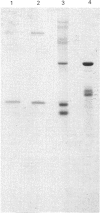
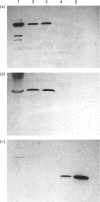
Abstract
A microsomal glutathione S-transferase (GST) was purified from human liver. This enzyme was shown to have characteristics similar to those of the rat microsomal GST described by Morgenstern & De Pierre [(1983) Eur. J. Biochem. 134, 591-597]. The specific activity of human microsomal GST towards 1-chloro-2,4-dinitrobenzene or cumene hydroperoxide can be stimulated by treating the enzyme with N-ethylmaleimide. This enhancement of activity is accompanied by increased sensitivity to inhibition by haematin and cholic acid. The subunit Mr values of the rat and human enzymes are similar (approx. 17,300), and the proteins are immunologically related. During purification, both human and rat microsomal GST enzymes are the only hepatic proteins obtained from Triton X-100-solubilized microsomal fractions that show activity towards the nephrotoxin hexachlorobuta-1,3-diene. The involvement of microsomal GST in toxification reactions is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson C., Söderström M., Mannervik B. Activation and inhibition of microsomal glutathione transferase from mouse liver. Biochem J. 1988 Feb 1;249(3):819–823. doi: 10.1042/bj2490819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer T. D., Vessey D. A., Kempner E. Radiation inactivation of microsomal glutathione S-transferase. J Biol Chem. 1986 Dec 25;261(36):16963–16968. [PubMed] [Google Scholar]
- Dao D. D., Partridge C. A., Kurosky A., Awasthi Y. C. Human glutathione S-transferases. Characterization of the anionic forms from lung and placenta. Biochem J. 1984 Jul 1;221(1):33–41. doi: 10.1042/bj2210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohn D. R., Anders M. W. The enzymatic reaction of chlorotrifluoroethylene with glutathione. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1339–1345. doi: 10.1016/0006-291x(82)91924-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., McLellan L. I., Stockman P. K., Chalmers J., Beckett G. J. Glutathione S-transferases in man: the relationship between rat and human enzymes. Biochem Soc Trans. 1987 Aug;15(4):721–725. doi: 10.1042/bst0150721. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Stockman P. K., Beckett G. J., Hayes J. D. Variations in the glutathione S-transferase subunits expressed in human livers. Biochim Biophys Acta. 1986 Nov 7;874(1):1–12. doi: 10.1016/0167-4838(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan L. I., Hayes J. D. Sex-specific constitutive expression of the pre-neoplastic marker glutathione S-transferase, YfYf, in mouse liver. Biochem J. 1987 Jul 15;245(2):399–406. doi: 10.1042/bj2450399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W. Microsomal glutathione transferase. Purification in unactivated form and further characterization of the activation process, substrate specificity and amino acid composition. Eur J Biochem. 1983 Aug 15;134(3):591–597. doi: 10.1111/j.1432-1033.1983.tb07607.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Morgenstern R., Lundqvist G., Andersson G., Balk L., DePierre J. W. The distribution of microsomal glutathione transferase among different organelles, different organs, and different organisms. Biochem Pharmacol. 1984 Nov 15;33(22):3609–3614. doi: 10.1016/0006-2952(84)90145-x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Meijer J., Depierre J. W., Ernster L. Characterization of rat-liver microsomal glutathione S-transferase activity. Eur J Biochem. 1980 Feb;104(1):167–174. doi: 10.1111/j.1432-1033.1980.tb04412.x. [DOI] [PubMed] [Google Scholar]
- Odum J., Green T. The metabolism and nephrotoxicity of tetrafluoroethylene in the rat. Toxicol Appl Pharmacol. 1984 Nov;76(2):306–318. doi: 10.1016/0041-008x(84)90012-7. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reddy C. C., Tu C. P., Burgess J. R., Ho C. Y., Scholz R. W., Massaro E. J. Evidence for the occurrence of selenium-independent glutathione peroxidase activity in rat liver microsomes. Biochem Biophys Res Commun. 1981 Aug 14;101(3):970–978. doi: 10.1016/0006-291x(81)91844-1. [DOI] [PubMed] [Google Scholar]
- Stockman P. K., Beckett G. J., Hayes J. D. Identification of a basic hybrid glutathione S-transferase from human liver. Glutathione S-transferase delta is composed of two distinct subunits (B1 and B2). Biochem J. 1985 Apr 15;227(2):457–465. doi: 10.1042/bj2270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman P. K., McLellan L. I., Hayes J. D. Characterization of the basic glutathione S-transferase B1 and B2 subunits from human liver. Biochem J. 1987 May 15;244(1):55–61. doi: 10.1042/bj2440055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Faulder C. G., Davis B. A., Hume R., Brown J. A., Cotton W., Hopkinson D. A. The human glutathione S-transferases: studies on the tissue distribution and genetic variation of the GST1, GST2 and GST3 isozymes. Ann Hum Genet. 1984 Jan;48(Pt 1):11–20. doi: 10.1111/j.1469-1809.1984.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Tahir M. K., Guthenberg C., Mannervik B. Inhibitors for distinction of three types of human glutathione transferase. FEBS Lett. 1985 Feb 25;181(2):249–252. doi: 10.1016/0014-5793(85)80269-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Berry P. N., Nash J. A., Green T., Lock E. A. Role of microsomal and cytosolic glutathione S-transferases in the conjugation of hexachloro-1:3-butadiene and its possible relevance to toxicity. J Pharmacol Exp Ther. 1984 Jan;228(1):202–208. [PubMed] [Google Scholar]
- Yalçin S., Jensson H., Mannervik B. A set of inhibitors for discrimination between the basic isozymes of glutathione transferase in rat liver. Biochem Biophys Res Commun. 1983 Jul 29;114(2):829–834. doi: 10.1016/0006-291x(83)90856-2. [DOI] [PubMed] [Google Scholar]