Abstract
This study investigated the effect of captopril (Cap) on spinal cord ischemia-reperfusion injury (SCII) in rats. Twenty-four adults male Wistar rats were randomly divided into four groups of six animals each: spinal cord ischemia-reperfusion (SCI-R) with Cap (SCI-R + Cap), SCI-R, sham-operated with Cap (SHAM + Cap), and SHAM. The 24 hr and 90 min before ischemia induction, Cap was administered intragastrically (100 mg kg-1) to the SHAM + Cap and SCI-R + Cap groups. Abdominal aortic clamping was performed in the SCI-R and SCI-R + Cap groups for 40 min. Hindlimb motor function was evaluated using the Tarlov Scale at 4, 6, 12, 24, 48, and 60 hr after SCII. The malondialdehyde (MDA), the ferric-reducing ability of plasma (FRAP) and prooxidant-antioxidant balance (PAB) values were also measured. Throughout the study period, the SCI-R group had significantly lower motor function scores compared to the other groups. The MDA and PAB levels were higher and the FRAP value was lower in the SCI-R group compared to in the SHAM group. The SCI-R + Cap had higher motor function scores compared to the SCI-R group at all time points. There were no significant differences in MDA concentration, FRAP and PAB values between the SCI-R + Cap and SCI-R groups. Captopril may act as a protective agent against SCII in rats based on hind limb motor function assessment.
Key Words: Captopril, Neurological status, Oxidative injury, Paraplegia
Introduction
Paraplegia is a common complication of surgical procedures related to the thoracic and thoracoabdominal aorta due to interruption of spinal cord blood flow.1 The requirement for aortic cross-clamping during surgery poses a threat of causing ischemia in various distal organs such as the liver, spleen, kidney, brain and spinal cord. The spinal cord has less collateral vasculature and cerebro-spinal fluid volume than the brain.2 On the other hand, the spinal cord high lipid content and active oxygen metabolism make it highly susceptible to damage caused by free radicals.3 Therefore, a given period of ischemia is likely to result in greater irreversible damage to the spinal cord than to the brain, and higher levels of malondialdehyde (MDA) are produced during oxidative stress damage.
The spinal cord is particularly sensitive to ischemia due to high levels of polyunsaturated fatty acids, high oxidative metabolic activity, low antioxidant capacity and weak neuronal antioxidant defenses.3
Despite the use of various clinical and experimental methods to reduce the occurrence of paraplegia, spinal cord injury remains a concern complication of these operations.4,5 Prevention of iatrogenic SCI injury (SCII) has remained an important medical issue in recent years.6,7 Numerous efforts have been made to prevent ischemic spinal cord injuries after aortic cross-clamping such as systemic or topical cooling, preoperative spinal angiography, distal aortic perfusion, intercostal artery reimplantation, ischemic preconditioning, cerebrospinal fluid drainage, intraoperative monitoring of spinal cord function and pharmacological preconditioning.8-11 Several pharma-cological agents including steroids, oxygen free-radical scavengers and vasodilators, have been used in preclinical experimental SCII models. However, to date, no effective treatment strategy can completely prevent SCII.12-14
Reactive oxygen species (ROS) are critical in the emergence of ischemia-reperfusion injury in various organs.15 The SCII is usually due to oxygen-free radical-induced lipid peroxidation, inflammation, leukocyte activation and neuronal apoptosis.6 Various bioactive agents with antioxidant properties have been proven to have neuroprotective effects by reducing ROS in numerous studies.12,16-19 Antioxidants can decrease oxidative stress by intercepting or removing ROS, thus avoiding the spread of oxidative chain reactions. These antioxidants include both endogenous antioxidant enzymes and exogenous sources, whether natural or synthetic, which maintain the biological system redox equilibrium. Antioxidant enzymes include superoxide dismutase, catalase and glutathione peroxidase. Thiol antioxidants (such as Glutathione and α-lipoic acid), uric acid and coenzyme Q10 are among the low-molecular-weight non-enzymatic antioxidants.20,21
The search for more effective and nontoxic antioxidant compounds is continued. This study focused on the free radical-scavenging agents and angiotensin-converting enzyme inhibitors (ACEIs) to prevent free radical formation. Angiotensin-converting enzyme inhibitors are usually used to treat hypertension, myocardial infarction and certain types of congestive heart failure.22,23 Also, ACEIs decrease oxidative stress under various pathophysiological conditions in many organs.24 Captopril (Cap; [2S]-N-[3-mercapto-2-methylpropionyl]-l-proline) is an ACEI with antioxidant properties due to its terminal sulfhydryl group that enhances enzymatic and non-enzymatic defenses in different tissues. It is a low-cost drug with few side effects.25 The transfer of Cap through the intact blood-brain barrier is typically negligible under normal conditions. However, Kuroiwa et al. discovered that ischemia could cause a transient blood-brain barrier opening.26 The present study chose Cap as it has been indicated to ameliorate both functional and biochemical outcomes in other models of ischemia-reperfusion injury by scavenging free radicals.25,28-30 This would provide novel therapeutic targets for the treatment of SCII in rats. To date, no studies have explored the effects of Cap in a rat model of SCII.
Materials and Methods
Animals. A total number of 24 healthy male Wistar rats were obtained from the animal center (Mashhad University of Medical Sciences, Iran) and were kept in an environment with a temperature of (21.00 ± 2.00 ˚C and humidity (60.00 ± 5.00%). Rats were exposed to a 12-hr light and 12-hr dark cycle for two weeks before the experiments. The study was approved by the institutional ethics committee of Ferdowsi University of Mashhad, Mashhad, Iran (No. 41807) in accordance with animal welfare standards.
Study design. Rats were randomly assigned to the following groups (n = 6): SCI-R with Cap (SCI-R + Cap), SCI-R, sham-operated with Cap (SHAM + Cap) and SHAM. In the SCI-R + Cap and the SHAM + Cap groups, Cap (100 mg kg-1, Sigma Alderich, St. Louis, USA) was administered intragastrically, 24 hr and 90 min before the surgical procedure. Also, the SHAM and the SCI-R groups were given an equivalent volume of distilled water saline instead of Cap. The dose of Cap used in this study was chosen based on a previous study conducted by Guo et al. 30 The SCI-R and the SCI-R + Cap groups underwent abdominal clamping surgery. In order to induce SCII, the abdominal aorta was occluded for 40 min. The SHAM and the SHAM + cap groups underwent a similar surgical procedure without the aorta being occluded.
Operation technique and ischemia-reperfusion injury induction. The animals were fasted for 12 hr and anesthetized by intramuscular administration of xylazine hydrochloride (5.00 mg kg-1, Alfasan, Woerden, The Netherlands) and 50.00 mg kg-1 of ketamine hydrochloride 10% (Alfasan). A midline laparotomy was performed after aseptic preparation of the surgical site. Heparin (Caspian Co., Rasht, Iran) was then administered intraperitoneally at 400 UI kg-1. To induce spinal cord ischemia, the abdominal aorta was occluded just below the left renal artery using an atraumatic microvascular clamp for 40 min. After the experimental procedure, 1.00 mg kg-1 protamine sulfate (Exir Co., Boroujerd, Iran) was injected intravenously (IV). Lactate ringer solution (1.00 mL) was administered intraperitoneally, and abdominal muscles and skin were closed. Cefazolin sodium (15.00 mg kg-1, q24hr, Alborz Darou Co., Qazvin, Iran) was administered intramuscularly (IM), preoperatively and postoperatively. Morphine HCl (400 µg kg-1, q24hr, IM, Darou Paksh Co., Tehran, Iran) was given to all the animals for postoperative analgesia.12
Evaluation of hind limb neurological status. At the end of 4, 6, 12, 24, 48, and 60 hr after reperfusion, the neurologic function was evaluated by two independent observers using the following criteria (modified Tarlov Scale: 0: Complete paralysis, 1: Move slightly but cannot move, 2: Ordinary moves but cannot walk or jump, 3: Can walk and jump with manifest ataxia, 4: Can usually jump, and 5: Complete recovery). 12,31
Biochemical examinations. At the end of the study (60 hr after reperfusion), the rats were sacrificed under deep anesthesia and spinal specimens were harvested from the 3rd to 5th lumbar vertebral segments. The specimens were flushed with normal saline solution and stored at – 80.00 ˚C to study the spinal cord oxidative stress status.12
Tissue MDA assay. One of the most common methods used to study oxidative stress is measuring the red complex resulting from the reaction of thiobarbituric acid with aldehydes during the lipid peroxidation process, particularly MDA. This research measured MDA using the Mihara and Uchiyama method.18,32 Briefly, the spinal samples were homogenized in 10.00% cold phosphate buffer (100 mM, pH = 7.40) using a homogenizer (Heidolph, Schwabach, Germany) and mixed with 1.00% phosphoric acid. After adding 1.00 mL of 0.67% thiobarbituric acid Sigma-Aldrich), the mixture was heated in a serological water bath (Behdad, Tehran, Iran) for 45 min. Then, the butanol layer was separated by centrifugation (Eppendorf CO., Leipzig, Germany). The optical density of the butanol layer was measured using a spectrophoto-meter at 523 nm (SPUV-26; SCO Tech, Plochingen, Germany). The MDA concentration in each sample was calculated using Lambert's law as follows. The results were reported in nmol per g of wet tissue.12
MDA Concentration = Absorbance / (MEC × LF) × DF
where, MEC is the molar extinction coefficient, LF is the light path and DF is the dilution factor.
Measurement of total antioxidant capacity by method of ferric-reducing ability of plasma (FRAP) assay. The FRAP assay was performed to assess the extent of total antioxidant power in spinal cord tissue after SCII. The principle of this test is based on the reduction of a colorless ferric-Tripyridyl-S-triazine (Fe3+-TPTZ) complex to a blue-colored ferrous (Fe2+) complex at low pH (pH = 3.60). The FRAP assay has an absorption peak at a wavelength of 593 nm. The FRAP value was determined using the method described by Benzie and Starin.33
Prooxidant –antioxidant balance (PAB) assay. Measuring total oxidant and total antioxidant capacity is necessary to determine the oxidant-antioxidant balance correctly. In the PAB assay, two reactions take place in one trial, and 3,3′,5,5′-Tetramethylbenzidine (TMB), and TMB cations are used as indicators of oxidation reduction. In an enzymatic reaction, the TMB chromogen is oxidized to a colored cation by pro-oxidants. The TMB cation is reduced to a colorless compound by antioxidants in a chemical reaction. Hydrogen peroxide represents oxidants and uric acid represents antioxidants. A modified PAB assay was used in this study based on a method previously described by Alamdari et al.34
Statistical analysis. The results were reported as mean ± standard deviation using SPSS Software (version 19.0; IBM Corp., Armonk, USA). Normality was assessed with the Kolmogorov-Smirnov test. The differences among the groups regarding Tarlov scores were determined using the Mann-Whitney U test. One-way ANOVA with post-hoc analyses using the Tukey method was used to compare the groups for biochemical analysis (MDA, FRAP, and PAB). A significance level of p < 0.05 was considered significant.
Results
Evaluation of hindlimb neurological status. The results indicated that the motor function score of the SCI-R group was significantly lower than those of the other groups during the entire study period (p < 0.05). The motor function score of the SCI-R + Cap group was significantly lower than those of the SHAM and the SHAM + Cap groups at 4 - 24 hr after surgery (p < 0.05), however, no significant difference was observed in the following time points (p > 0.05). The motor function score of the SHAM + Cap group was not significantly different from that of the SHAM group at any point in time (p > 0.05). The SCI-R + Cap group had a higher motor function score than the SCI-R group at 4 - 60 hr (p < 0.05; Fig. 1).
Fig. 1.
Neurological status evaluation after spinal cord ischemia-reperfusion. Data are shown as mean ± SD.
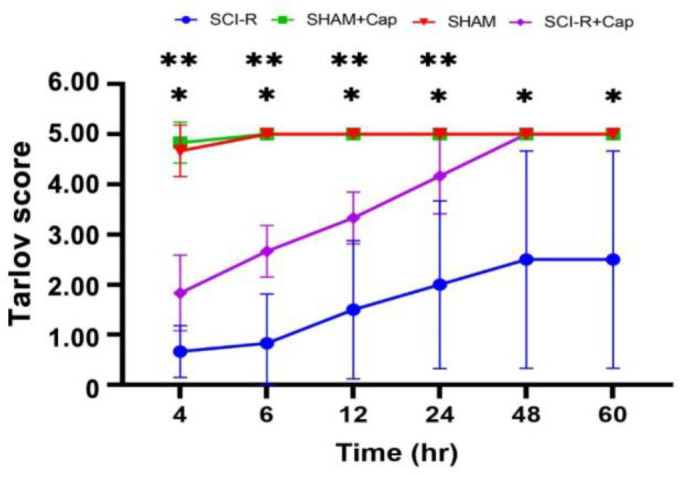
* Means spinal cord ischemia-reperfusion (SCI-R) group was significant with SHAM, SHAM + captopril (Cap) and spinal cord ischemia-reperfusion (SCI-R) + captopril (Cap) groups at all time.
** Means spinal cord ischemia-reperfusion (SCI-R) + captopril (Cap) group was significant with SHAM and SHAM + captopril (Cap) groups up to 24 hours.
Tissue MDA assay. A significant increase in MDA content was observed in the SCI-R (16.98 ± 0.68) and the SCI-R + Cap groups (14.71 ± 0.67) compared to that in the SHAM group (10.98 ± 1.02; p < 0.05). The MDA content in the SHAM group was significantly different from that in the SHAM + Cap group (8.05 ± 0.17; p < 0.05). The MDA concentration in the SCI-R + Cap group had lower levels than that in the SCI-R group, however, the difference was not significant (p > 0.05; Fig. 2).
Fig. 2.
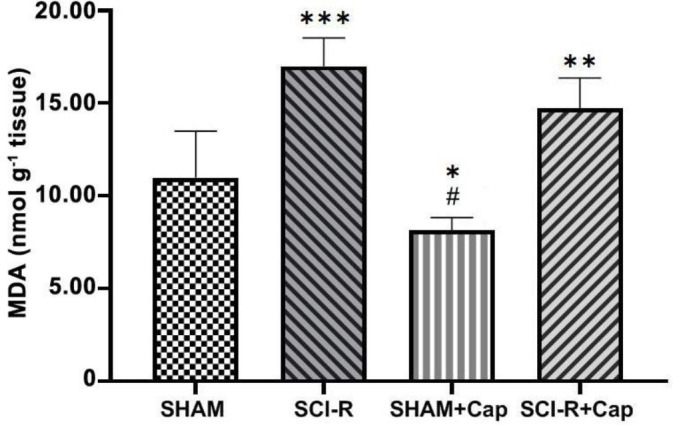
Malondialdehyde (MDA) content in spinal cord segments following ischemia-reperfusion injury. Data are shown as mean ± SD. Statistically significant differences between groups: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.0001 versus the SHAM group. # p ≤ 0.0001 versus the spinal cord ischemia-reperfusion (SCI-R) + captopril (Cap) group.
Ferric-reducing ability of plasma assay. The FRAP values showed a significant decrease in the SCI-R (1.14 ± 0.26) and SCI-R + Cap groups (2.58 ± 0.62) compared to the SHAM group (4.61 ± 0.41; p < 0.05), however, the FRAP value of the SHAM group was not significantly different from that of the SHAM + Cap group (4.94 ± 0.63; p > 0.05). Also, the total antioxidant capacity of the SCI-R + Cap group was not significantly different from that of the SCI-R group (p > 0.05; Fig. 3).
Fig. 3.
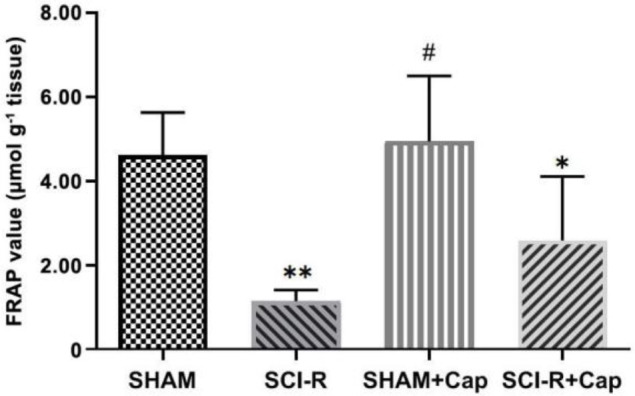
Ferric-reducing ability of plasma (FRAP) levels in the spinal cord following ischemic-spinal cord injury. Data are shown as mean ± SD. Statistically significant differences between groups: *p ≤ 0.05 and **p ≤ 0.001 versus the SHAM group. # p ≤ 0.05 versus the spinal cord ischemia-reperfusion (SCI-R) + captopril (Cap) group.
Prooxidant–antioxidant balance assay. The results indicated that the ratio of oxidant to antioxidant significantly was increased in the SCI-R (1.25 ± 0.23) and SCI-R + Cap groups (1.08 ± 0.09) compared to the SHAM group (0.51 ± 0.03; p < 0.05), however, the PAB level in the SHAM group was not significantly different from the SHAM + Cap group (0.48 ± 0.06; p > 0.05). Also, the PAB level of the SCI-R + Cap group was not significantly different from those in the SCI-R group (p > 0.05; Fig. 4).
Fig. 4.
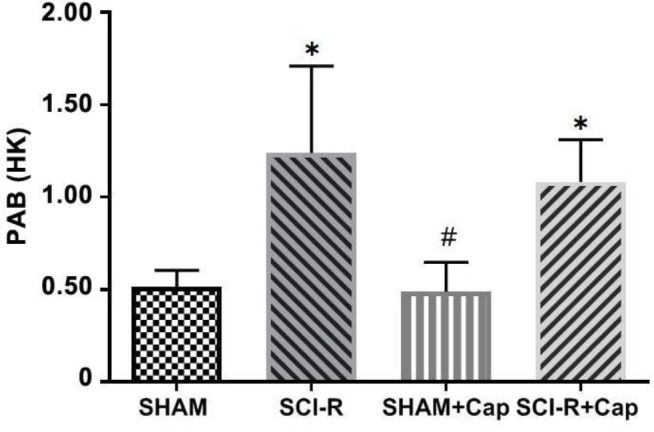
Prooxidant-antioxidant balance (PAB) in spinal cord following ischemic-reperfusion injury. Data are shown as mean ± SD. Statistically significant differences between groups: * p ≤ 0.01 versus the SHAM group. # p ≤ 0.01 versus the spinal cord ischemia-reperfusion (SCI-R) + captopril (Cap) group. The values of PAB assay were expressed in an arbitrary Hamidi-Koliakos (HK) unit.
Discussion
The results of this study showed that acute administration of Cap could protect the spinal cord as demonstrated by improved hind limb neurological function. However, the levels of MDA, FRAP, and PAB values were not significantly different between the SCI + Cap and SCI-R groups.
Motor dysfunction and hindlimb paralysis are one of the main disorders associated with SCII.35 Basso Beattie, Bresnahan, and Tarlov scales are commonly used to assess motor function after SCII.36 The Tarlov score is used to evaluate the weight-bearing, movements and walking ability of the hind limbs.37 This score reflects the degree of neurological impairment associated with SCII with scores ranging from 0 (paralysis) to 5 (no neurological deficit).6,38,39 In this study, the Tarlov scoring system was used to assess the effects of Cap on functional recovery in the SCII rats. Similar to Liu et al.,9 our results indicated that abdominal aortic clamping surgery (40 min) led to spinal cord injury. In this study, the results indicated that Cap had a significant effect on improving neurological function. Hence, the SCI-R + Cap group had higher motor function scores than the SCI-R group at 4 - 60 hr after SCII. Werner et al. also observed that Cap could significantly improve neurologic status compared to the control group over 3 days after brain ischemia.40
This study measured ROS biomarkers to evaluate biological responses to therapeutic intervention. The levels of MDA, FRAP and PAB were assessed. So far, FRAP and PAB assays have not been used to assess the protective effect of Cap against the SCII in vivo. Similar to other studies, MDA concentrations and PAB levels significantly were increased, and FRAP levels significantly was decreased in the SCI-R group compared to those in the SHAM group.9,12,40-42 According to our results, an SCII model was successfully established.
Lipid peroxidation has been reported as an important contributor to the loss of cellular function under oxidative stress conditions.43 We indirectly measured the amount of ROS and Lipid peroxidation using the MDA assay. The MDA is an end product of lipid peroxidation and is the most commonly used marker for measuring lipid per-oxidation. It has attracted significant interest in studying physiological mechanisms and has the potential to be used in routine clinical laboratory testing.43 Karimani et al. showed that chronic oral administration of Cap (10.00 mg kg-1, q24hr, for 7 weeks) reduced the concentration of MDA in kidney and liver tissues.44 However, Gurer et al. stated that the brain MDA concentration in the Lead + Cap group (administration of Cap (10.00 mg kg-1, q24hr) one week after the injury) was not significantly different from that in the Lead group.45 Similar to the study by Gurer et al., our findings indicated that pretreatment with Cap did not significantly attenuate the increase in MDA levels in the SCI-R + Cap group, and MDA levels were not lower than those in the SCI-R group.45 The MDA levels were lower in the SHAM + Cap group compared to the SHAM group, indicating that Cap could significantly decrease MDA levels in healthy spinal cord tissue by strengthening the antioxidant defenses of the spinal cords.
The imbalance between the production of free radicals and antioxidant defense causes oxidative stress, therefore, it is essential to evaluate antioxidant components in addition to oxidants.43 By this approach, an opportunity is provided to monitor the effectiveness of antioxidant interventions and it is also possible to obtain information about the involvement of the general state of oxidative stress in the pathophysiology of the spinal cord. The FRAP test, a non-radical set-based method, has been reported to have a low correlation with radical extinction in lipid systems and other antioxidant activity tests46 which assesses reducing capacity by reacting sample anti-oxidants with inorganic oxidants such as Fe3+ resulting in the formation of a colored ferrous complex.43 Gurer et al. showed that Cap increased the antioxidant capacity (glutathione) of the brain.45 Nevertheless, in our study, acute administration of Cap did not increase the anti-oxidant capacity of the spinal cord in any of the groups. We considered the FRAP assay to evaluate oxidative stress status because it is fast, inexpensive easy to handle and gives highly reproducible results. Cellular antioxidant glutathione, Cu and zinc superoxide dismutase play the most important roles in removing ROS after SCII.21 Gaggini et al. suggested that the FRAP assay was reliable for uric acid, α-tocopherol, ascorbic acid and bilirubin (endogenous antioxidants). However, it could not evaluate thiol-type antioxidants (e.g., antioxidant glutathione).43 Munteanu and Apetrei indicated that the ability of an antioxidant test to detect both hydrophilic and lipophilic antioxidants was an important factor to consider, however, FRAP assay only measures hydrophilic anti-oxidants such as uric acid and ascorbic acid.46 Therefore, this test could not detect many important antioxidants in the spinal cord tissue. Also, the reaction of this method is nonspecific, and the FRAP assay has low specificity.43
The PAB assay is a currently available test that simultaneously measures the balance of oxidants and antioxidants in one experiment. The PAB assay values are expressed in arbitrary units based on the percentage of hydrogen peroxide in a standard solution. In the PAB assay, low values indicate a greater concentration of antioxidants than oxidants and high values indicate the opposite. Our experimental study showed that Cap did not modulate the PAB value in the spinal cord tissue in SCI-R + Cap group and antioxidant-oxidant status was present at higher concentrations of oxidants compared to anti-oxidants. The PAB assay is not routinely used to assess oxidative stress status. The sensitivity and specificity of the PAB assay are still not well known and it may not be powerful enough to replace other tests for oxidative stress status.47
There have been conflicting reports regarding the antioxidant properties of Cap. A study performed by Johnson et al., which examined the effect of two anti-hypertensive drugs (Cap and hydralazine) in preventing apoptosis in neurons, found that Cap was ineffective in reducing the production of MDA in the spinal cord tissue.48 Captopril also did not affect the early phase of apoptosis in neurons while hydralazine reduced the production of free radicals in neurons.48 However, there have been reports that treatment with Cap reduces the level of lipid peroxidation and increases antioxidant defenses in various rat tissues.30,49,50 Werner et al. demonstrated that administering Cap (10.00 mg kg-1, IV) 30 min before the ischemic period attenuated brain oxidative in rats.40 The present study examined the effects of Cap following ischemic damage in the rat spinal cord. We showed that administering Cap prior to ischemia-reperfusion injury could protect the spinal cord and improve hind limb neurological function. However, acute administration of Cap did not affect biochemical analysis in the SCI-R + Cap group compared to the SCI-R group. The reason for the differences between this study and previous studies is unclear, however, it may be due to variations in the markers used or in the tissues in which they were measured. Additionally, the methods used to evaluate protection in this study might be different from those used in previous studies on the other tissues.
Motor function is an objective measure of the degree of spinal cord injury and recovery. The biochemical measurements are only one aspect of the overall effects of antioxidants on spinal cord injury and they may not always closely correlate with changes in motor function. However, the results from the neurological deficit score suggested that Cap might have a beneficial effect on the outcome of SCII.
The present study showed that abdominal aortic clamping led to SCII, and an acute administration of Cap (100 mg kg-1) reduced SCII based on hind limb motor function assessment. Future studies should use combination therapy strategies and additional tests such as histopathology and cellular examinations to better determine whether this treatment may be amenable to human patients. It is important to consider the limitations of these assays such as the small sample size, short-term administration of Cap before ischemia induction and single dose Cap administration.
Acknowledgments
This study was supported by the funds granted (No. 41807) by the Applied Research Centre, Vice-chancellor for Research of Ferdowsi University of Mashhad, Mashhad, Iran.
Conflict of interests
The authors declare no conflict of interest.
References
- 1.Fang H, Li HF, Pan Q, et al. MiR-132-3p modulates MEKK3-dependent NF-κB and p38/JNK signaling pathways to alleviate spinal cord ischemia-reperfusion injury by hindering M1 polarization of macrophages. Front Cell Dev Bio. 2021;9:570451. doi: 10.3389/fcell.2021.570451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford MW, Lerman J, Pilato M, et al. Haemodynamic and organ blood flow responses to sevoflurane during spontaneous ventilation in the rat: a dose-response study. Can J Anaesth. 1992;39(3):270–276. doi: 10.1007/BF03008788. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco C, Naziroǧlu M, Rodríguez AB, et al. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol. 2018;9:95. doi: 10.3389/fphys.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amanollahi S, Bahrami AR, Haghighitalab A, et al. Immediate administration of hTERT-MSCs-IDO1-EVs reduces hypoalbuminemia after spinal cord injury. Vet Res forum. 2024;15: 27–34. doi: 10.30466/vrf.2023.2003942.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodabakhshi Rad A, Kazemi Mehrjerdi H, Pedram MS, et al. Clinical evaluation of the effect of methyl-prednisolone sodium succinate and meloxicam in experimental acute spinal cord injury. Iran J Vet Med. 2023;17(2):129–138. [Google Scholar]
- 6.Ji Y, Meng B, Yuan C, et al. Monitoring somatosensory evoked potentials in spinal cord ischemia-reperfusion injury. Neural Regen Res. 2013;8(33):3087–3094. doi: 10.3969/j.issn.1673-5374.2013.33.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakai H, Fujita Y, Masuda S, et al. Intravenous injection of adult human bone marrow mesenchymal stromal cells attenuates spinal cord ischemia/reperfusion injury in a murine aortic arch crossclamping model. JTCVS Open. 2021;7:23–40. doi: 10.1016/j.xjon.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell MT, Puskas F, Smith PD, et al. Attenuation of spinal cord ischemia-reperfusion injury by specific α-2a receptor activation with dexmedetomidine. J Vasc Surg. 2012;56(5):1398–1402. doi: 10.1016/j.jvs.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Huang W, Xiao X, et al. Neuroprotective effect of ulinastatin on spinal cord ischemia-reperfusion injury in rabbits. Oxid Med Cell Longev. 2015;2015:624819. doi: 10.1155/2015/624819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17(2):357–368. [PubMed] [Google Scholar]
- 11.Tabayashi K. Spinal cord protection during thoracoabdominal aneurysm repair. Surg Today. 2005;35(1):1–6. doi: 10.1007/s00595-004-2889-z. [DOI] [PubMed] [Google Scholar]
- 12.Erkut B, Onk OA. Effect of N-acetylcysteine and allopurinol combination to protect spinal cord ischemia/reperfusion injury induced by aortic cross-clamping in rat model. J Cardiothorac Surg. 2015;10:95 . doi: 10.1186/s13019-015-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazli Y, Colak N, Alpay MF, et al. Neuroprotective effect of atorvastatin in spinal cord ischemia-reperfusion injury. Clinics (Sao Paulo) 2015;70(1):52–60. doi: 10.6061/clinics/2015(01)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Kazui T, Terada H, et al. Experimental study on the protective effects of edaravone against ischemic spinal cord injury. J Thorac Cardiovasc Surg. 2005;130(6):1586–1592. doi: 10.1016/j.jtcvs.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82(4):1026–1060. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Boga M, Discigil B, Ozkisacik EA, et al. The combined effect of iloprost and N-acetylcysteine in preventing spinal cord ischemia in rabbits. Eur J Vasc Endovasc Surg. 2006;31(4):366–372. doi: 10.1016/j.ejvs.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Federico A, Morgillo F, Tuccillo C, et al. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 18.Kubesy AAM, Yehia SG, Salem SI, et al. Altered blood oxidative stress markers in association with antioxidant supplemented therapy for mange, tick, and flea allergic dermatitis of dogs. Comp Clin Path. 2020;29:937–943. [Google Scholar]
- 19.Saito T, Tsuchida M, Umehara S, et al. Reduction of spinal cord ischemia/reperfusion injury with sim-vastatin in rats. Anesth Analg. 2011;113(3):565–571. doi: 10.1213/ANE.0b013e318224ac35. [DOI] [PubMed] [Google Scholar]
- 20.Ashok A, Andrabi SS, Mansoor S, et al. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants (Basel) 2022;11(2):408. doi: 10.3390/antiox11020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Z, Zhu H, Li J, et al. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50(4):264–274. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- 22.Perdomo-Pantoja A, Chara A, Kalb S, et al. The effect of renin-angiotensin system blockers on spinal cord dysfunction and imaging features of spinal cord compression in patients with symptomatic cervical spondylosis. Spine J. 2020;20(4):519–529. doi: 10.1016/j.spinee.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Przyklenk K, Kloner RA. Angiotensin converting enzyme inhibitors improve contractile function of stunned myocardium by different mechanisms of action. Am Heart J. 1991;121(5):1319–1330. doi: 10.1016/0002-8703(91)90134-4. [DOI] [PubMed] [Google Scholar]
- 24.Ambreen A, Jahan S, Malik S. Effect of angiotensin-converting enzyme inhibitor, lisinopril on morphological and biochemical aspects of fibrotic liver regeneration. Saudi J Gastroenterol. 2016;22(6):428–434. doi: 10.4103/1319-3767.195559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapenna D, De Gioia S, Ciofani G, et al. Captopril has no significant scavenging antioxidant activity in human plasma in vitro or in vivo. Br J Clin Pharmacol. 1996;42(4):451–456. doi: 10.1046/j.1365-2125.1996.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroiwa T, Xi G, Hua Y, et al. Brain edema and blood-brain barrier opening after photothrombotic ischemia in rat. Acta Neurochir Suppl. 2013;118:11–15. doi: 10.1007/978-3-7091-1434-6_2. [DOI] [PubMed] [Google Scholar]
- 27.Aldahmash BA, El-Nagar DM. Antioxidant effects of captopril against lead acetate-induced hepatic and splenic tissue toxicity in Swiss albino mice. Saudi J Biol Sci. 2016;23(6):667–673. doi: 10.1016/j.sjbs.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartosz M, Kedziora J, Bartosz G. Antioxidant and prooxidant properties of captopril and enalapril. Free Radic Biol Med. 1997;23(5):729–735. doi: 10.1016/s0891-5849(97)00014-2. [DOI] [PubMed] [Google Scholar]
- 29.Petrov L, Atanassova M, Alexandrova A. Comparative study of the antioxidant activity of some thiol-containing substances. Cent Eur J Med. 2012;7:269–273. [Google Scholar]
- 30.Guo L, Richardson KS, Tucker LM, et al. Role of the renin-angiotensin system in hepatic ischemia reperfusion injury in rats. Hepatology. 2004;40(3):583–589. doi: 10.1002/hep.20369. [DOI] [PubMed] [Google Scholar]
- 31.Tarlov IM. Acute spinal cord compression paralysis. J Neurosurg. 1972;36(1):10–20. doi: 10.3171/jns.1972.36.1.0010. [DOI] [PubMed] [Google Scholar]
- 32.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 33.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 34.Alamdari DH, Ghayour-Mobarhan M, Tavallaie S, et al. Prooxidant–antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem. 2008;41(6):375–380. doi: 10.1016/j.clinbiochem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Sun Z, Zhao T, Lv S, et al. Dexmedetomidine attenuates spinal cord ischemia-reperfusion injury through both anti-inflammation and anti-apoptosis mechanisms in rabbits. J Transl Med. 2018;16(1):209. doi: 10.1186/s12967-018-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim WR, Kang M, Park H, et al. Functional test scales for evaluating cell-based therapies in animal models of spinal cord injury. Stem Cells Int. 2017;2017:5160261. doi: 10.1155/2017/5160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang W, Han B, Hai Y, et al. The role of microglia/ macrophages activation and TLR4/NF-κB/MAPK pathway in distraction spinal cord injury-induced inflammation. Front Cell Neurosci. 2022;16:926453. doi: 10.3389/fncel.2022.926453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisdas T, Panuccio G, Sugimoto M, et al. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2015;61(6):1408–1416. doi: 10.1016/j.jvs.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 39.McMurray J, Chopra M. Influence of ACE inhibitors on free radicals and reperfusion injury: pharmacological curiosity or therapeutic hope? Br J Clin Pharmacol. 1991;31(4):373–379. doi: 10.1111/j.1365-2125.1991.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner C, Hoffman WE, Kochs E, et al. Captopril improves neurologic outcome from incomplete cerebral ischemia in rats. Stroke. 1991;22(7):910–914. doi: 10.1161/01.str.22.7.910. [DOI] [PubMed] [Google Scholar]
- 41.Fu D, Liu H, Li S, et al. Antioxidative and antiapoptotic effects of delta-opioid peptide [D-Ala2, D-Leu5] enkephalin on spinal cord ischemia-reperfusion injury in rabbits. Front Neurosci. 2017;11:603. doi: 10.3389/fnins.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D, Liu B, Xiao X, et al. The effect of safflower yellow on spinal cord ischemia reperfusion injury in rabbits. Oxid Med Cell Longev. 2013;2013:692302. doi: 10.1155/2013/692302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaggini M, Sabatino L, Vassalle C. Conventional and innovative methods to assess oxidative stress biomarkers in the clinical cardiovascular setting. Biotechniques. 2020;68(4):223–231. doi: 10.2144/btn-2019-0138. [DOI] [PubMed] [Google Scholar]
- 44.Karimani A, Mamashkhani Y, Moghadam Jafari A, et al. Captopril attenuates diazinon-induced oxidative stress: a subchronic study in rats. Iran J Med Sci. 2018;43(5):514–522. [PMC free article] [PubMed] [Google Scholar]
- 45.Gurer H, Neal R, Yang P, et al. Captopril as an antioxidant in lead-exposed Fischer 344 rats. Hum Exp Toxicol. 1999;18(1):27–32. doi: 10.1177/096032719901800104. [DOI] [PubMed] [Google Scholar]
- 46.Munteanu IG, Apetrei C. Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci. 2021;22(7):3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alamdari DH, Paletas K, Pegiou T, et al. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem. 2007;40(3-4):248–254. doi: 10.1016/j.clinbiochem.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Johnson P, Wei Y, Huentelman MJ, et al. Hydralazine, but not captopril, decreases free radical production and apoptosis in neurons and thymocytes. Free Radic Res. 1998;28(4):393–402. doi: 10.3109/10715769809070808. [DOI] [PubMed] [Google Scholar]
- 49.Fouad AA, Al-Mulhim AS, Jresat I, et al. Protective effects of captopril in diabetic rats exposed to ischemia/reperfusion renal injury. J Pharm Pharmacol. 2013;65(2):243–252. doi: 10.1111/j.2042-7158.2012.01585.x. [DOI] [PubMed] [Google Scholar]
- 50.Vargas AV, Robinson AV, Schulak JA. Captopril amelioration of renal reperfusion injury. J Surg Res. 1994;57(1):28–32. doi: 10.1006/jsre.1994.1104. [DOI] [PubMed] [Google Scholar]


