Abstract
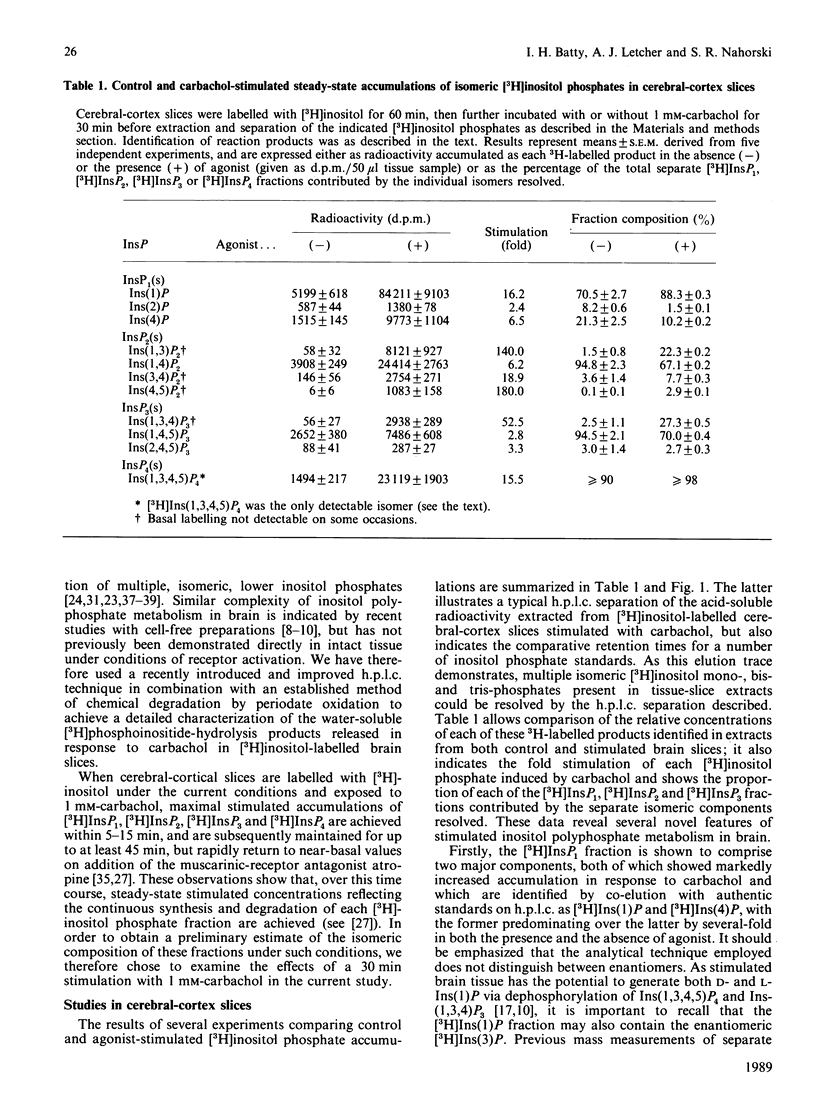
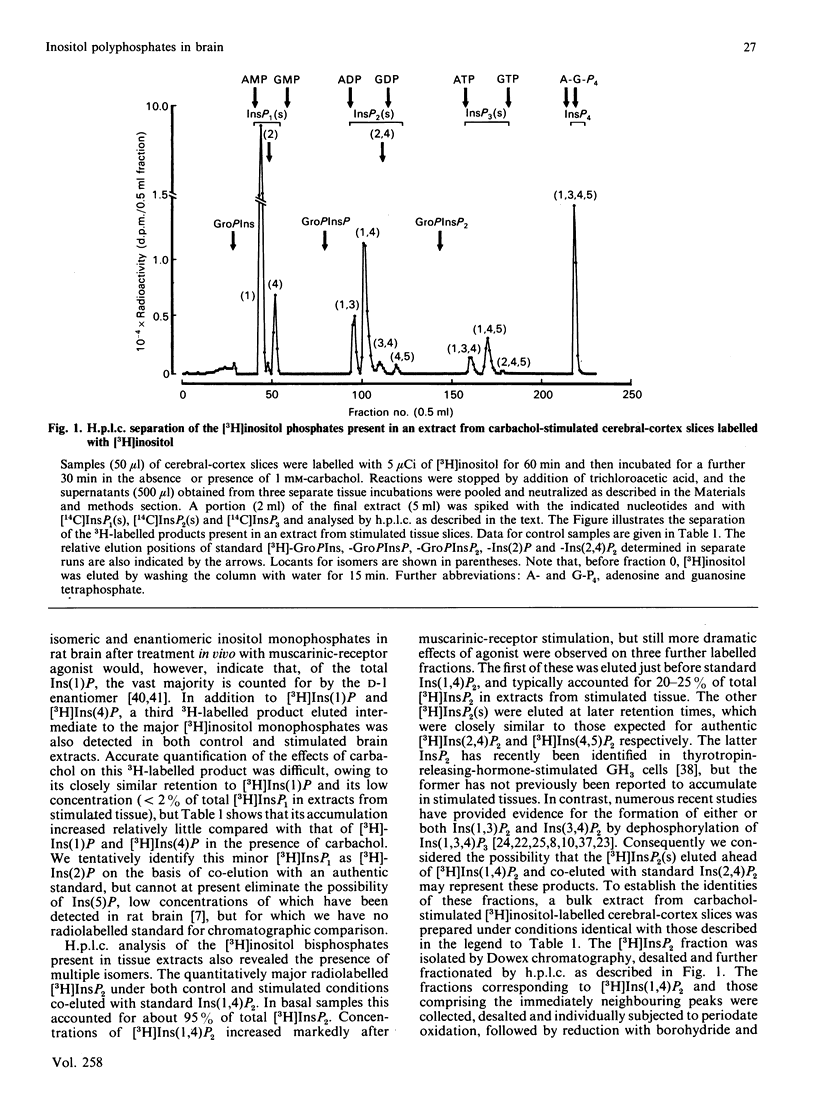
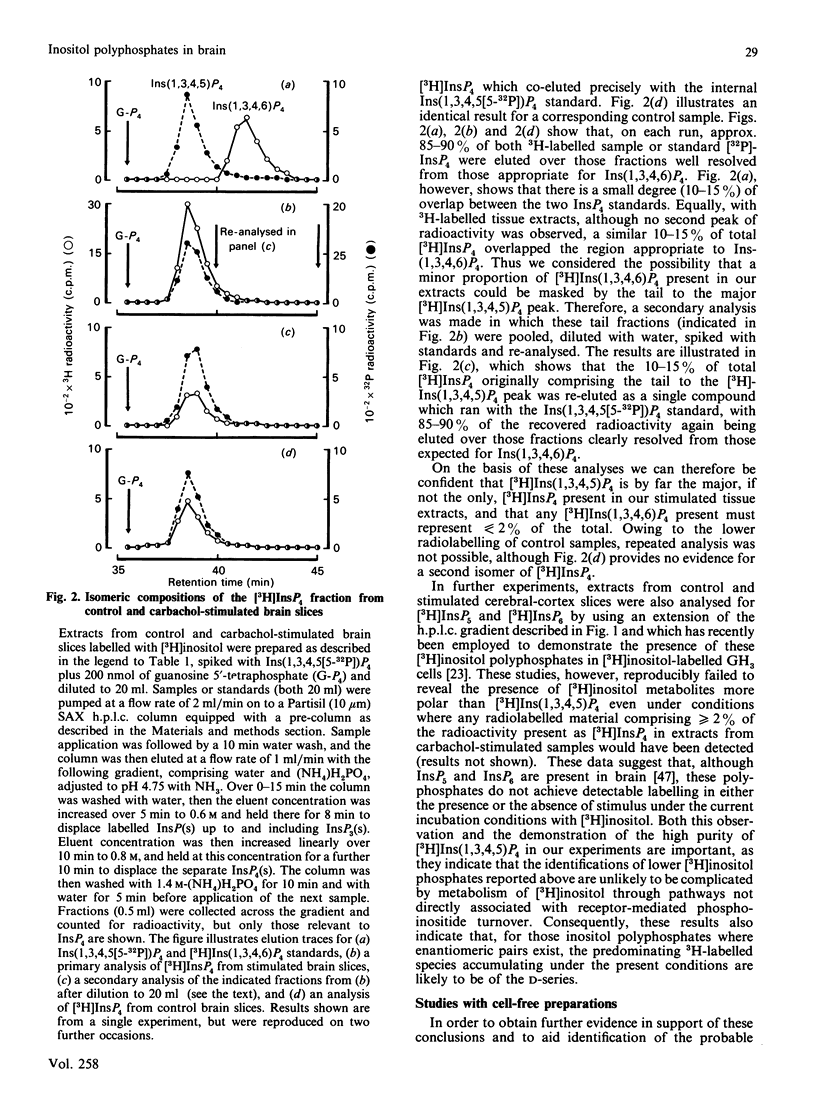
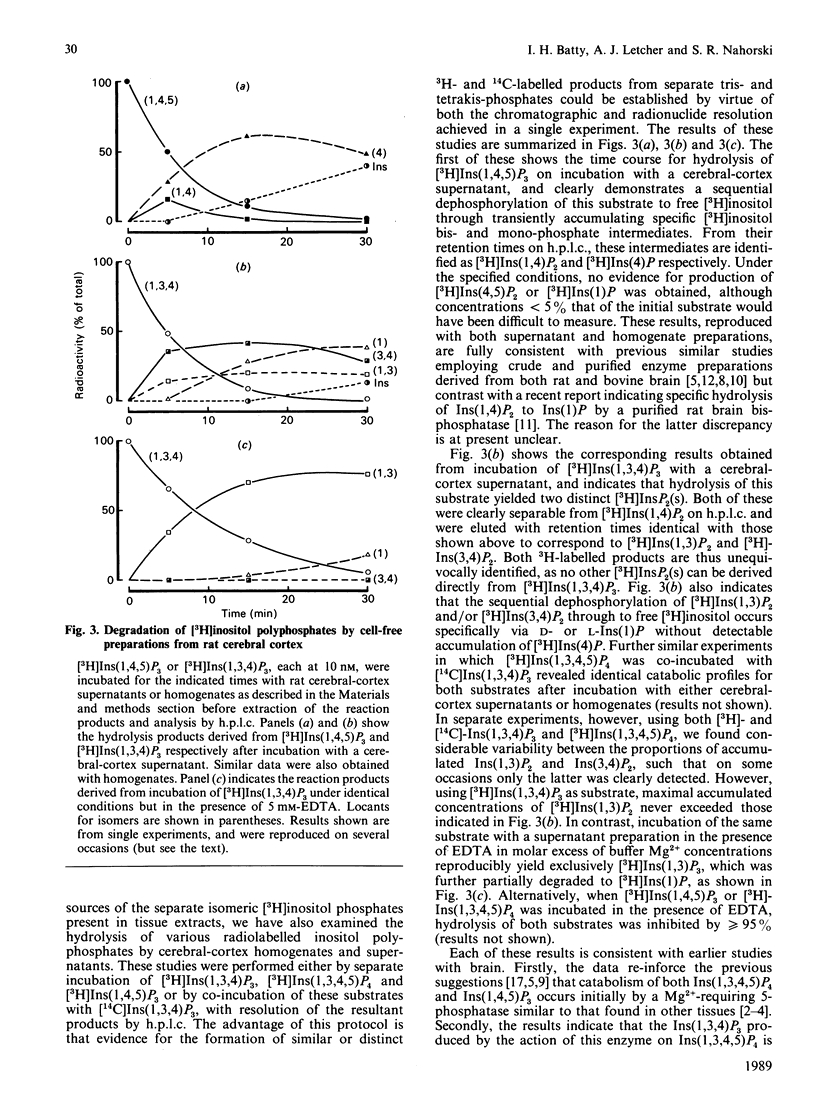
1. Basal and carbachol-stimulated accumulations of isomeric [3H]inositol mono-, bis-, tris- and tetrakis-phosphates were examined in rat cerebral-cortex slices labelled with myo-[2-3H]inositol. 2. In control samples the major [3H]inositol phosphates detected were co-eluted on h.p.l.c. with Ins(1)P, Ins(4)P (inositol 1- and 4-monophosphate respectively), Ins(1,4)P2 (inositol 1,4-bisphosphate), Ins(1,4,5)P3 (inositol 1,4,5-tris-phosphate) and Ins(1,3,4,5)P4 (inositol 1,3,4,5-tetrakisphosphate). 3. After stimulation to steady state with carbachol, accumulation of each of these products was markedly increased. 4. Agonist stimulation, however, also evoked much more dramatic increased accumulations of a second [3H]inositol trisphosphate, which was co-eluted on h.p.l.c. with authentic Ins(1,3,4)P3 (inositol 1,3,4-trisphosphate) and of three further [3H]inositol bisphosphates ([3H]InsP2(s]. 5. Examination of the latter by chemical degradation by periodate oxidation and/or h.p.l.c. allowed identification of these as [3H]Ins(1,3)P2, [3H]Ins(3,4)P2 and [3H]Ins(4,5)P2 (inositol 1,3-, 3,4- and 4,5-bisphosphates respectively), which respectively accounted for about 22%, 8% and 3% of total [3H]InsP2 in extracts from stimulated tissue slices. 6. By using a h.p.l.c. method which clearly resolves Ins(1,3,4,5)P4 and Ins(1,3,4,6)P4 (inositol 1,3,4,6-tetrakisphosphate), only the former isomer could be detected in extracts from either control or stimulated tissue slices. Similarly, [3H]inositol pentakis- and hexakis-phosphates were not detectable either in the presence or absence of carbachol under the radiolabelling conditions described. 7. The catabolism of [3H]Ins(1,4,5)P3 and [3H]Ins(1,3,4)P3 by cell-free preparations from cerebral cortex was also studied. 8. In the presence of Mg2+, [3H]Ins(1,4,5)P3 was specifically dephosphorylated via [3H]Ins(1,4)P2 and [3H]Ins(4)P to free [3H]inositol, whereas [3H]Ins(1,3,4)P3 was degraded via [3H]Ins(3,4)P2 and, to a lesser extent, via [3H]Ins(1,3)P2 to D- and/or L-[3H]Ins(1)P and [3H]inositol. 9. In the presence of EDTA, hydrolysis of [3H]Ins(1,4,5)P3 was greater than or equal to 95% inhibited, whereas [3H]Ins(1,3,4)P3 was still degraded, but yielded only a single [3H]InsP2 identified as [3H]Ins(1,3)P2. 10. The significance of these observations with cell-free preparations is discussed in relation to the proportions of the separate isomeric [3H]inositol phosphates measured in stimulated tissue slices.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann K. E., Gish B. G., Honchar M. P., Sherman W. R. Evidence that inositol 1-phosphate in brain of lithium-treated rats results mainly from phosphatidylinositol metabolism. Biochem J. 1987 Mar 1;242(2):517–524. doi: 10.1042/bj2420517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J. G., Nahorski S. R. Potassium depolarisation markedly enhances muscarinic receptor stimulated inositol tetrakisphosphate accumulation in rat cerebral cortical slices. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1130–1137. doi: 10.1016/s0006-291x(86)80161-9. [DOI] [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Catt K. J. Multiple pathways of inositol polyphosphate metabolism in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem. 1988 Mar 25;263(9):4083–4091. [PubMed] [Google Scholar]
- Balla T., Guillemette G., Baukal A. J., Catt K. J. Metabolism of inositol 1,3,4-trisphosphate to a new tetrakisphosphate isomer in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem. 1987 Jul 25;262(21):9952–9955. [PubMed] [Google Scholar]
- Balsinde J., Diez E., Mollinedo F. Phosphatidylinositol-specific phospholipase D: a pathway for generation of a second messenger. Biochem Biophys Res Commun. 1988 Jul 29;154(2):502–508. doi: 10.1016/0006-291x(88)90168-4. [DOI] [PubMed] [Google Scholar]
- Bansal V. S., Inhorn R. C., Majerus P. W. The metabolism of inositol 1,3,4-trisphosphate to inositol 1,3-bisphosphate. J Biol Chem. 1987 Jul 15;262(20):9444–9447. [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I., Nahorski S. R. Differential effects of lithium on muscarinic receptor stimulation of inositol phosphates in rat cerebral cortex slices. J Neurochem. 1985 Nov;45(5):1514–1521. doi: 10.1111/j.1471-4159.1985.tb07221.x. [DOI] [PubMed] [Google Scholar]
- Batty I., Nahorski S. R. Lithium inhibits muscarinic-receptor-stimulated inositol tetrakisphosphate accumulation in rat cerebral cortex. Biochem J. 1987 Nov 1;247(3):797–800. doi: 10.1042/bj2470797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: I. Receptor characterisation. J Neurochem. 1984 May;42(5):1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Metabolism of inositol bis-, tris-, tetrakis- and pentakis-phosphates in GH3 cells. Biochem J. 1988 Mar 1;250(2):493–500. doi: 10.1042/bj2500493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., Murray J. J., Verghese M. W., Snyderman R. Regulation of inositol phosphate metabolism in chemoattractant-stimulated human polymorphonuclear leukocytes. Definition of distinct dephosphorylation pathways for IP3 isomers. J Biol Chem. 1987 Aug 25;262(24):11546–11552. [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. The formation of inositol 1,2-cyclic phosphate on agonist stimulation of phosphoinositide breakdown in mouse pancreatic minilobules. Evidence for direct phosphodiesteratic cleavage of phosphatidylinositol. J Biol Chem. 1985 Dec 25;260(30):16068–16071. [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat brain. Biochem Biophys Res Commun. 1986 Jan 14;134(1):351–358. doi: 10.1016/0006-291x(86)90570-x. [DOI] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. The dephosphorylation pathway of D-myo-inositol 1,3,4,5-tetrakisphosphate in rat brain. Biochem J. 1987 Nov 1;247(3):635–639. doi: 10.1042/bj2470635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Hanley M. R., Lee C. M., Michell R. H., Jones L. M. Similar effects of substance P and related peptides on salivation and on phosphatidylinositol turnover in rat salivary glands. Mol Pharmacol. 1980 Jul;18(1):78–83. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Hughes P. J., Drummond A. H. Formation of inositol phosphate isomers in GH3 pituitary tumour cells stimulated with thyrotropin-releasing hormone. Acute effects of lithium ions. Biochem J. 1987 Dec 1;248(2):463–470. doi: 10.1042/bj2480463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Bansal V. S., Majerus P. W. Pathway for inositol 1,3,4-trisphosphate and 1,4-bisphosphate metabolism. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2170–2174. doi: 10.1073/pnas.84.8.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Fatty acid stimulation of membrane phosphatidylinositol hydrolysis by brain phosphatidylinositol phosphodiesterase. Biochem J. 1979 Feb 15;178(2):497–500. doi: 10.1042/bj1780497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Heslop J. P., Berridge M. J. Inositol(3,4)bisphosphate and inositol(1,3)bisphosphate in GH4 cells--evidence for complex breakdown of inositol(1,3,4)trisphosphate. Biochem Biophys Res Commun. 1987 Feb 27;143(1):353–359. doi: 10.1016/0006-291x(87)90672-3. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Inositol(1,3,4,5)tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate. Biochem Biophys Res Commun. 1987 Jul 15;146(1):284–290. doi: 10.1016/0006-291x(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Connolly T. M., Bross T. E., Majerus P. W. Inositol cyclic triphosphate [inositol 1,2-(cyclic)-4,5-triphosphate] is formed upon thrombin stimulation of human platelets. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6397–6401. doi: 10.1073/pnas.83.17.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Watling K. J., Gee N. S., Aspley S., Jackson R. G., Reid G. G., Baker R., Billington D. C., Barnaby R. J., Leeson P. D. The dephosphorylation of inositol 1,4-bisphosphate to inositol in liver and brain involves two distinct Li+-sensitive enzymes and proceeds via inositol 4-phosphate. Biochem J. 1988 Jan 1;249(1):143–148. doi: 10.1042/bj2490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfred M. A., Farrell L. E., Wells W. W. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat liver plasma membranes. J Biol Chem. 1984 Nov 10;259(21):13204–13208. [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J., Michell R. H. The pathway of myo-inositol 1,3,4-trisphosphate dephosphorylation in liver. Biochem J. 1987 Dec 15;248(3):977–980. doi: 10.1042/bj2480977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Parry J. B., Tang E. K., Irvine R. F., Michell R. H., Kirk C. J. Metabolism of D-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J. 1987 Aug 15;246(1):139–147. doi: 10.1042/bj2460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W. R., Leavitt A. L., Honchar M. P., Hallcher L. M., Phillips B. E. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J Neurochem. 1981 Jun;36(6):1947–1951. doi: 10.1111/j.1471-4159.1981.tb10819.x. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Munsell L. Y., Gish B. G., Honchar M. P. Effects of systemically administered lithium on phosphoinositide metabolism in rat brain, kidney, and testis. J Neurochem. 1985 Mar;44(3):798–807. doi: 10.1111/j.1471-4159.1985.tb12886.x. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Morris A. J., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate (3-hydroxy)kinase. Biochem J. 1988 Jan 1;249(1):283–292. doi: 10.1042/bj2490283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Takimoto K., Motoyama N., Okada M., Nakagawa H. Purification and properties of inositol-1,4-bisphosphate 4-phosphohydrolase from rat brain. Biochim Biophys Acta. 1987 Jul 29;929(3):327–335. doi: 10.1016/0167-4889(87)90260-6. [DOI] [PubMed] [Google Scholar]
- Vallejo M., Jackson T., Lightman S., Hanley M. R. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987 Dec 17;330(6149):656–658. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Erneux C., Van Eijk R., Van Haastert P. J. Two dephosphorylation pathways of inositol 1,4,5-trisphosphate in homogenates of the cellular slime mould Dictyostelium discoideum. Biochem J. 1988 Sep 1;254(2):343–350. doi: 10.1042/bj2540343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]


