Abstract
The cardiovascular and respiratory effects, and the quality of anesthesia of alfaxalone administered intramuscularly (IM) to cats sedated with dexmedetomidine and hydromorphone were evaluated. Twelve healthy adult cats were anesthetized, with six cats receiving dexmedetomidine (0.01 mg/kg IM) followed by alfaxalone (5 mg/kg IM; group DA) and six receiving dexmedetomidine (0.01 mg/kg IM) plus hydromorphone (0.1 mg/kg IM) followed by alfaxalone (5 mg/kg IM; group DHA). Cardiorespiratory (pulse rate, blood pressure, respiratory rate, saturation of oxygen with hemoglobin, end tidal carbon dioxide partial pressure) and bispectral index (BIS) data were collected every 10 mins for 90 mins starting immediately after intubation. The quality of anesthesia was scored by a blinded researcher at induction and at 5 and 60 mins after extubation. Recovery scores ranged from 1 (prolonged struggling) to 4 (no struggling). There were no clinically significant (P >0.05) differences in any data between groups or over time. Physiologic parameters were within normal limits for cats at all times. BIS values were consistent with light anesthesia in both groups. However, recovery was prolonged and marked with excitement, ataxia and hyper-reactivity in all cats. Thus, although cardiovascular and respiratory parameters are stable following IM injection of alfaxalone to cats sedated with dexmedetomidine and hydromorphone, recovery is extremely poor and this route of administration is not recommended for anesthesia in cats.
Introduction
Cats often resent restraint and handling, which can make the administration of anesthetic drugs difficult. With fractious, fearful or excited cats, intravenous (IV) injections can be challenging or even impossible. Inhalant anesthetics can be delivered by mask or chamber to induce anesthesia, but the use of inhalants alone increases the risk of anesthesia-related mortality.1,2 Thus, drugs that can be administered intramuscularly (IM) are often used when IV injections are not possible. Alfaxalone, a fairly new injectable anesthetic drug, is approved in some countries for both IV and IM administration in cats. 3 Intramuscular injections of alfaxalone have been used alone or in combination with other injectable anesthetics or tranquilizers to produce anesthesia in a variety of mammals, including wallabies, 4 marmosets 5 and rabbits, 6 but no descriptive studies of the actions of the drug following IM administration in the cat have been published. When used IV in cats, alfaxalone provides anesthesia of short duration with cardiorespiratory effects, and quality of induction and recovery that is similar to that of propofol, 7 although there is some evidence that recovery from alfaxalone may not be quite as smooth as that following propofol. 8
In an attempt to design a balanced anesthetic protocol using alfaxalone, we chose to administer the drug to cats sedated with dexmedetomidine with or without hydromorphone. Our goals were to evaluate the (i) cardiovascular effects (using pulse rate and arterial blood pressure), (ii) respiratory effects [using respiratory rate, saturation of oxygen with hemoglobin (SpO2) and end-tidal carbon dioxide partial pressure (ETCO2)], and (iii) anesthetic quality [using anesthesia scoring systems and bispectral index (BIS) values] of IM administered alfaxalone and dexmedetomidine with or without hydromorphone in cats. Our hypothesis was that alfaxalone administered IM to sedated cats would produce a light plane of anesthesia without producing adverse cardiorespiratory or behavioral effects.
Materials and methods
Animals
Twelve neutered adult cats (seven males; five females) weighing 5.5 ± 2.5 kg and aged 5.7 ± 0.7 years were used in this study. The cats were members of a university research colony. This study was approved by the university’s Animal Care and Use Committee.
Study design
The cats were randomized into two groups and all cats were anesthetized once, with six cats receiving dexmedetomidine and alfaxalone (group DA) and six cats receiving dexmedetomidine, hydromorphone and alfaxalone (group DHA).
Anesthesia
On the day of the study, food was withheld for 6 h before induction of anesthesia, but water was freely available. Following a physical examination, all cats were determined to be American Society of Anesthesiologists I status (healthy, normal patient). Blood (complete blood count) and serum chemistry analysis were done approximately 30 days prior to this study as part of a routine examination, and all analyses were normal. The cats were weighed, lightly restrained and 0.01 mg/kg dexmedetomidine (Dexdomitor; Orion Corporation) with or without 0.1 mg/kg hydromorphone (Hydromorphone HCl; Baxter Health Care Corporation) was administered in the right quadriceps muscle using a 22-gauge needle. Then, the cats were placed in a holding cage and observed continuously. When both drugs were used, they were combined in the same syringe immediately prior to injection. Saline was added to the dexmedetomidine so that the volume of the drug used alone was equal to the volume of dexmedetomidine plus hydromorphone, and the contents of the syringes were not revealed to the blinded observer. Fifteen minutes following the dexmedetomidine or dexmedetomidine/hydromorphone injection, the cats were scored by a blinded observer for quality of sedation (See Supplementary data) and 5 mg/kg alfaxalone (Alfaxan; Vetoquinol) was administered in the left quadriceps muscles using a 22-gauge needle. The cats were restrained lightly as they begin to lose consciousness. Time from injection of drug to loss of withdrawal in response to toe pinch (LWD) were recorded. Endotracheal intubation with a 5.0 mm endotracheal tube was attempted 10 mins after the alfaxalone was injected and was attempted every 1 min until successful. Success was defined as the ability to insert the endotracheal tube through the larynx without the cat chewing or swallowing. The time from drug injection to successful endotracheal intubation (ETI) was recorded and an intubation score was assigned by a blinded observer (see Supplementary data). The tube was secured behind the cat’s ears with a plastic tie, but the tube cuff was not inflated.
Instrumentation
Following induction, electrical leads were placed on the cat’s thorax and left stifle for recording of a lead II electrocardiogram, a pulse oximeter probe was placed on the cat’s tongue for measurement of SpO2, a small-volume sampling line was connected to a port at the oral end of the endotracheal tube for the side-stream measurement of ETCO2, and an oscillometric cuff (size 2) was placed over the dorsal pedal artery for indirect measurement of systolic (SAP), diastolic (DAP) and mean arterial blood pressures (MAP). All physiologic data were recorded by the same monitor (Datascope Panorama; Mindray DS USA). The pulse rate (PR) was counted manually by palpation of the femoral artery and the respiratory rate (RR) was counted manually by watching thoracic excursions. BIS was measured by placing subdermal needle electrodes connected to the BIS monitor (A-2000 BIS monitor; Aspect Medical System) on the cranium of each animal, as described previously.9–11 Briefly, the primary lead was placed on the midline approximately a third of the distance from an imaginary line connecting the zygomatic processes of the frontal bone and the most caudal portion of the external frontal crest that was palpable. The secondary lead (lead 2) and the ground lead (lead 4) were placed over the left temporal bone and near the base of the left ear, respectively. An additional lead (lead 3) was placed caudolateral to the secondary lead for muscle artifact (blink) detection (Figure 1).
Figure 1.
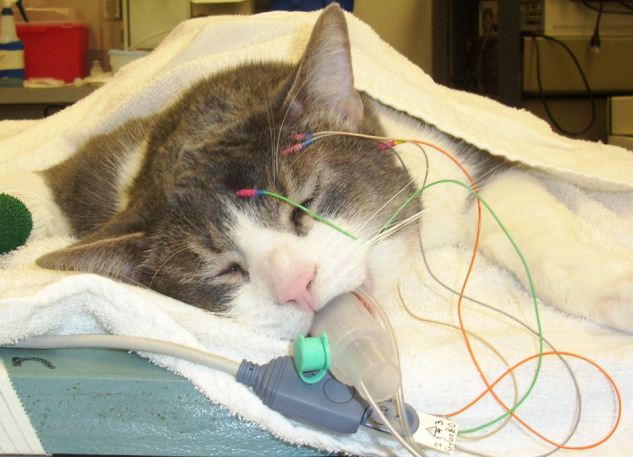
Electrode placement for acquiring bispectral index data in the cat. The primary lead was placed on the midline, approximately a third of the distance from an imaginary line connecting the zygomatic processes of the frontal bone and the most caudal portion of the external frontal crest that was palpable. The secondary lead (lead 2) and the ground lead (lead 4) were placed over the left temporal bone and near the base of the left ear, respectively. An additional lead (lead 3) was placed caudolateral to the secondary lead for muscle artifact (blink) detection
Data were collected every 10 mins starting immediately after intubation and instrumentation, and ending 90 mins later for a total of nine data collection times designated as T10–T90. Data collected during this time included PR, RR, ETCO2, SpO2, MAP, DAP and SAP. At 90 mins, physiologic data collection ceased, but the cats were monitored and time to return of the withdrawal reflex in response to toe pinch and time to extubation (ETO) were recorded. Extubation occurred when the cat spontaneously swallowed with no manipulation of the tube. Cats were scored by a blinded observer 5 mins and 60 mins after extubation using a scoring system modified from a study assessing a different anesthetic protocol administered IM to cats (Supplementary data). 12 BIS data were collected continuously using proprietary software and a laptop computer. Body temperature was measured every 15 mins and was maintained between 38°C and 39°C using a commercial warming system (Hot Dog, Augustine Biomedical+ Design). Supplemental oxygen was available and was used if the SpO2 was less than 85% for more than 10 mins.
Statistics
Normality of errors was tested with the Shapiro–Wilk test. Physiologic data were analyzed using repeated measures of analysis of variance (ANOVA), with treatment as the independent variable for between-group analysis and two-way repeated measures for among-group analysis. For BIS, the median of 60 BIS values at each data collection time was calculated and means of these median BIS values were compared using repeated measures ANOVA. For physiologic data and BIS Bonferroni’s post hoc analysis was planned if significant differences occurred. Time data were analyzed between groups at each time point with paired t-tests. Mean ± SD were calculated for physiologic, BIS and time data. Anesthetic quality scores are categorical data thus median and range were computed and analyzed with the Pearson’s χ2 test. All analyses were performed with a commercial statistics program (GraphPad Prism, GraphPad Software). Significance was set at P <0.05.
Results
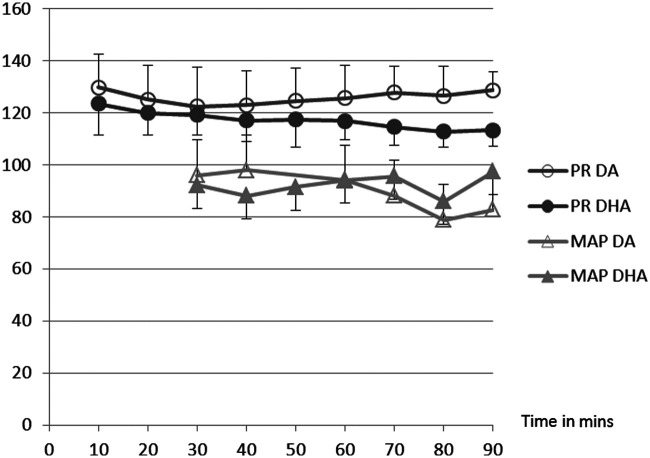
There was no difference in age, weight or sex between the two groups of cats. Select data are presented in Tables 1 and 2, and Figures 1 and 2. All cats were moderately-to-profoundly sedated following premedication. All cats except for one in group DA were intubated on the first attempt and that cat was intubated on the second attempt 1 min later. The addition of hydromorphone did not change induction or recovery scores, but time to loss of withdrawal and to intubation was shorter in DHA than DA (Table 1). Time to return of withdrawal was longer in DHA than DA. Recovery was prolonged and marked by hyper-reactivity, excitement and ataxia in both groups. Physiologic data were normally distributed. There were no differences between groups or over time for pulse rate (PR), SpO2, ETCO2, MAP, SAP, DAP (Figures 2 and 3), BIS (Table 2) or body temperature. BIS values were consistent with light anesthesia in both groups. All data were collected at each time point except for MAP, SAP and DAP, which were not attainable until T30. One cat in the DA group had a SpO2 of <85% for longer than 10 mins and received supplemental oxygen for 15 mins. After 15 mins, the supplemental oxygen was removed and the cat was able to maintain SpO2 >85% of the duration of the study. The SpO2 data for this cat was not analyzed during the delivery of supplemental oxygen.
Table 1.
Selective data for cats receiving dexmedetomidine and alfaxalone (DA) or dexmedetomidine, hydromorphone and alfaxalone (DHA) intramuscularly. The starting time for all times in the table is the time of injection of DA or DHA. Time is reported in minutes. Time data are reported as mean ± SD and quality scores are reported as range and (median)
| Cat group | Sedation score | Time to LWD (mins) | Time to ETI (mins) | Score at intubation | Time to RWD (mins) | Time to ETO (mins) | Score 5 mins post-extubation | Score 1 h post-extubation |
|---|---|---|---|---|---|---|---|---|
| DA | 2–3(3) | 7.2 ± 2.5 | 15.5 ± 4.7 | 1–3(2) | 93.5 ± 27.2 | 108.5 ± 22.5 | 1–3(2) | 2–3(3) |
| DHA | 2–3(3) | 4.0 ± 1.9 | 11.2 ± 1.2 | 1–2(1) | 115.5 ± 21.3 | 123.3 ± 26.8 | 1–3(2) | 2–3(3) |
LWD = loss of withdrawal reflex; ETI = intubation; RWD = return of withdrawal reflex; ETO = extubation
Table 2.
Bispectral index (BIS) scores for cats receiving dexmedetomidine and alfaxalone (DA) or dexmedetomidine, hydromorphone and alfaxalone (DHA) intramuscularly. Data are reported as mean ± SD. Time 0 data were collected immediately after intubation
| Time in mins | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cat group | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| DA | 84.5 ± 9.7 | 83.8 ± 10.8 | 83.7 ± 9.0 | 78.4 ± 4.1 | 77.7 ± 3.0 | 75.8 ± 6.6 | 77.6 ± 5.8 | 79.3 ± 3.4 | 79.5 ± 5.6 | 76.3 ± 5.5 |
| DHA | 71.7 ± 19.8 | 60.3 ± 31.0 | 55.5 ± 34.7 | 60.6 ± 30.6 | 64.0 ± 30.8 | 64.1 ± 32.1 | 64.5 ± 31.5 | 64.1 ± 33.0 | 64.0 ± 31.6 | 63.6 ± 34.4 |
Figure 2.
Cardiovascular data for cats receiving dexmedetomidine and alfaxalone (DA) or dexmedetomidine, hydromorphone and alfaxalone (DHA) intramuscularly. Data are reported as mean. Standard deviation bars are not shown because no significant differences occurred. Pulse rate (PR) units are beats/min and mean arterial pressure (MAP) units are mmHg
Figure 3.
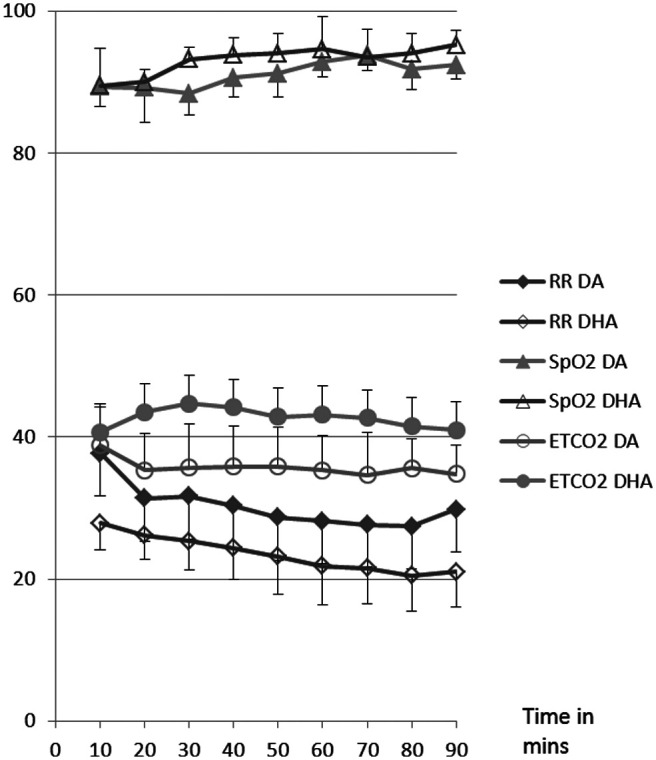
Respiratory data for cats receiving dexmedetomidine and alfaxalone (DA) or dexmedetomidine, hydromorphone and alfaxalone (DHA) intramuscularly. Data are reported as mean. Standard deviation bars are not shown because no significant differences occurred. End-tidal carbon dioxide (ETCO2) units are mmHg, oxygen hemoglobin saturation (SpO2) units are % saturation and respiratory rate (RR) is in breaths/min
Discussion
Following IM administration of alfaxalone, the cats in our study were reactive to sound throughout the anesthetic period, and the time of recovery from anesthesia was unpredictable, with the cats progressing rapidly and inconsistently from LWD to ETO. The recoveries were marked by excitement, incoordination and hyper-reactivity to stimuli. The addition of hydromorphone did not change the quality of anesthesia or recovery, although the time to LWD and ETI was shortened. The volume of the alfaxalone, even at the low end of the recommended dose 3 was excessive for an IM injection and the cats reacted, even when sedated, to the injection. In contrast to the poor quality of anesthesia and recovery, all cardiopulmonary parameters were within normal limits and remained stable throughout the anesthetic period.
Ideally, the anesthetic and cardiopulmonary effects of alfaxalone would have been assessed without the confounding effects of the dexmedetomidine and hydromorphone. We did conduct a pilot study using alfaxalone alone in three cats. Based on the dosing recommendations from the product information sheet describing IM use in cats, 3 10 mg/kg alfaxalone alone was administered IM to two cats. Although discomfort during IV injection was not mentioned in cats,7,8 and was specifically found to be absent when administered IV to dogs, 13 IM injection seemed to cause moderate- to-profound discomfort in our cats, who reacted fairly violently to the injection. In addition, the volume of drug at this dose was 1 ml/kg body weight, which was not a practical volume to inject IM in a cat. Because of the volume and the discomfort, we tried another pilot study with 10 mg/kg alfaxalone administered subcutaneously (SC) to one cat, but this resulted in a fairly prolonged period (several hours) of hyper-reactivity and ataxia, but did not result in an anesthetic plane that would allow intubation. Thus, we determined that alfaxalone administered alone, either IM or SC, was inappropriate for induction and maintenance of anesthesia, and we chose to evaluate the administration of alfaxalone following the administration of dexmedetomidine with or without hydromorphone. In actuality, this would be a more appropriate clinical recommendation for use of the drug as premedicant sedation allows a decreased dose of anesthetic induction drugs, including alfaxalone,14,15 which would, presumably, lead to a decreased incidence of dose-dependent adverse effects. As occurred in our study, alfaxalone administered IM alone at approximately 5 mg/kg to wallabies did not provide anesthesia, but premedication with medetomidine followed by alfaxalone induced an anesthetic plane adequate for physical examination, blood collection and transport to another facility. 7 In spite of the reduction in volume and addition of premedicant sedative drugs, 8/12 cats in our study still reacted to the injection of alfaxalone. Reaction included attempts at evasive movement, paddling and/or growling.
Although the cats seemed to be at a light plane of anesthesia and were easily endotracheally intubated, with all cats but one intubated on the first attempt, the cats exhibited hyper-reactivity during and after anesthesia. During anesthesia the cats would move their ears in response to sound, even when response to toe pinch was absent. In addition, the time of recovery from anesthesia was unpredictable and the cats would progress rapidly through response to toe pinch to apparent complete consciousness and rejection of the endotracheal tube without warning. Although the dexmedetomidine premedication should have reduced any excitatory reactions in the cats during anesthesia, the dose of dexmedetomidine that we used (10 μg/kg) was lower than the Food and Drugs Administration (FDA)-approved dose (40 μg/kg), so perhaps the dose was inadequate for elimination of excitatory effects. The dose of alfaxalone may also have been inadequate to produce a moderate-to-deep plane of anesthesia and the cats were potentially merely sedated or only very lightly anesthetized and, thus, more likely to react to sound. Response to sound can occur in apparently anesthetized patients, including feline patients 16 and is even suggested as a measure of anesthetic depth. 17 Thus, our cats could have been at a very light plane of anesthesia throughout the research period. BIS is a monitor that uses an algorithm of the electroencephalogram to measure brain activity. BIS has been used to predict hypnotic and anesthetic depth in a variety of species, including cats.9–11 For both groups in our study, BIS values were comparable to a light plane of inhalant anesthesia in cats.10,11 However, this low-dose theory may be incorrect as it was higher dosages of alfaxalone that were associated with worse recoveries following IV administration of alfaxalone to cats. 8
The inadequate dosage theory might explain reactivity during the anesthetic period, but would not explain the prolonged excitement and hyper-reactivity during the recovery period. During recovery, the cats moved almost incessantly, at first thrashing in the cage and then pacing once they were more awake. They were ataxic and hyper-reactive to sound and touch at both the 5- and 60-min recovery assessment times, although they were improved at 60 mins. The cats were not officially scored at 2 h post recovery, but were still being observed and all were still hyper-reactive. In fact, we were not comfortable returning the cats to the research colony until approximately 4 h post extubation. We would expect the presence of sedatives to improve the recovery scores, and cats premedicated with acepromazine and butorphanol had recovery scores that were ‘moderate’ (23% of 35 cats) at worst, while those receiving alfaxalone, without acepromazine or butorphanol, were graded ‘moderate’ (55%) or ‘poor’ (22%). 15 Although the dexmedetomidine premedication should have reduced any excitatory reactions in our cats, the effects of the drug may have dissipated before the recovery period. The mean elimination half-life of dexmedetomidine administered IM at the FDA-approved dose of 40 μg/kg in the cat is 74 mins (1.24 h). 18 No information is available for administration of 10 μ/kg, which was the dose used in this study, but the time from dexmedetomidine injection to extubation of the cats averaged 120–131 mins (ETO + 15 mins from dexmedetomidine injection to alfaxalone injection). Although only 50% of the drug is eliminated in one half-life, the low dose administered in our study and the long duration between administration and recovery mean that the amount of dexmedetomidine in the cats was probably very low. However, we cannot rule out that there was some dexmedetomidine effect in recovery and that the cats might have been even more hyper-reactive without the drug. Sedation does not always improve recovery following the administration of alfaxalone, and cats recovering from an anesthetic protocol of acepromazine, buprenorphine, alfaxalone and isoflurane had more episodes of trembling and paddling during recovery than cats anesthetized with the same protocol, but with propofol as the induction drug. 8 Premedication with buprenorphine alone also may have decreased hyperexcitability during recovery from IV alfaxalone in some, but not all, cats. 19 However, premedication with a more potent sedative (acepromazine) followed by either alfaxalone or propofol, and isoflurane provided recovery scores that were not different between groups. 7 However, there was one cat in the alfaxalone group that could not be scored because of ‘considerable excitement in the early phase of recovery’.
Although a brief excitatory period during recovery from anesthesia is not unusual and can be caused by a wide variety of anesthetic drugs, 20 the excitement and hyper-reactivity in the study reported here were exaggerated and prolonged. The cause of the exaggerated and prolonged response in our study is unclear, but could be owing to route of administration, species of interest, adverse effects of concurrently administered drugs and/or adverse effects of alfaxalone. The quality of induction and recovery following IM administration of alfaxalone has not been investigated previously in either dogs or cats, but has been investigated in other species. IM alfaxalone (4 mg/kg) did not cause excitement at induction or recovery in wallabies sedated concurrently with medetomidine. 4 Marmosets received 10 mg/kg alfaxalone IM, which would require a very large volume, but no comments were made regarding discomfort or reaction to the injection, and most of the marmosets were euthanased, so no recovery data are available. 5 Rabbits sedated with medetomidine received 5 mg/kg alfaxalone IM followed by isoflurane, and the induction and recovery were reported to be ‘uneventful’, but the recovery was actually not observed continuously. 6 The IV administration of alfaxalone has resulted in calm recoveries in both dogs21,22 and cats,7,8,15,19,23 and excitatory recoveries in both dogs 24 and cats.8,15,19 Thus, the problem does not seem to be limited to either IM injections or to cats, although the IM route seems to produce a more profound response. As for concurrently administered drugs, hydromorphone was used in our study, and opioids can cause excitement in cats. However, there was no difference in recovery scores between the DA and DHA groups.
Because of the facts just presented, it appears that alfaxalone itself may be the cause of excitement during recovery. In fact, the product insert for the drug even offers a cautionary warning: ‘During recovery, it is preferable that animals are not handled or disturbed. This may lead to paddling, minor muscle twitching or movements that are more violent which, while better avoided, are clinically insignificant’. 3 Based on the responses of the cats in the study reported here, we would disagree that the responses in recovery are clinically insignificant in cats receiving IM alfaxalone, and we would recommend that alfaxalone be administered IV and that drugs other than alfaxalone be used for IM anesthetic protocols in cats. Cats recovering from anesthesia induced by combinations of a2 agonists and dissociative drugs (ketamine 12 or tiletamine 25 ) with our without opioids are generally calm. We used a scoring system (Supplementary data) similar to the one used by Ko et al, 12 and cats in that study scored mostly 4 (best possible recovery), with a few scoring 3, whereas our cats scored primarily 1 (worst possible recovery) and 2. However, a limitation of our study is that we did not compare IM administration of other anesthetic protocols in this same group of cats and it is possible that all 12 of our cats would also have reacted adversely to a different IM anesthetic protocol.
In opposition to the poor and unpredictable anesthetic responses, the cardiopulmonary parameters were within normal limits and stable throughout the anesthetic period. Although this stability has previously been reported following IV administration of alfaxalone alone to cats, 26 in our study the stability may have been due to the combination of the drugs and not just to alfaxalone alone. Dose-dependent hypotension has been reported following the IV administration of alfaxalone to cats in some studies,15,23 but blood pressure in our cats was normal-to-slightly high for anesthetized cats from T30 to T90. The normal-to-high pressures may be due to the dexmedetomidine, which causes vasoconstriction and normal to elevated blood pressures. 27 We were unable to obtain pressure readings in the majority of the cats at T10 and T20, presumably owing to the initial intense vasoconstriction caused by the dexmedetomidine. A limitation of our study is that we chose to measure blood pressure indirectly using an oscillometric technique as opposed to measuring blood pressure directly with an arterial catheter. Measurement of oscillometric blood pressure in cats may result in values that are slightly lower than actual pressure. 28 However, because pressures were consistently normal or high in this study, the fact that the measurement technique may have slightly underestimated the actual pressures only adds to our conclusion that the combination of drugs that we chose did not cause hypotension. Hypoventilation and apnea appear to be the most common alfaxalone-induced side effects in cats.15,23 One cat from the DA group in our study had a brief period of hypoxemia, as determined by SpO2 <85%, but this was treated successfully by the administration of supplemental oxygen. This cat was overweight with a body condition score of 4 out of 5, and this could adversely affect respiratory function in sedated or anesthetized cats. Thus, the need for oxygen support was probably owing to the effect of the cat’s weight in combination with sedation and not to a sole effect of the anesthetic drugs. Although a SpO2 of <85% is very low and we would have intervened earlier in a clinical case, we allowed the SpO2 to decrease lower than normal because we were trying to thoroughly assess the physiologic effects of the drugs. A limitation of this study is that we did not measure physiologic parameters pre-operatively, so we could not determine the effect of alfaxalone on the physiologic parameters. However, in both groups of cats, all parameters were within normal limits for sedated cats throughout the study period.
Conclusions
Alfaxalone promoted cardiovascular and respiratory stability when administered IM to sedated cats with or without hydromorphone. However, because of the large volume of injectate with subsequent discomfort on injection and pronounced and prolonged hyper-reactivity following injection, we do not recommend this route of administration in cats.
Supplemental Material
Sedation, intubation and recovery
Acknowledgments
We would like to thank Mary Albi LVT, Janel Bingman LVT, Shelley Ensign LVT, Shona Meyer LVT and Nicole Valdez for their expert cat-wrangling skills and for keeping the clinic running while we were locked in the research laboratory.
Footnotes
Supplementary data: Sedation, intubation and recovery score criteria.
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 16 January 2012
References
- 1. Brodbelt D. Perioperative mortality in small animal anaesthesia. Vet J 2009; 182: 152–161. [DOI] [PubMed] [Google Scholar]
- 2. Clarke KW, Hall LW. A survey of anaesthesia in small animal practice. AVA/BSAVA report. J Vet Anaesth 1990: 17; 4–10. [Google Scholar]
- 3. Anon. Jurox Alfaxan CD-RTU (package insert). Hong Kong: Alfamedic Ltd, 2012. [Google Scholar]
- 4. Bouts T, Karunaratna D, Berry K, et al. Evaluation of medetomidine-alfaxalone and medetomidine-ketamine in semi-free ranging Bennett’s wallabies (Macropus rufogriseus). J Zoo Wildl Med 2011; 42: 617–622. [DOI] [PubMed] [Google Scholar]
- 5. Thomas AA, Leach MC, Flecknell PA. An alternative method of endotracheal intubation of common marmosets (Callithrix jacchus). Lab Anim 2012; 46: 71–76. [DOI] [PubMed] [Google Scholar]
- 6. Marsh MK, McLeod SR, Hansen A, et al. Induction of anaesthesia in wild rabbits using a new alfaxalone formulation. Vet Rec 2009; 164: 122–123. [DOI] [PubMed] [Google Scholar]
- 7. Taboada FM, Murison PJ. Induction of anaesthesia with alfaxalone or propofol before isoflurane maintenance in cats. Vet Rec 2010; 167: 85–89. [DOI] [PubMed] [Google Scholar]
- 8. Mathis A, Pinelas R, Brodbelt DC, et al. Comparison of quality of recovery from anaesthesia in cats induced with propofol or alfaxalone. Vet Anaesth Analg 2012; 39: 282–290. [DOI] [PubMed] [Google Scholar]
- 9. Greene SA, Tranquilli WJ, Benson GJ, et al. Effect of medetomidine administration on bispectral index measurements in dogs during anesthesia with isoflurane. Am J Vet Res 2003; 64: 316–320. [DOI] [PubMed] [Google Scholar]
- 10. Lamont LA, Greene SA, Grimm KA, et al. Relationship of bispectral index to minimum alveolar concentration multiples of sevoflurane in cats. Am J Vet Res 2004; 65: 93–98. [DOI] [PubMed] [Google Scholar]
- 11. Lamont LA, Greene SA, Grimm KA, et al. Relationship of feline bispectral index to multiples of isoflurane minimum alveolar concentration. Comp Med 2005; 55: 269–274. [PubMed] [Google Scholar]
- 12. Ko JC, Austin BR, Barletta M, et al. Evaluation of dexmedetomidine and ketamine in combination with various opioids as injectable anesthetic combinations for castration in cats. J Am Vet Med Assoc 2011; 239: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 13. Michou JN, Leece EA, Brearley JC. Comparison of pain on injection during induction of anaesthesia with alfaxalone and two formulations of propofol in dogs. Vet Anaesth Analg 2012; 39: 275–281. [DOI] [PubMed] [Google Scholar]
- 14. Maddern K, Adams VJ, Hill NAT, et al. Alfaxalone induction dose following administration of medetomidine and butorphanol in the dog. Vet Anaesth Analg 2010; 37: 7–13. [DOI] [PubMed] [Google Scholar]
- 15. Zaki S, Ticehurst K, Miyaki Y. Clinical evaluation of Alfaxan-CD as an intravenous anaesthetic in young cats. Aust Vet J 2009; 87: 82–87. [DOI] [PubMed] [Google Scholar]
- 16. Moshitch D, Las L, Ulanovsky N, et al. Responses of neurons in primary auditory cortex (A1) to pure tones in the halothane-anesthetized cat. J Neurophysiol 2006; 95: 3756–3769. [DOI] [PubMed] [Google Scholar]
- 17. Bonhomme V, Plourde G, Meuret P, et al. Auditory steady-state response and bispectral index for assessing level of consciousness during propofol sedation and hypnosis. Anesth Analg 2000; 91: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 18. Anon. Scientific discussion. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/veterinary/000070/WC500062496.pdf (2002, accessed July 2012).
- 19. Bösing B, Tünsmeyer J, Mischke R, et al. Clinical usability and practicability of alfaxalone for short-term anaesthesia in the cat after premedication with buprenorphine. Tierarztl Prax Ausg K Kleintiere Heimtiere 2012; 40: 17–25. [PubMed] [Google Scholar]
- 20. O’Brien D. Acute postoperative delirium: definitions, incidence, recognition, and interventions. J Perianesth Nurs 2002; 17: 384–392. [DOI] [PubMed] [Google Scholar]
- 21. Muir W, Lerche P, Wiese A, et al. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet Anaesth Analg 2008; 35: 451–462. [DOI] [PubMed] [Google Scholar]
- 22. Ambros B, Duke-Novakovski T, Pasloske KS. Comparison of the anesthetic efficacy and cardiopulmonary effects of continuous rate infusions of alfaxalone-2-hydroxypropyl-beta-cyclodextrin and propofol in dogs. Am J Vet Res 2008; 69: 1391–1398. [DOI] [PubMed] [Google Scholar]
- 23. Muir W, Lerche P, Wiese A, et al. The cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in cats. Vet Anaesth Analg 2009; 36: 42–54. [DOI] [PubMed] [Google Scholar]
- 24. Jiménez CP, Mathis A, Mora SS, et al. Evaluation of the quality of the recovery after administration of propofol or alfaxalone for induction of anaesthesia in dogs anaesthetized for magnetic resonance imaging. Vet Anaesth Analg 2012; 39: 151–159. [DOI] [PubMed] [Google Scholar]
- 25. Ko JC, Abbo LA, Weil AB, et al. A comparison of anesthetic and cardiorespiratory effects of tiletamine-zolazepam-butorphanol and tiletamine-zolazepam-butorphanol-medetomidine in cats. Vet Ther 2007; 8: 164–176. [PubMed] [Google Scholar]
- 26. Whittem T, Pasloske KS, Heit MC, et al. The pharmacokinetics and pharmacodynamics of alfaxalone in cats after single and multiple intravenous administration of Alfaxan at clinical and supraclinical doses. J Vet Pharmacol Ther 2008; 31: 571–579. [DOI] [PubMed] [Google Scholar]
- 27. Pypendop BH, Barter LS, Stanley SD, et al. Hemodynamic effects of dexmedetomidine in isoflurane-anesthetized cats. Vet Anaesth Analg 2011; 38: 555–567. [DOI] [PubMed] [Google Scholar]
- 28. Caulkett NA, Cantwell SL, Houston DM. A comparison of indirect blood pressure monitoring techniques in the anesthetized cat. Vet Surg 1998; 27: 370–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sedation, intubation and recovery