Abstract
Carbohydrate recognition by bovine serum conglutinin has been investigated by inhibition and direct binding assays using glycoproteins and polysaccharides from Saccharomyces cerevisiae (baker's yeast), and neoglycolipids derived from N-acetylglucosamine oligomers, mannobiose and human milk oligosaccharides. The results clearly show that conglutinin is a lectin which binds terminal N-acetylglucosamine, mannose and fucose residues as found in chitobiose (GlcNAc beta 1-4GlcNAc), mannobiose (Man alpha 1-3Man) and lacto-N-fucopentaose II [Fuc alpha 1-4(Gal beta 1-3)GlcNAc beta 1-3Gal beta 1-4Glc] respectively.
Full text
PDF
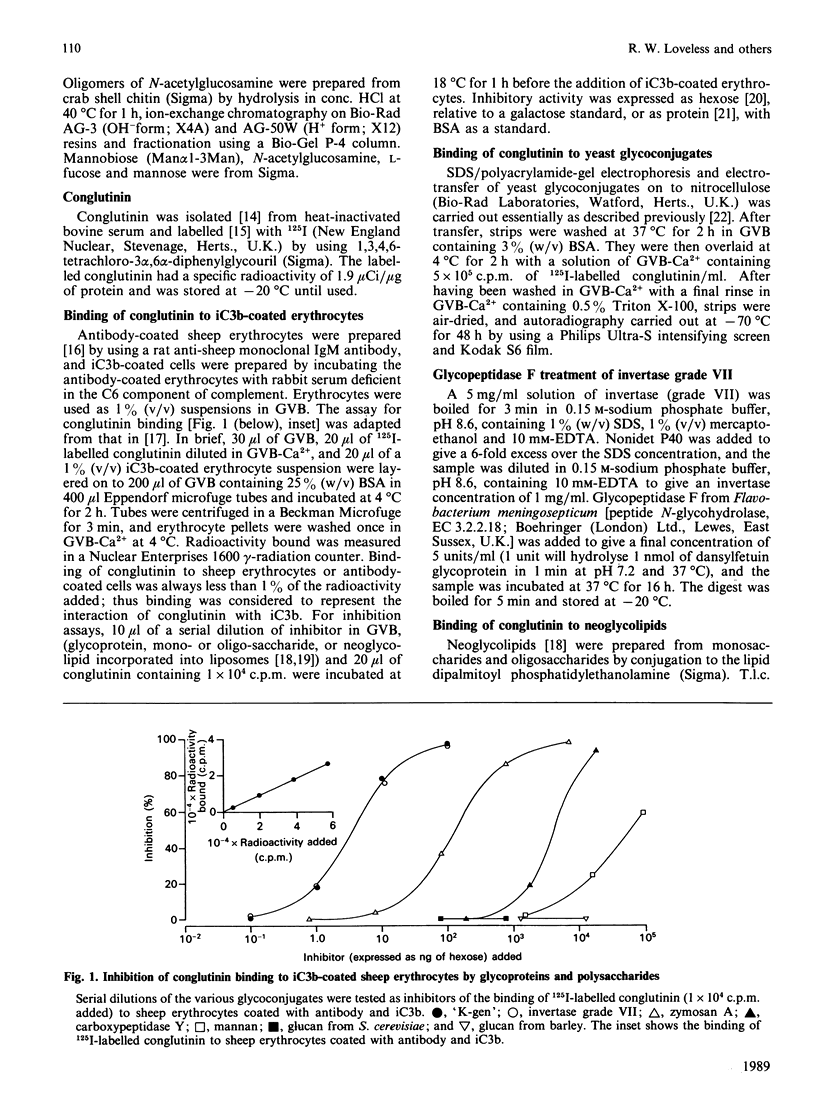
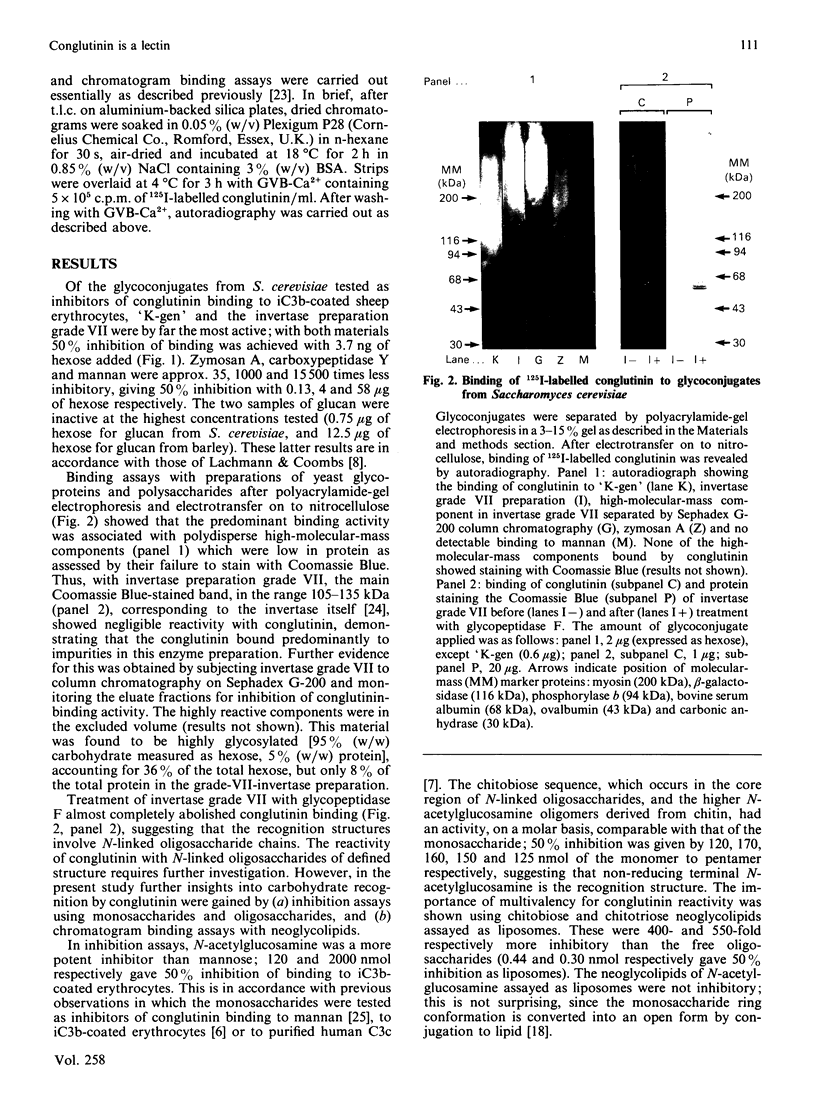
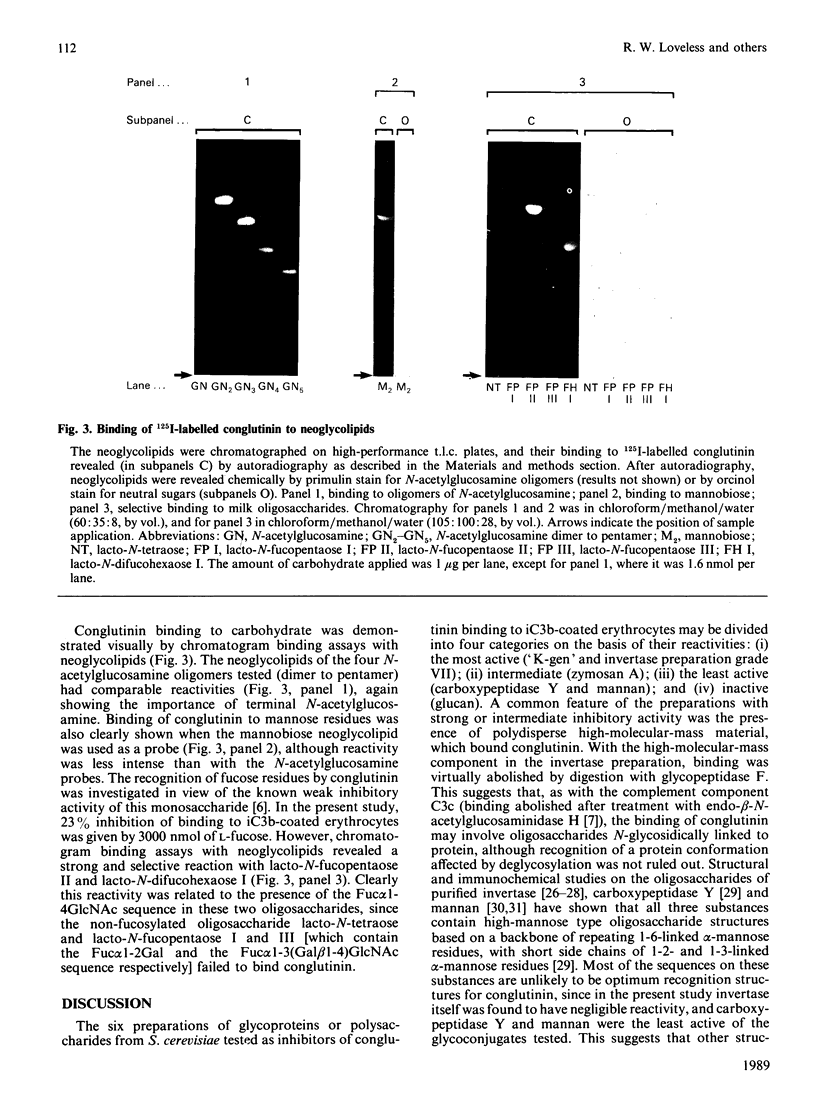

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Childs R. A., Pennington J., Uemura K., Scudder P., Goodfellow P. N., Evans M. J., Feizi T. High-molecular-weight glycoproteins are the major carriers of the carbohydrate differentiation antigens I, i and SSEA-1 of mouse teratocarcinoma cells. Biochem J. 1983 Dec 1;215(3):491–503. doi: 10.1042/bj2150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Lachmann P. J. Bovine conglutinin is a collagen-like protein. Biochemistry. 1984 May 8;23(10):2139–2144. doi: 10.1021/bi00305a006. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hase S., Kikuchi N., Ikenaka T., Inoue K. Structures of sugar chains of the third component of human complement. J Biochem. 1985 Oct;98(4):863–874. doi: 10.1093/oxfordjournals.jbchem.a135366. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Hirani S., Lambris J. D., Müller-Eberhard H. J. Localization of the conglutinin binding site on the third component of human complement. J Immunol. 1985 Feb;134(2):1105–1109. [PubMed] [Google Scholar]
- Hirani S., Lambris J. D., Müller-Eberhard H. J. Structural analysis of the asparagine-linked oligosaccharides of human complement component C3. Biochem J. 1986 Jan 15;233(2):613–616. doi: 10.1042/bj2330613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki N., Kawasaki T., Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem. 1983 Sep;94(3):937–947. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- Kawasaki N., Kawasaki T., Yamashina I. Mannan-binding protein and conglutinin in bovine serum. J Biochem. 1985 Nov;98(5):1309–1320. doi: 10.1093/oxfordjournals.jbchem.a135398. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- LACHMANN P. J. A comparison of some properties of bovine conglutinin with those of rabbit immuno-conglutinin. Immunology. 1962 Nov;5:687–705. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lachmann P. J. Conglutinin and immunoconglutinins. Adv Immunol. 1967;6:479–527. doi: 10.1016/s0065-2776(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Lehle L., Cohen R. E., Ballou C. E. Carbohydrate structure of yeast invertase. Demonstration of a form with only core oligosaccharides and a form with completed polysaccharide chains. J Biol Chem. 1979 Dec 10;254(23):12209–12218. [PubMed] [Google Scholar]
- Leon M. A., Yokohari R. Conglutination: Specific Inhibition by Carbohydrates. Science. 1964 Mar 20;143(3612):1327–1328. doi: 10.1126/science.143.3612.1327. [DOI] [PubMed] [Google Scholar]
- Linscott W. D., Ranken R., Triglia R. P. Evidence that bovine conglutinin reacts with an early product of C3b degradation, and an improved conglutination assay. J Immunol. 1978 Aug;121(2):658–664. [PubMed] [Google Scholar]
- Mizuno Y., Kozutsumi Y., Kawasaki T., Yamashina I. Isolation and characterization of a mannan-binding protein from rat liver. J Biol Chem. 1981 May 10;256(9):4247–4252. [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Characterization of the carbohydrate fragments obtained from Saccharomyces cerevisiae mannan by alkaline degradation. J Biol Chem. 1974 Dec 10;249(23):7679–7684. [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Microheterogeneity of the inner core region of yeast manno-protein. Biochem Biophys Res Commun. 1975 Sep 16;66(2):870–879. doi: 10.1016/0006-291x(75)90590-2. [DOI] [PubMed] [Google Scholar]
- Oka S., Ikeda K., Kawasaki T., Yamashina I. Isolation and characterization of two distinct mannan-binding proteins from rat serum. Arch Biochem Biophys. 1988 Jan;260(1):257–266. doi: 10.1016/0003-9861(88)90448-1. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Shepherd V. L., Lee Y. C., Schlesinger P. H., Stahl P. D. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Ballou C. E. Immunochemical characterization of the mannan component of the external invertase (beta-fructofuranosidase) of Saccharomyces cerevisiae. Biochemistry. 1974 Jan 15;13(2):355–361. doi: 10.1021/bi00699a021. [DOI] [PubMed] [Google Scholar]
- Stoll M. S., Mizuochi T., Childs R. A., Feizi T. Improved procedure for the construction of neoglycolipids having antigenic and lectin-binding activities, from reducing oligosaccharides. Biochem J. 1988 Dec 1;256(2):661–664. doi: 10.1042/bj2560661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang C. J., Slayter H. S., Lachmann P. J., Davis A. E., 3rd Ultrastructure and composition of bovine conglutinin. Biochem J. 1986 Mar 1;234(2):381–389. doi: 10.1042/bj2340381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P. W., Feizi T. Neoglycolipid micro-immunoassays applied to the oligosaccharides of human milk galactosyltransferase detect blood-group related antigens on both O- and N-linked chains. Carbohydr Res. 1987 Mar 15;161(1):133–143. doi: 10.1016/0008-6215(87)84012-0. [DOI] [PubMed] [Google Scholar]
- Tang P. W., Gool H. C., Hardy M., Lee Y. C., Feizi T. Novel approach to the study of the antigenicities and receptor functions of carbohydrate chains of glycoproteins. Biochem Biophys Res Commun. 1985 Oct 30;132(2):474–480. doi: 10.1016/0006-291x(85)91158-1. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Atkinson P. H. Structure of yeast external invertase Man8-14GlcNAc processing intermediates by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1986 Jul 25;261(21):9815–9824. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 25;252(12):4409–4412. [PubMed] [Google Scholar]
- Young N. M., Leon M. A. The carbohydrate specificity of conglutinin and its homology to proteins in the hepatic lectin family. Biochem Biophys Res Commun. 1987 Mar 13;143(2):645–651. doi: 10.1016/0006-291x(87)91402-1. [DOI] [PubMed] [Google Scholar]