Abstract
A relaxation of previous recommendations on the numbering of the atoms of myo-inositol is suggested, so that substituents are not necessarily numbered so that the smallest possible locant is used. This allows an alternative designation to be used, when authors wish, to bring out structural relationships.
Full text
PDF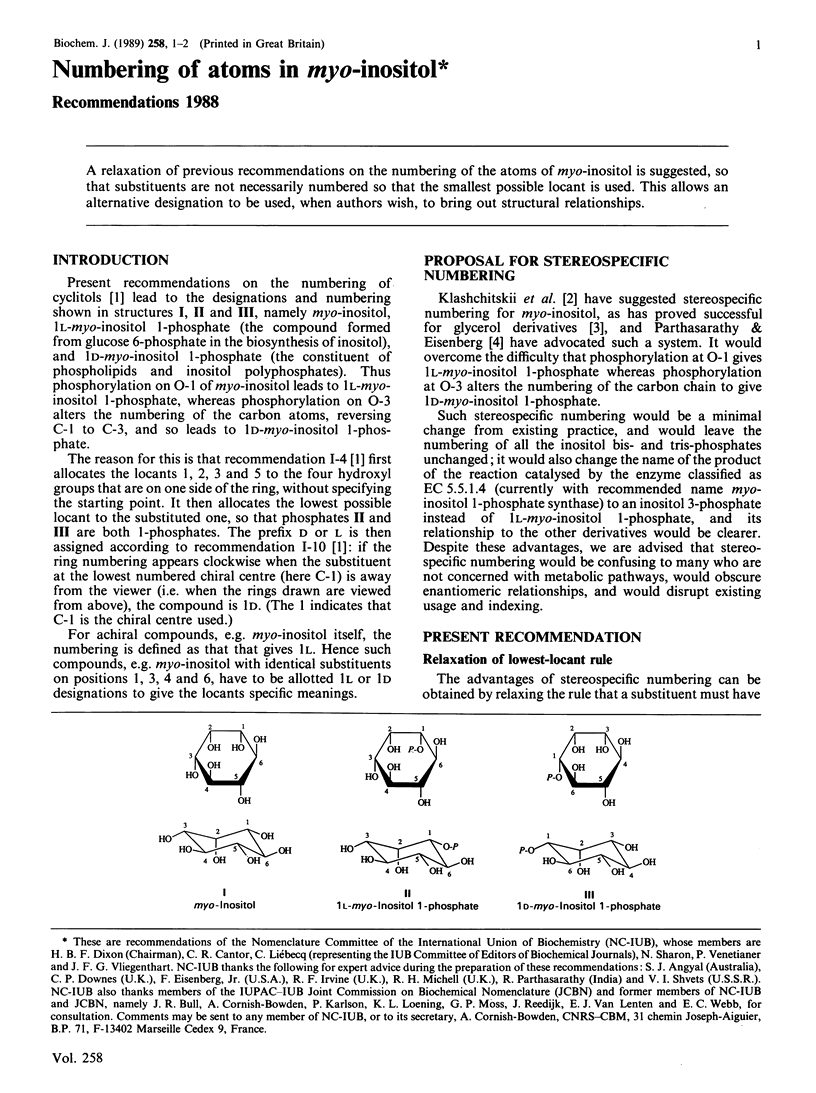

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Irvine R. F., Letcher A. J., Lander D. J., Heslop J. P., Berridge M. J. Inositol(3,4)bisphosphate and inositol(1,3)bisphosphate in GH4 cells--evidence for complex breakdown of inositol(1,3,4)trisphosphate. Biochem Biophys Res Commun. 1987 Feb 27;143(1):353–359. doi: 10.1016/0006-291x(87)90672-3. [DOI] [PubMed] [Google Scholar]
- Klyashchitskii B. A., Shvets V. I., Preobrazhenskii N. A. On the nomenclature of asymmetrically substituted myoinositol derivatives with particular reference to phosphatidylinositols. Chem Phys Lipids. 1969 Dec;3(4):393–400. doi: 10.1016/0009-3084(69)90044-9. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R., Eisenberg F., Jr The inositol phospholipids: a stereochemical view of biological activity. Biochem J. 1986 Apr 15;235(2):313–322. doi: 10.1042/bj2350313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]


