is scanty and there are only a few reports about changes in plasma minerals with growth.2,4 Moreover, apart from scattered measurements obtained in healthy young animals to compare with clinical cases, 10 no information is available on changes in calciotropic hormones in growing cats.
We hypothesised that significant changes in the values of blood parameters related to mineral metabolism should be present along the growing process of the cat. Thus, the purpose of the work reported here was to describe changes in calcium, phosphorus, magnesium, PTH, calcitriol and calcidiol in cats from the age of 3 to 15 months, which was defined as adulthood.
Materials and methods
Fourteen European shorthair cats of both sexes (seven males, seven females) were studied. All cats were sexually intact. Cats were kept in a cattery belonging to the Animal House Facility of the University of Cordoba and were socialised by frequent human contact to prevent stress associated with handling. The animals had been monitored since birth and, at the time of entry in the experiment (age = 3 months), were subjected to a detailed physical examination and a blood analysis (complete blood count and biochemical profile) to assure clinical normality. All experimental procedures were approved by the ethics committee of the University of Cordoba.
Study design
After weaning, cats were raised on a commercial dry food for kittens (BabyCat; Royal Canin) containing 1.1% calcium, 0.97% phosphorus and 700 IU/kg vitamin D. At the time the experiments were started cats were switched to a junior diet (Kitten Advance; Affinity-Petcare SA) with the following content: 1.2% calcium, 1% phosphorus and 1500 IU/kg vitamin D. They were maintained on this diet for the whole experimental protocol. The first blood samples were obtained when the cats were 3 months old; subsequently, cats were sampled every 3 months until the age of 15 months. Blood samples were obtained with minimal restrain from the jugular vein using heparinised syringes and were handled anaerobically.
Analytical methods
Blood ionised calcium (iCa) was measured immediately after collection using selective electrodes (Bayer Diagnostics); then, samples were centrifuged and plasma was frozen at −20ºC. No changes in the analytes under study have been reported after freezing for 2 months at −20ºC.11–13 Total calcium (tCa), phosphorus, magnesium and albumin were quantified by spectrophotometry (BioSystems SA). These techniques are used routinely in our hospital for the measurement of feline samples. PTH was measured in plasma samples within 2 months of collection using an immunoradiometric assay (IRMA) (Duo PTH Kit; Scantibodies Laboratory). This kit is designed for the quantitative determination of human ‘whole’ PTH (W-PTH) and ‘intact’ PTH (I-PTH), and has been recently validated by our laboratory for measurement of feline PTH. 14 The kit contains two IRMA assays. Both assays share a polyclonal antibody (anti-PTH 39-84) coated on to the surface of polystyrene beads as a solid phase. The IRMA for W-PTH uses a tracer antibody directed against the most N-terminal PTH (1-4) region. The use of this antibody guarantees that only biologically active PTH is detected. The IRMA for I-PTH uses a specific polyclonal antibody directed against PTH (7-34) as a tracer. With this antibody both W-PTH (1-84) and N-truncated PTH fragments (7-84) are detected. In addition to W-PTH and I-PTH measurements, the ratio between both parameters (W-PTH:I-PTH ratio) was calculated. Calcitriol and calcidiol were determined in plasma samples using a radioimmunoassay (Immunodiagnostic Systems). Both kits are complete assay procedures intended for the extraction and quantification of calcitriol and calcidiol, respectively. Intra- and interassay variation for these assays is: calcitriol, 9% and 12% respectively; and calcidiol, 6% and 8%, respectively. The sensitivity of the assays is <3 pg/ml for calcitriol and <2 ng/ml for calcidiol. Both assays showed linearity during dilution of feline plasma samples and the percentages of spiking recovery were 90 ± 6 % (calcitriol) and 107 ± 3 % (calcidiol). No matrix effect was detected when comparing dilutional parallelism of feline and human plasma samples.
Statistical analysis
Statistical analysis was performed by using the computer package SPSS for Windows 15.0. A Kolmogorov–Smirnov test was carried out to test for normality. All data sets, except albumin and PTH (I-PTH and W-PTH), passed normality testing. The effect of time on the normally distributed variables was assessed by repeated measures of analysis of variance using a general linear model with Fisher’s least significant difference as a post hoc test. Kruskal–Wallis analysis followed by a post hoc rank total test was used for albumin and PTH. P-values <0.05 were considered significant. Values are given as the mean ± standard error.
Results
Total calcium concentration ranged between 9.8 and 10.2 mg/dl and did not change along the first 9 months of the study. At 12 months tCa started to decrease and was significantly lower at 15 months of age (8.6 ± 0.3 mg/dl). In contrast with tCa, a constant trend to decrease in iCa was observed along the experiments. Younger cats had higher iCa concentrations (5.28 ± 0.08 mg/dl, at 3 months of age) and iCa decreased progressively during the growing period, reaching a minimum of 4.96 ± 0.04 mg/dl at 12 months. Ionised calcium concentration remained steady from 12 to 15 months of age. Albumin concentration was around 3 g/dl at all sampling times except at 3 months — at this age a significantly lower albumin concentration (2.3 ± 0.1 g/dl) was measured (Figure 1).
Figure 1.
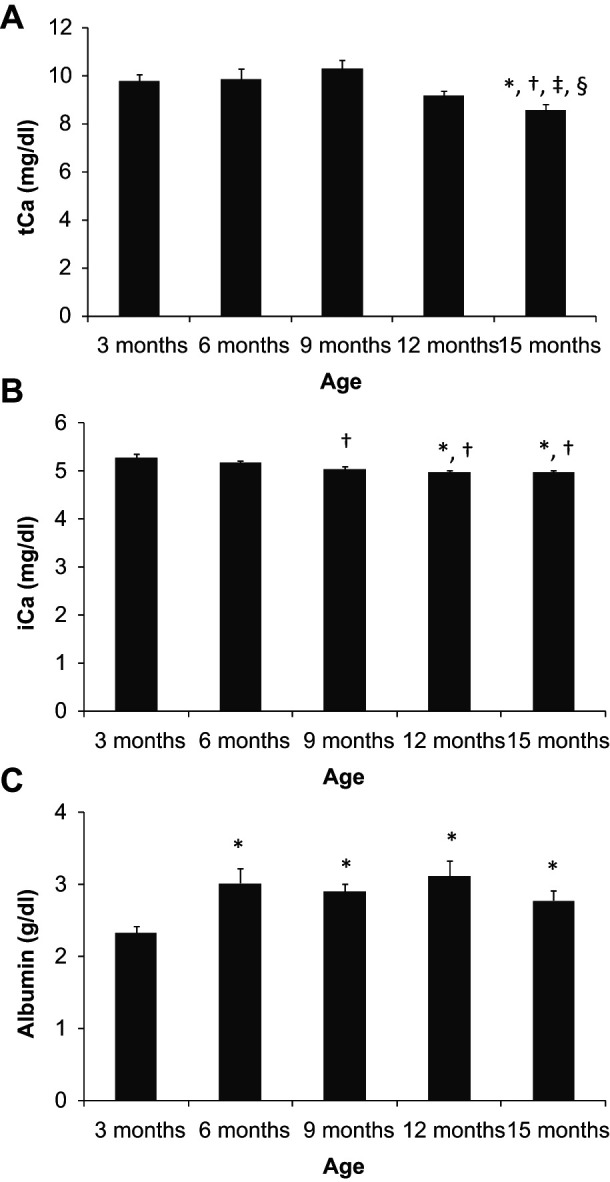
Plasma concentrations of total calcium (tCa) (a), ionised calcium (iCa) (b) and albumin (c) in cats from 3 to 15 months of age. *P <0.05 versus 3 months, †P <0.05 versus 6 months, ‡P <0.05 versus 9 months, §P <0.05 versus 12 months
Plasma phosphorus concentration was stable between 3 and 9 months of age at values ranging from 7.6 to 7.9 mg/dl. A significant decrease in phosphorus concentration was first detected at 12 months, 6.9 ± 0.3 mg/dl, and the decrease was more accentuated at 15 months, 5.3 ± 0.1 mg/dl. Plasma magnesium also decreased with age, but, in contrast to phosphorus, lower values were only identified at 15 months (2.7 ± 0.1 mg/dl), while between 3 and 12 months magnesium concentrations ranged between 3.3 ± 0.3 and 3.7 ± 0.2 mg/dl (Figure 2).
Figure 2.
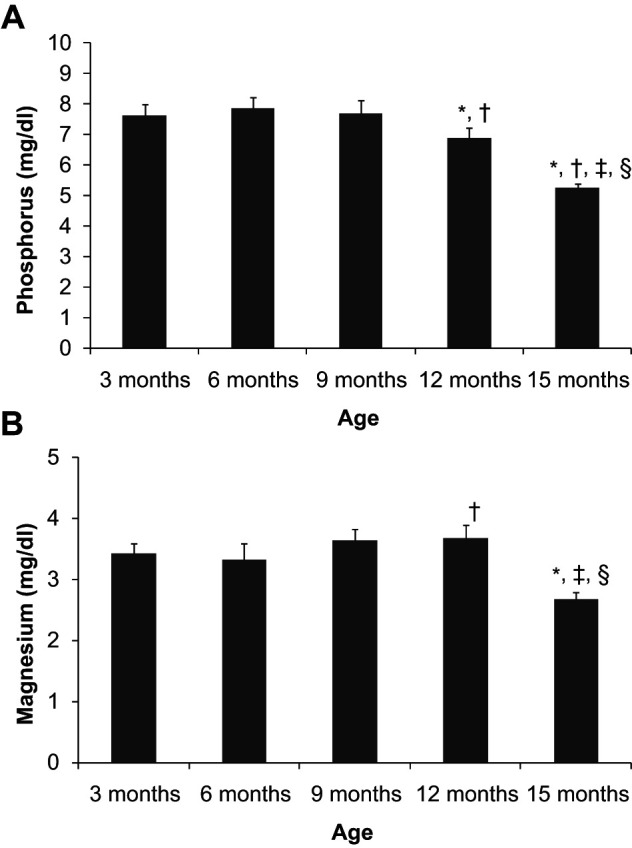
Plasma concentrations of phosphorus (a) and magnesium (b) in cats from 3 to 15 months of age. *P <0.05 versus 3 months, †P <0.05 versus 6 months, ‡P <0.05 versus 9 months, §P <0.05 versus 12 months
Figure 3 depicts plasma PTH concentrations. Both I-PTH and W-PTH remained constant from 3 to 9 months of age: I-PTH in the region of 5 pg/ml and W-PTH around 10 pg/ml. A non-significant peak was identified at 12 months in I-PTH (7.4 ± 1.2 pg/ml) and W-PTH (13.9 ± 2.9 pg/ml), and was followed by a small decrease at 15 months: 6.9 ± 0.9 pg/ml and 10.8 ± 2.4 pg/ml, respectively. A significant (P = 0.027) decline in the ratio W-PTH:I-PTH was observed with ageing (from 1.81 ± 0.14 at 3 months to 1.47 ± 0.15 at 15 months).
Figure 3.
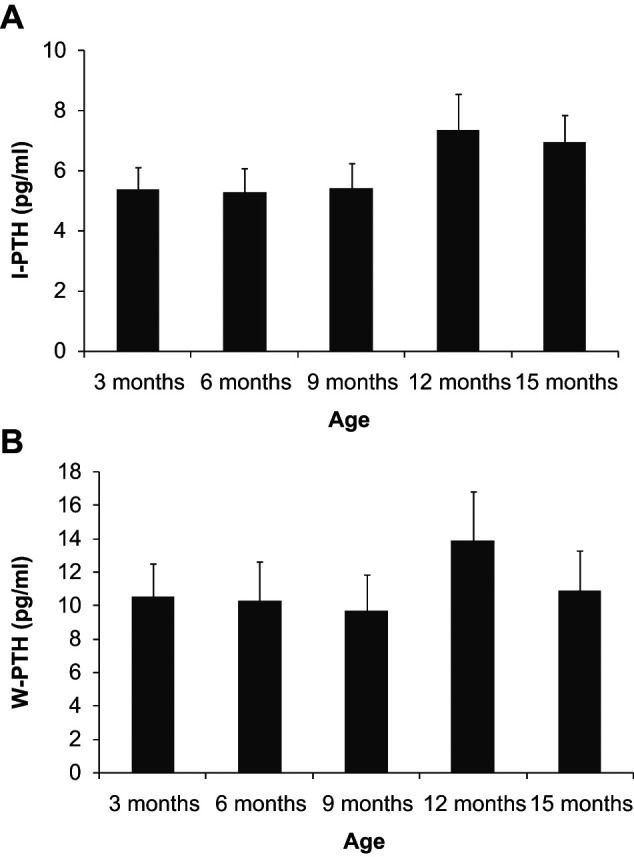
Plasma concentrations of intact parathyroid hormone (I-PTH) (a) and whole parathyroid hormone (W-PTH) (b) in cats from 3 to 15 months of age
Calcitriol concentration was high at 3 and 6 months (180.3 ± 11 pg/ml and 182.9 ± 11.7 pg/ml, respectively). Plasma calcitriol concentration significantly decreased at 9 months (122.9 ± 15.7 pg/ml) and kept decreasing until 15 months (103.2 ± 11.5 pg/ml), although no significant differences were found between 9, 12 and 15 months of age. In contrast, plasma calcidiol concentration was significantly lower at 3 months of age (43.3 ± 7.4 ng/ml) when compared with other sampling times. Calcidiol concentrations recorded between 6 and 15 months were in the range of 68.2 ± 10.1 to 80.5 ± 10.6 ng/ml and did not differ when compared statistically (Figure 4).
Figure 4.
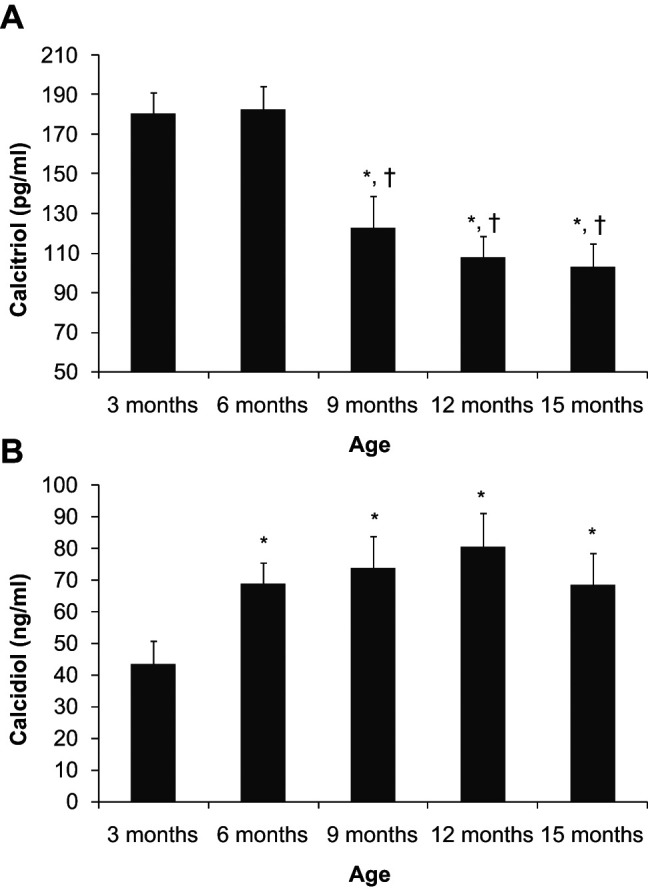
Plasma concentrations of calcitriol (a) and calcidiol (b) in cats from 3 to 15 months of age. *P <0.05 versus 3 months, †P <0.05 versus 6 months
Discussion
Concentrations of blood parameters related to mineral metabolism change along the growing process of the cat. From 3 months of age to adulthood, cats show a decrease in plasma minerals: calcium, phosphorus and magnesium; a decrease in calcitriol and an increase in calcidiol.
The adult (15 months) levels of all parameters under study were within the reference intervals of our laboratory for feline samples.
Kittens younger than 8 weeks have been reported previously to have higher plasma calcium than adults. 4 Our results show that between 3 and 12 months of age tCa concentration is significantly higher than values measured at 15 months. Thus, it would seem that, as in other species, 3 adult levels are not reached until the cat is at least 1 year old. Moreover, the biologically active calcium fraction, iCa, which, to our knowledge, has not been studied in growing kittens, shows a steady decrease during the first year of life and stabilises between 12 and 15 months of age. The discrepancy in the decline of iCa and tCa at younger ages may be explained by changes in plasma albumin. Lower albumin concentrations, as measured in younger cats, 4 will result in lesser tCa values and thus may explain why tCa is proportionally lower than iCa at these young ages.
In our study a decrease in both plasma phosphorus and magnesium concentrations, which follows the same time-frame than the decline in tCa (reduction at 12–15 months) has been identified. Previous studies have also reported that immature cats tend to have higher serum phosphorus concentrations. 4 However, age variations for serum magnesium concentrations in kittens had not been reported previously. 2
Plasma PTH concentrations — both I-PTH and W-PTH — remained stable along the study period with only a small peak, which does not seem relevant for diagnostic purposes, around 1 year of age. The only reference to PTH values in growing cats that we have been able to find was provided by Tomsa et al 10 who measured I-PTH in seven healthy cats 4–6 months old and used them as control values to compare with cases of nutritional secondary hyperparathyroidism. The I-PTH values reported by Tomsa et al 10 are comparable with the I-PTH concentrations measured in our study. As described previously in adult cats, 14 W-PTH concentrations are also higher than I-PTH concentrations in growing kittens. We have reported an increase in the ratio W-PTH:I-PTH in hypocalcaemic adult cats, supporting the notion that this ratio tends to increase when more ‘active’ PTH is needed. 14 Thus, although these ratios should be interpreted with caution when using heterologous assays, the decline in the ratio W-PTH:I-PTH with age detected in kittens would be consistent with a decrease in the need for ‘active’ PTH once the skeletal growth is completed.
In our study reciprocal changes in calcidiol and calcitriol have been identified. These changes may be explained by the vitamin D requirements during growth. Our interpretation, based on the knowledge about calcitriol and calcidiol metabolism obtained in other species,8,15,16 is that at younger ages the pro-vitamin D, calcidiol, is lower because it is being used to produce calcitriol, and calcitriol is higher to facilitate skeletal growth. When the growing needs decrease calcitriol concentration also declines and, owing to reduced demand, calcidiol increases.
In addition to the fluctuations in calcitriol and calcidiol concentrations detected during growth, it is very important to discuss their absolute values because they are clinically relevant in the diagnosis of juvenile bone disease. Evaluation of calcitriol and calcidiol concentrations is essential in the diagnosis of vitamin D-dependent rickets.17–24 Two major subtypes of vitamin D-dependent rickets have been reported in kittens: type I and type II. Type I is due to a genetic defect affecting 1-alpha-hydroxylase, the enzyme that converts calcidiol to calcitriol, 17 while type II is associated with mutations in the gene encoding the vitamin D receptor (VDR).18–21 In type I rickets calcitriol is decreased because it cannot be synthesised and the substrate (calcidiol) is increased, while in type II rickets calcitriol is increased because there is tissue resistance owing to the VDR defect, and calcidiol is either normal or decreased. 24
As the diagnosis of feline vitamin D-dependent rickets is based in the evaluation of calcitriol and calcidiol plasma concentrations, it is obvious that to provide a correct interpretation of vitamin D status reference values adequate to the age of the kitten are needed. However, to our knowledge, these reference intervals have not been available before and most authors have likely used adult reference values. Our results seem to indicate that calcitriol values in growing kittens are higher than previously thought.
Reference intervals for calcidiol used previously in the diagnosis of rickets are more similar to our results. Our data are quite comparable to the results reported by Morris et al, 25 who investigated the effect of dietary vitamin D content on plasma calcidiol concentrations. They also found a tendency of increasing calcidiol with age, and this tendency was more accentuated when diets with abnormally high vitamin D content were fed.
Thus, the main contribution of our work is to provide, for the first time, values for calcitriol and calcidiol in growing kittens at different stages of skeletal maturity. In addition, given the fact that the concentrations of these metabolites change with growth, when used for diagnostic purposes, values should be compared with the range corresponding to the age of the kitten. There are insufficient animals in this study to determine a reference interval. The animals were also a specific non-neutered population that lived in a controlled environment with standardised feeding regimes, so the values obtained should not be extrapolated to other populations. The principles established regarding the changes in parameters during the growth period apply to young cats, but reference intervals for this age group need to be developed by any laboratory performing these tests on their specific populations of young cats.
Conclusions
From 3 months of age to adulthood, cats show a decrease in plasma minerals: calcium (both tCa and iCa), phosphorus and magnesium. No major changes in PTH are evident, although the W-PTH:I-PTH ratio decreases significantly with age. A reciprocal change in vitamin D metabolites (decrease in calcitriol and increase in calcidiol) has been identified during the growing process. Our results reinforce the need to use adequate age-related reference values for diagnostic purposes, especially when evaluating the vitamin D status in the diagnosis of feline rickets.
Footnotes
Funding: The work was made possible with financial support from Junta de Andalucia: PAIDI-Grupo CTS-179.
The authors do not have any potential conflicts of interest to declare.
Accepted: 18 January 2013
References
- 1. Schoenmakers I, Nap RC, Mol JA, et al. Calcium metabolism: an overview of its hormonal regulation and interrelation with skeleteal integrity. Vet Q 1999; 21: 147–153. [DOI] [PubMed] [Google Scholar]
- 2. Kraft W, Hartmann K, Dereser R. Age dependency of laboratory values in dogs and cats. Part II: Serum electrolytes. Tierarztl Prax 1996; 24: 169–173 [in German]. [PubMed] [Google Scholar]
- 3. Harper EJ, Hackett RM, Wilkinson J, et al. Age-related variations in hematologic and plasma biochemical test results in Beagles and Labrador Retrievers. J Am Vet Med Assoc 2003; 223: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 4. Levy JK, Crawford PC, Werner LL. Effect of age on reference intervals of serum biochemical values in kittens. J Am Vet Med Assoc 2006; 228: 1033–1037. [DOI] [PubMed] [Google Scholar]
- 5. Arnaud SB, Goldsmith RS, Stickler GB, et al. Serum parathyroid hormone and blood minerals: interrelationships in normal children. Pediatr Res 1973; 7: 485–493. [DOI] [PubMed] [Google Scholar]
- 6. Root AW, Harrison HE. Recent advances in calcium metabolism. I. Mechanisms of calcium homeostasis. J Pediatr 1976; 88: 1–18. [DOI] [PubMed] [Google Scholar]
- 7. Chesney RW, Rosen JF, Hamstra AJ, et al. Serum 1,25 dihydroxyvitamin D levels in normal children and in vitamin D disorders. Am J Dis Child 1980; 134: 135–139. [DOI] [PubMed] [Google Scholar]
- 8. Kano K, Yoshida H, Yata J, et al. Age and seasonal variations in the serum levels of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in normal humans. Endocrinol Jpn 1980; 27: 215–221. [DOI] [PubMed] [Google Scholar]
- 9. Lichtenstein P, Specker BL, Tsang RC, et al. Calcium-regulating hormones and minerals from birth to 18 months of age: a cross-sectional study. I. Effects of sex, race, age, season, and diet on vitamin D status. Pediatrics 1986; 77: 883–890. [PubMed] [Google Scholar]
- 10. Tomsa K, Glaus T, Hauser B, et al. Nutritional secondary hyperparathyroidism in six cats. J Small Anim Pract 1999; 40: 533–539. [DOI] [PubMed] [Google Scholar]
- 11. Torrance AG, Nachreiner R. Human-parathormone assay for use in dogs: validation, sample handling studies, and parathyroid function testing. Am J Vet Res 1989; 50: 1123–1127. [PubMed] [Google Scholar]
- 12. Unterer S, Lutz H, Gerber B, et al. Evaluation of an electrolyte analyzer for measurement of ionized calcium and magnesium concentrations in blood, plasma, and serum of dogs. Am J Vet Res 2004; 65: 183–187. [DOI] [PubMed] [Google Scholar]
- 13. Agborsangaya C, Toriola AT, Grankvist K, et al. The effects of storage time and sampling season on the stability of serum 25-hydroxyvitamin D and androstenedione. Nutr Cancer 2010; 62: 51–57. [DOI] [PubMed] [Google Scholar]
- 14. Pineda C, Aguilera-Tejero E, Raya AI, et al. Feline parathyroid hormone: validation of hormonal assays and dynamics of secretion. Domest Anim Endocrinol 2012; 42: 256–264. [DOI] [PubMed] [Google Scholar]
- 15. Mundy GR, Guise TA. Hormonal control of calcium homeostasis. Clin Chem 1999; 45: 1347–1352. [PubMed] [Google Scholar]
- 16. Seino Y, Shimotsuji T, Yamaoka K, et al. Plasma 1,25-dihydroxyvitamin D concentrations in cords, newborns, infants, and children. Calcif Tissue Int 1980; 30: 1–3. [DOI] [PubMed] [Google Scholar]
- 17. Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med 2009; 23: 196–199. [DOI] [PubMed] [Google Scholar]
- 18. Schreiner CA, Nagode LA. Vitamin D-dependent rickets type 2 in a four-month-old cat. J Am Vet Med Assoc 2003; 222: 337–339. [DOI] [PubMed] [Google Scholar]
- 19. Godfrey DR, Anderson RM, Barber PJ, et al. Vitamin D-dependent rickets type II in a cat. J Small Anim Pract 2005; 46: 440–444. [DOI] [PubMed] [Google Scholar]
- 20. Tanner E, Langley-Hobbs SJ. Vitamin D-dependent rickets type 2 with characteristic radiographic changes in a 4-month-old kitten. J Feline Med Surg 2005; 7: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacKenzie JM, Crawford J, Ghantous S. Successful therapy of vitamin D-dependant rickets in a kitten. J Am Anim Hosp Assoc 2011; 47: 290–293. [DOI] [PubMed] [Google Scholar]
- 22. Phillips AM, Fawcett AC, Allan GS, et al. Vitamin D-dependent non-type 1, non-type 2 rickets in a 3-month-old Cornish Rex kitten. J Feline Med Surg 2011; 13: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henik RA, Forrest LJ, Friedman AL. Rickets caused by excessive renal phosphate loss and apparent abnormal vitamin D metabolism in a cat. J Am Vet Med Assoc 1999; 215: 1644–1649. [PubMed] [Google Scholar]
- 24. Dittmer KE, Thompson KG. Vitamin D metabolism and rickets in domestic animals: a review. Vet Pathol 2011; 48: 389–407. [DOI] [PubMed] [Google Scholar]
- 25. Morris JG, Earle KE, Anderson PA. Plasma 25-hydroxyvitamin D in growing kittens is related to dietary intake of cholecalciferol. J Nutr 1999; 129: 909–912. [DOI] [PubMed] [Google Scholar]


