Abstract
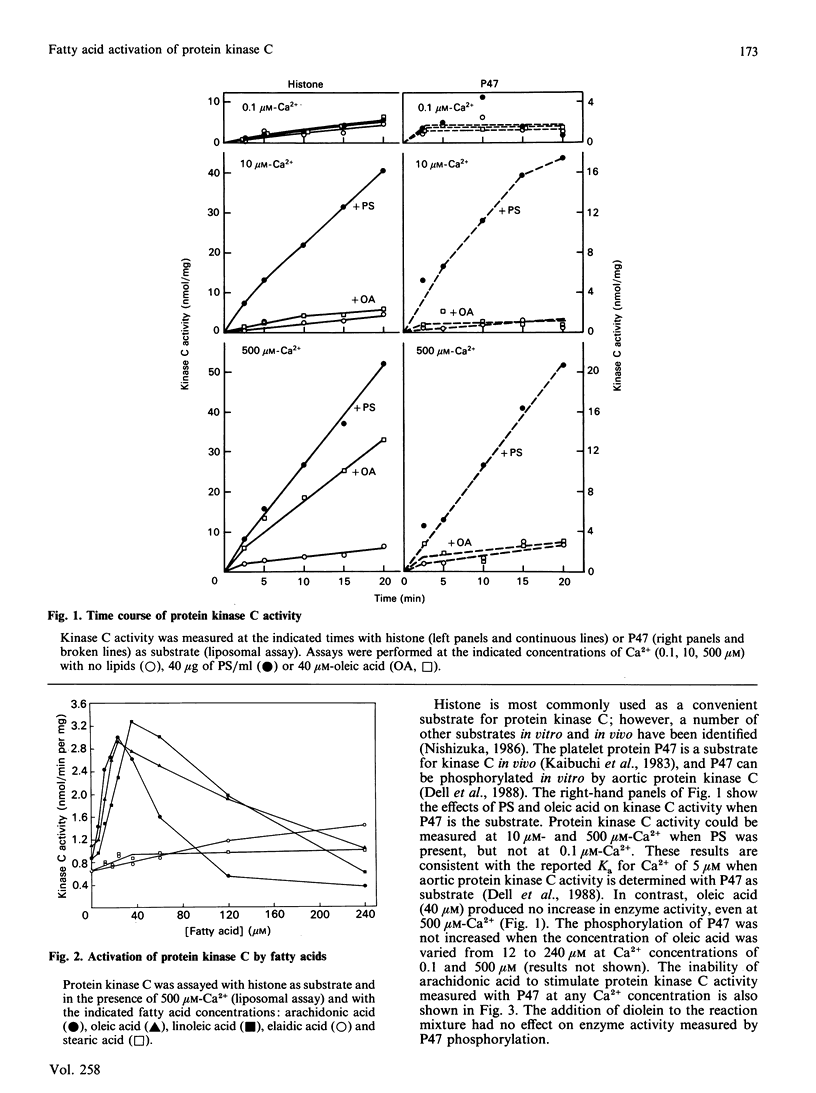
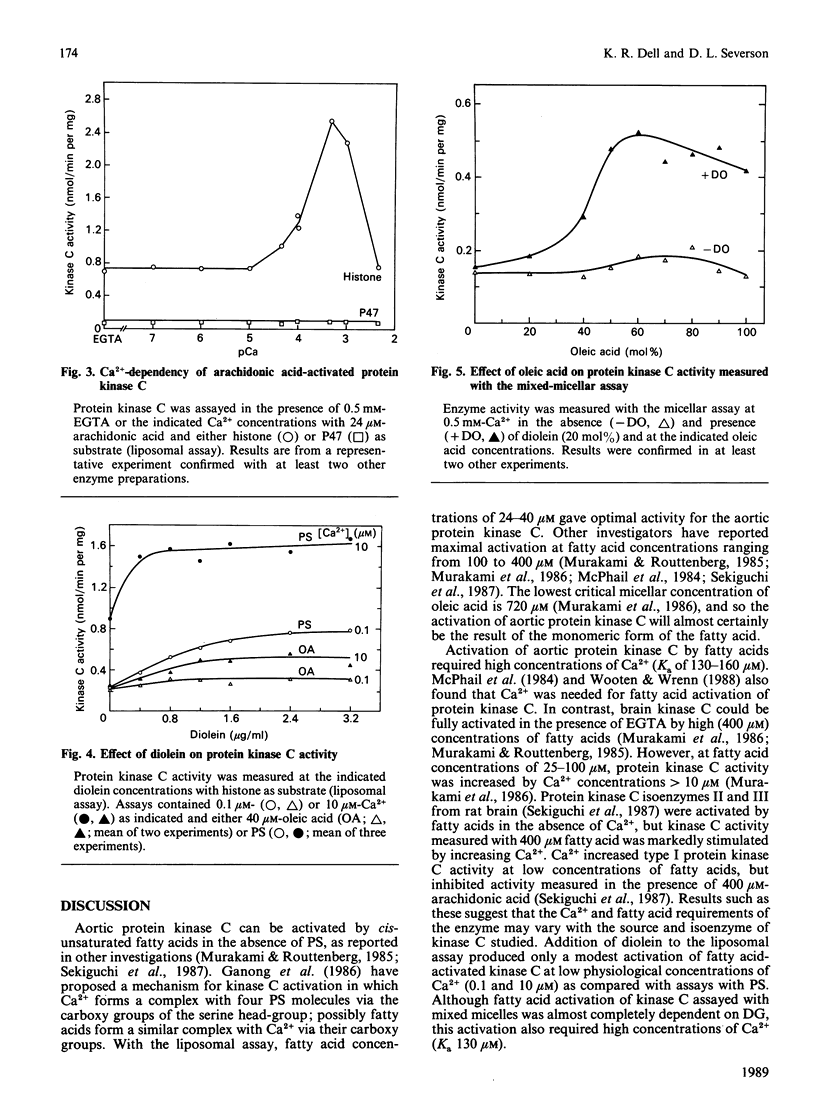
Long-chain cis-unsaturated fatty acids could substitute for phosphatidylserine and activate bovine aortic protein kinase C in assays with histone as substrate. The optimal concentration was 24-40 microM for oleic, linoleic and arachidonic acids. With arachidonic acid, the Ka for Ca2+ was 130 microM and kinase activity was maximal at 0.5 mM-Ca2+. Diolein only slightly activated the oleic acid-stimulated enzyme at low physiological Ca2+ concentrations (0.1 and 10 microM). Oleic acid also stimulated kinase C activity, determined with a Triton X-100 mixed-micellar assay. Under these conditions, the fatty acid activation was absolutely dependent on the presence of diolein, but a Ca2+ concentration of 0.5 mM was still required for maximum kinase C activity. The effect of fatty acids on protein kinase C activity was also investigated with the platelet protein P47 as a substrate, since the properties of kinase C can be influenced by the choice of substrate. In contrast with the results with histone, fatty acids did not stimulate the phosphorylation of P47 by the aortic protein kinase C. Activation of protein kinase C by fatty acids may allow the selective phosphorylation of substrates, but the physiological significance of fatty acid activation is questionable because of the requirement for high concentrations of Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Role of substrate in determining the phospholipid specificity of protein kinase C activation. Biochemistry. 1987 Aug 11;26(16):5002–5008. doi: 10.1021/bi00390a018. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Role of substrate in imparting calcium and phospholipid requirements to protein kinase C activation. Biochemistry. 1987 Apr 7;26(7):1974–1982. doi: 10.1021/bi00381a029. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Dell K. R., Walsh M. P., Severson D. L. Characterization of bovine aortic protein kinase C with histone and platelet protein P47 as substrates. Biochem J. 1988 Sep 1;254(2):455–462. doi: 10.1042/bj2540455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillone L. R., Clark M. A., Godfrey R. W., Stassen F., Crooke S. T. Vasopressin induces V1 receptors to activate phosphatidylinositol- and phosphatidylcholine-specific phospholipase C and stimulates the release of arachidonic acid by at least two pathways in the smooth muscle cell line, A-10. J Biol Chem. 1988 Feb 25;263(6):2658–2663. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Hunneman D. H., Schweickhardt C. Mass fragmentographic determination of myocardial free fatty acids. J Mol Cell Cardiol. 1982 Jun;14(6):339–351. doi: 10.1016/0022-2828(82)90249-8. [DOI] [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Murakami K., Chan S. Y., Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986 Nov 25;261(33):15424–15429. [PubMed] [Google Scholar]
- Murakami K., Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+. FEBS Lett. 1985 Nov 18;192(2):189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ogita K., Kikkawa U., Nishizuka Y. Three distinct forms of rat brain protein kinase C: differential response to unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Jun 15;145(2):797–802. doi: 10.1016/0006-291x(87)91035-7. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Verkest V., McArthur M., Hamilton S. Fatty acid activation of protein kinase C: dependence on diacylglycerol. Biochem Biophys Res Commun. 1988 Apr 29;152(2):825–829. doi: 10.1016/s0006-291x(88)80112-8. [DOI] [PubMed] [Google Scholar]
- Wooten M. W., Wrenn R. W. Linoleic acid is a potent activator of protein kinase C type III-alpha isoform in pancreatic acinar cells; its role in amylase secretion. Biochem Biophys Res Commun. 1988 May 31;153(1):67–73. doi: 10.1016/s0006-291x(88)81190-2. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Roemen T. H., Prinzen F. W., Coumans W. A., Reneman R. S. Uptake and tissue content of fatty acids in dog myocardium under normoxic and ischemic conditions. Circ Res. 1982 Apr;50(4):538–546. doi: 10.1161/01.res.50.4.538. [DOI] [PubMed] [Google Scholar]


