Abstract
Mismatch repair (MMR) is involved in the removal of mispaired bases from DNA and thus plays an important role in the maintenance of genomic stability and the prevention of mutations and cancer. Moreover, MMR triggers genotoxicity and apoptosis upon processing of DNA lesions such as O6-methylguanine. Whereas the enzymology of MMR has been elucidated in great detail, only limited data are available concerning its regulation. Here we show that the major mismatch-binding proteins MSH2 and MSH6, forming the MutSα complex, are phosphorylated in vitro by protein kinase C and casein kinase II, but not by protein kinase A. Phosphorylation of MSH2 and MSH6 was also found within the cell, with MSH6 being more extensively phosphorylated than MSH2. Lack of MSH2 and MSH6 phosphorylation in vivo due to phosphate depletion, kinase inhibition (by H7 and quercetin) and treatment with phosphatases (CIP, SAP and λ-PPase) significantly reduced mismatch-binding activity of MutSα. It also prevented methylation-induced nuclear translocation of the repair complex, indicating that nuclear translocation of MutSα upon mutagen treatment is dependent on protein phosphorylation. The finding that MSH2 and MSH6 are subject to phosphorylation resulting in increased mismatch binding by MutSα indicates a novel type of post-translational regulation of MMR which might be involved in the response of cells to genotoxic stress.
INTRODUCTION
Repair of DNA damage is a process essentially required for maintaining genomic stability and cellular function. One of the DNA repair processes is mismatch repair (MMR), which is responsible for removal of base mismatches caused by spontaneous and induced base deamination, oxidation, methylation and replication errors, thus increasing the fidelity of DNA replication and avoiding mutations (1,2). Base mismatches such as GT, arising from deamination of 5-methylcytosine, are recognised by the MutSα complex, which is composed of the mismatch repair proteins MSH2 and MSH6 (3,4). MutSα not only binds to spontaneously occurring base mismatches but also to various chemically induced DNA lesions such as O6-methylguanine (O6-MeG) paired with cytosine or thymine (5), 1,2-intrastrand (GpG) crosslinks generated by cisplatin (6), UV-induced photoproducts (7) and purine adducts of benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxides (8), 2-aminofluorene and N-acetyl-2-aminofluorene (9). For many of these adducts it is unclear which role binding of MutSα plays in damage repair and processing. For O6-MeG/T mispairs, however, evidence is available to indicate that this lesion is responsible for genotoxic effects and apoptosis mediated by MutSα (10–13), which is supposed to process the lesion by futile MMR cycles (14). Whether for other lesions MutSα acts as a sensor of DNA damage triggering apoptotic functions or causes erroneous processing leading to secondary lesions that are genotoxic is still a matter of debate. The importance of MMR in maintaining genomic stability and reducing mutation load is clearly illustrated by MMR deficiency syndromes such as HNPCC, which are related to microsatellite instability and increased spontaneous tumour incidence (15). An attractive hypothesis would be that MMR not only prevents mutations but also signals non-repairable mutagenic lesions, thus eliminating heavily damaged cells by programmed cell death. MMR has also been implicated in the development of tumour cell resistance, counteracting successful tumour therapy (16–18).
Despite the importance of MMR for preventing mutagenic and carcinogenic effects and its involvement in cell killing and apoptosis, only limited data are available concerning the regulation of MMR. Thus it was shown that doxorubicin and alkylating agents increase the nuclear level of MSH2 and MSH6 (19,20). For alkylating agents inducing O6-MeG in DNA it was further shown that they cause an increase in nuclear GT-binding activity, which was due to alkylation-induced nuclear translocation of MutSα (20). The mechanism behind this remained unknown. Here we have studied whether MSH2 and MSH6 are subject to post-translational modification, notably phosphorylation, which could have an impact on mismatch binding and nuclear translocation. Phosphorylation is involved in the regulation of many physiological processes. Thus it was shown to be associated with alterations in DNA binding and transactivation of transcription factors (21,22), with activation of intracellular signalling cascades (23,24) and the regulation of nuclear translocation of various proteins (25). Phosphorylation has also been reported to be involved in the regulation of DNA repair proteins such as alkyltransferase (26) and apurinic endonuclease (27,28). Among various kinases involved in protein phosphorylation, Ca2+-dependent protein kinase C (PKC), casein kinase II (CKII) and protein kinase A (PKA) are the best characterised. Here we show that the MMR proteins MSH2 and MSH6 are phosphorylated in vitro by PKC and CKII, but not PKA. We also demonstrate that MSH2 and MSH6 become phosphorylated within the cell in vivo, which results in increased MutSα GT-binding activity and is involved in nuclear translocation of the MutSα complex. According to our knowledge this is the first demonstration that MMR proteins are subject to post-translational modification by phosphorylation.
MATERIALS AND METHODS
Cell lines
HeLa-MR cells (obtained from the ATCC) are deficient for the repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) and were used previously to demonstrate nuclear translocation of MutSα (20). Cells were cultivated in 7% CO2 at 37°C in F12/Dulbecco’s minimal essential medium (DMEM) containing 5% fetal bovine serum.
EMSA
Nuclear extracts suitable for EMSA were prepared as previously described (20). The amount of protein was determined as described (29). For gel retardation assays, 29-nt oligomers with the sequence 5′-GGGCTCGAGCTGCAGCTGCTAGTAGATCT-3′ were annealed to oligomers with the sequence 5′-GGGAGATCTACTAGNAGCTGCAGCTCGAG-3′ (N = C or T) and labelled with [α-32P]dCTP using Klenow enzyme. An aliquot of 5 µg nuclear proteins was incubated in 10% glycerol, 10 mM HEPES–KOH, pH 7.9, 50 mM KCl, 4 mM MgCl2, 4 mM Tris–HCl, 0.5 mM DTT, 0.5 mM EDTA, 1 µg BSA, 1 µg poly(dI·dC) with 25 fmol radioactively labelled duplex DNA for 30 min at 27°C, after which the DNA–protein complexes were separated on 4% polyacrylamide gels using 0.25× TBE buffer.
Western blot analysis
Nuclear and cytoplasmic extracts suitable for western blot analysis were prepared as previously described (20). The amount of protein was determined (30) and samples of 25 µg nuclear or cytoplasmic protein extracts were loaded onto a 10% SDS–polyacrylamide gel and run for 2 h at 35 mA. Separated proteins were transferred to a 0.2 mm cellulose nitrate membrane (Schleicher and Schüll, Dassel, Germany) in a BioRad Blot cell using buffer consisting of 25 mM Tris–HCl, 100 mM glycine and 20% methanol. To avoid non-specific binding, the filters were incubated in 5% non-fat dry milk, 0.1% Tween-20 in phosphate-buffered saline (PBS) for 3 h. Thereafter filters were incubated with monoclonal mouse antibodies against hMSH2 (Calbiochem) and hMSH6 (Transduction Laboratories, Heidelberg, Germany) or with polyclonal antibody against ERK2 (Santa Cruz, Heidelberg, Germany) diluted in the same solution (1:750) overnight at 4°C and afterwards with horseradish peroxidase-conjugated donkey anti-mouse or anti-rabbit IgG (Amersham, Braunschweig, Germany; dilution 1:4000) for 1 h. The protein–antibody complexes were visualised by ECL (Amersham) according to the manufacturer’s protocol.
Immunoprecipitation
Nuclear, cytoplasmic and whole cell extracts suitable for immunoprecipitation were prepared as previously described (20). For separate immunoprecipitation of MSH2 or MSH6, cells were lysed with Triton X-100 (final concentration 1%) and SDS (final concentration 0.1%) for 10 min on ice. For co-immunoprecipitation experiments, NP-40 (0.5%) was added instead of Triton X-100 and SDS and incubated for 20 min on ice. The cell extracts were incubated on ice for 30 min together with 20 µl of protein G and centrifuged (400 g, 2 min) to remove proteins that non-specifically bind protein G. The supernatant was incubated on ice together with 15 µl of the specific antibody. One hour later 25 µl of protein G was added and incubated for an additional 2 h. The protein–antibody complex was further purified by washing five times with 1 ml of RIPA and centrifugation (400 g, 2 min) and subjected to western blot analysis.
In vitro phosphorylation
To examine the phosphorylation of MSH2 and MSH6 in vitro, 0.5 µg recombinant human MutSα produced in Sf9 cells (31) (kindly provided by Drs J. Jiricny and P. Dufner, Zürich) was incubated with 10 µCi [γ-32P]dATP and 1 U recombinant protein kinases (CKII, PKA or PKC) in appropriate buffer (PKC buffer: 25 mM Tris–HCl pH 7.4, 6.25 mM MgCl2, 125 µM CaCl2, 250 µM ATP, 0.03% Triton X-100, 300 µg/ml l-phosphatidyl-l-serine and 100 µg/ml 1-oleoyl-2-acetyl-sn-glycerol (OAG); PKA buffer: 100 mM Tris–HCl pH 7.4, 50 mM MgCl2, 10 µM cAMP, 1.5 mM CaCl2 and 250 µM ATP; CKII buffer: 100 mM Tris–HCl pH 7.4, 50 mM MgCl2, 25 mM DDT, 250 mM KCl and 250 µM ATP) in a reaction volume of 50 µl for 1 h at 37°C. The reaction was stopped by addition of 10 µl of 4× SDS loading buffer (Sigma) and subjected to western blot analysis.
In vivo phosphorylation
In vivo phosphorylation experiments on the basis of phosphate depletion were performed as previously described (32), using a modified protocol. HeLa-MR cells (1 × 106) were grown on 6 cm dishes for 36 h in phosphate-depleted medium (DMEM without phosphate, supplemented with 0.5% glucose, 0.5% serum, 0.5% sodium pyruvate) at 37°C. Thereafter, 0.2 mCi radioactively 32P-labelled orthophosphoric acid was added per ml of medium and cells were incubated for an additional 6 h at 37°C. Cells were trypsinised and subjected to immunoprecipitation.
Kinase inhibitors and dephosphorylation experiments
HeLa-MR cells were cultivated in phosphate-depleted medium for 36 h at 37°C and pretreated for 30 min with different protein kinase inhibitors (50 µM H7 or 100 µM quercetin). Thereafter, phosphate-free medium was replaced with normal medium containing the protein kinase inhibitors and incubated for an additional 2 h. Nuclear extracts were prepared and 5 µg each were subjected to EMSA. To examine the effect of MutSα dephosphorylation on GT-binding activity, 5 µg nuclear extract suitable for EMSA or 0.5 µg recombinant MutSα protein was incubated with 2 U calf intestinal phosphatase (CIP), PKC or protein-specific λ phosphatase (λ-PPase) (La Roche, Mannheim, Germany) in the appropriate buffer in a reaction volume of 15 µl for 30 min at 37°C. Inactivation of phosphatases in the control experiment was performed by heating the solution for 20 min at 80°C. The dephosphorylated proteins were adjusted to 10% glycerol, 10 mM HEPES–KOH, pH 7.9, 50 mM KCl, 4 mM MgCl2, 4 mM Tris–HCl, 0.5 mM DTT, 0.5 mM EDTA, 1 µg BSA, 1 µg poly(dI·dC) and incubated together with 25 fmol radioactively labelled duplex DNA (labelled by Klenow polymerase and [α-32P]dCTP) for 30 min at 27°C. Thereafter the DNA–protein complexes were seperated on a 4% polyacrylamide gel using 0.25× TBE buffer.32P
RESULTS
MSH2 and MSH6 are phosphorylated in vitro
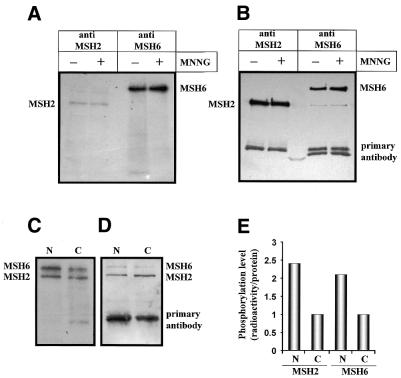
To elucidate whether MSH2 and MSH6 contain potential post-translational modification sites, the amino acid sequences of the proteins were analysed by means of the program PROSITE (33). The search revealed several putative phosphorylation sites in MSH2 and MSH6 for PKC and CKII. In addition, MSH6 but not MSH2 contains potential phosphorylation sites for cAMP- and cGMP-dependent protein kinases (data not shown). To determine whether MSH2 and MSH6 are phosphorylated by PKC or CKII, in vitro phosphorylation experiments were performed. In these experiments recombinant MutSα was incubated with purified protein kinases and subjected to western blot analysis. Autoradiographic analysis of the proteins revealed significant phosphorylation of MSH6 by PKC and CKII (Fig. 1A). MSH2 was also phosphorylated by these kinases, but the phosphorylation level was much lower compared to MSH6. In contrast, no phosphorylation of either MSH2 or MSH6 was found on incubation with PKA. In this assay histone H1 was used as a positive control of phosphorylation because it is known to be a substrate of PKA (34). Under the conditions applied, the protein was clearly found to become phosphorylated by PKA (data not shown). The identity of the proteins MSH2 and MSH6 in the radioactive phosphorylation assay was confirmed by western blot analysis (Fig. 1B).
Figure 1.
In vitro phosphorylation of MSH2 and MSH6 by CKII and PKC. Human recombinant MutSα was incubated with [γ-32P]ATP and recombinant protein kinases, subjected to SDS gel electrophoresis and transferred to nitrocellulose membrane. (A) Autoradiographic detection of phosphorylated MSH2 and MSH6 upon incubation of MutSα with either PKC, PKA or CKII. The lower band in the PKC blot very likely represents an autophosphorylated form of PKC. (B) The same filter was incubated with a mixture of antibodies against human MSH2 and MSH6 to demonstrate the positions of MSH2 and MSH6 on the filter. Data of one representative experiment are shown.
MSH2 and MSH6 are phosphorylated in vivo
To analyse whether phosphorylation of MSH2 and MSH6 also occurs within the cell, phosphorylation of the proteins was analysed by in vivo labelling experiments. To this end, human cells (HeLa-MR) were cultivated in phosphate-free medium for 36 h. Thereafter, radioactively labelled orthophosphoric acid (0.2 mCi/ml medium) was added to the cells for 6 h. In addition, HeLa-MR cells were non-treated or treated with the methylating agent N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) for 2 h to analyse the influence of mutagen exposure on phosphorylation. Cells were harvested, whole cell extracts were prepared and MSH2 and MSH6 were separately immunoprecipitated by means of specific monoclonal antibodies. The immunoprecipitated proteins were subjected to western blot analysis and detected by autoradiography. As shown in Figure 2A, two distinct radioactively labelled bands were visualised, representing phosphorylated proteins of 102 and 160 kDa, corresponding to the molecular weights of MSH2 and MSH6, respectively. To verify that the detected phosphorylated proteins were indeed MSH2 and MSH6 and to determine the level of phosphorylation, the proteins were visualised by reincubation of the filter with antibodies directed against MSH2 and MSH6. The results, shown in Figure 2B, clearly confirmed that the proteins detected by autoradiography were MSH2 and MSH6. A comparison of the level of phosphorylation with the amount of immunoprecipitated protein bound to the filter revealed that MSH6 was more efficiently phosphorylated than MSH2 in vivo. This is in line with the data shown above obtained with in vitro phosphorylated MutSα (see Fig. 1). The results also demonstrate that treatment of cells with MNNG does not significantly affect the overall level of MSH2 and MSH6 phosphorylation.
Figure 2.
In vivo phosphorylation of MSH2 and MSH6. HeLa-MR cells grown in phoshate-depleted medium were labelled with radioactive orthophosphoric acid and phosphorylation of MMR proteins was analysed. (A) HeLa-MR cells were non-treated (–) or treated (+) with 25 µM MNNG for 2 h, total cell extracts were prepared and MSH2 and MSH6 were separately immunoprecipitated by specific monoclonal antibodies. The immunoprecipitated proteins were electrophoretically separated and transferred to a nitrocellulose membrane. Phosphorylated proteins were detected by autoradiography. (B) The same membrane was incubated with a mixture of antibodies against human MSH2 and MSH6 to demonstrate the position of MSH2 and MSH6 on the filter. The lower bands are due to the presence of primary antibodies used for immunoprecipitation. The experiments shown in (A) and (B) were repeated twice and blots of one representative experiment are shown. (C–E) Nuclear and cytoplasmic extracts were prepared and MSH2 was immunoprecipitated by a specific monoclonal antibody. The immunoprecipitated proteins were electrophoretically separated and transferred to a nitrocellulose membrane. The filter was used for autoradiographic detection of phosphorylated proteins (C) and reincubated with a mixture of antibodies against human MSH2 and MSH6 to localise the immunoprecipitated MSH2 and the co-immunoprecipitated MSH6 (D). Both filters were quantified by densitometric measurement of the bands and the relative level of phosphorylation was determined and shown in relation to the amount of the respective protein in a column diagram. The phosphorylation level of cytoplasmic protein was set to 1. Columns designated by N and C correspond to nuclear and cytoplasmic extracts, respectively (E).
Phosphorylated MutSα is predominantly localised in the nucleus
To elucidate the physiological role of phosphorylation, it is important to determine the cellular compartment in which phosphorylation of MSH2 and MSH6 takes place. To address this, HeLa-MR cells were cultivated in phosphate-free medium and, after addition of radioactively labelled orthophosphoric acid to the medium for 6 h, nuclear and cytoplasmic extracts were prepared and MSH2 was immunoprecipitated by means of a specific monoclonal antibody. The autoradiographic analysis revealed that phosphorylated MSH2 and the co-immunoprecipitated phosphorylated MSH6 are present both in the cytoplasmic and the nuclear extract (Fig. 2C and D). We should mention that in this experimental series a slightly changed experimental protocol was used to prepare cell extracts which allowed co-precipitation of MSH2 together with MSH6 (see Materials and Methods). Therefore, both proteins were detectable at once (in comparison to Fig. 2A). Because control experiments had shown that unphosphorylated and phosphorylated recombinant MutSα is equally detected by monoclonal antibodies against MSH2 and MSH6 (data not shown), it was possible to quantify the level of phosphorylation by densitometric measurements of band intensities. Quantification showed that the MSH2 and MSH6 proteins present in the nucleus were more efficiently phosphorylated (∼2.5-fold) than the corresponding cytoplasmic proteins (Fig. 2E; phosphorylation expressed as radioactivity per unit of protein).
Phosphorylation is associated with nuclear translocation and increased GT-binding activity of MutSα
In a previous study we showed that treatment of cells with O6-MeG-generating agents such as MNNG leads to translocation of the cytoplasmatically preformed MutSα complex from the cytoplasm into the nucleus, which is due to a yet unknown mechanism signalled by the presence of O6-MeG in DNA (20). Bearing in mind the finding that MSH2 and MSH6 are subject to phosphorylation and that the phosphorylated proteins were present at a higher level in the nucleus than in the cytoplasm, it is tempting to speculate that post-translational modification by phosphorylation is involved in triggering the response. To analyse the role of phosphorylation in nuclear translocation of MutSα, HeLa-MR cells were cultivated in either phosphate-free, serum-free or normal medium for 36 h. Thereafter the cells were incubated with MNNG (25 µM) for 2 h and nuclear extracts were prepared and subjected to western blot analysis. If cells were cultivated in normal (phosphate-containing) medium an increase in nuclear MSH2 amount was clearly detectable after the incubation with MNNG (Fig. 3A). This is in line with previous findings demonstrating MNNG-induced nuclear translocation of MutSα (20). In contrast, MNNG treatment of cells cultivated in phosphate-free medium did not result in an increase in nuclear MSH2 protein level (Fig. 3A). Phosphate depletion did not cause detectable unwished side-effects such as cytotoxicity. However, it slightly inhibited cell proliferation. Cells inhibited in growth by serum depletion also responded with an increase in nuclear MSH2 protein upon MNNG treatment, indicating a lack of MSH2 accumulation observed under phosphate-depleted conditions not due to growth inhibition (see Fig. 3A for a representative experiment; for quantification of several western blots see the right panel). Similar results were obtained for the nuclear MSH6 protein (Fig. 3B; for quantification see right panel). Since an MNNG-induced increase in nuclear MMR protein level was not observed under non-phosphorylating conditions, the data indicate that MSH2 and MSH6 phosphorylation is involved in MNNG-induced nuclear translocation of MutSα.
Figure 3.
Effect of phosphorylation on nuclear translocation of MutSα. HeLa-MR cells were cultivated for 36 h in normal (control) F12/DMEM or in phosphate-free (designated as –phosphate) or serum-free (designated as –serum) medium. Two hours after incubation with 25 µM MNNG, nuclear extracts were prepared and aliquots of 25 µg each were subjected to western blot analysis. The filter was incubated with antibody against human MSH2 (A) or MSH6 (B). Incubation with antibody against ERK2 was used as a loading control and for quantification. The MNNG-induced relative amounts of MSH2 and MSH6 protein are shown in the right panel (in relation to the untreated control which was set to 1). Means and standard deviations of three independent western blots are presented.
A reduced level of phosphorylation of MSH2/MSH6 upon cultivation of cells in phosphate-free medium could result in decreased formation or reduced stability of the MutSα complex, notably if phosphorylation occurred in the MSH2–MSH6 interaction region. A lower level of formation of the MutSα complex due to inhibition of phosphorylation of MSH2 and MSH6 can be excluded, however, because we were able to immunoprecipitate the MutSα complex in equal amounts from nuclear extracts of HeLa-MR cells cultivated for 48 h in both normal and phosphate-free medium using a monoclonal anti-MSH6 antibody. Similar results were obtained by immunoprecipitation of MutSα from whole cell extracts (Fig. 4A).
Figure 4.
Effect of phosphorylation on complex formation and GT-binding activity of MutSα. (A) HeLa-MR cells were cultivated for 36 h in normal or phosphate-free medium. Whole cell extracts and nuclear extracts were prepared and the MutSα complex was imSunoprecipitated using a specific antibody against MSH6. The immunoprecipitated proteins were subjected to western blot analysis. The filter was incubated with antibodies against human MSH2 and MSH6. (B) HeLa-MR cells were cultivated for 36 h in either normal or phosphate-free medium. After 2 h incubation with 25 µM MNNG, nuclear extracts were prepared and 4 µg protein each were subjected to EMSA using a GT-containing oligonucleotide as described. The specific binding complex is indicated by an arrow; the lower band represents a non-specific binding complex. (C) Aliquots of 5 µg nuclear extract were not treated or mixed together with a monoclonal antibody against MSH6 for 30 min and subjected to EMSA using a GT-containing oligonucleotide as described. The specific supershift is indicated by an asterik; the lower band is non-specific.
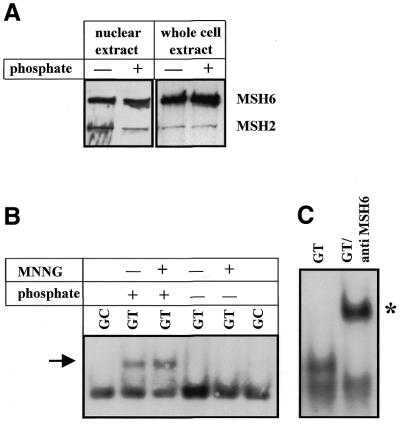
It would be attractive to hypothesise that MutSα phosphorylation affects mismatch binding, which could influence the MNNG-induced translocation of MutSα. To test the influence of phosphorylation of MSH2 and MSH6 on mismatch-binding activity, HeLa-MR cells were cultivated for 36 h in normal or phosphate-free medium and thereafter treated with MNNG (25 µM) for 2 h. Nuclear extracts of the cells were incubated with specific GT mismatch-containing oligonucleotides and subjected to mobility shift analysis (EMSA). In HeLa-MR cells cultivated in normal medium containing phosphate GT-binding activity was clearly detectable, whereas cells cultivated in phosphate-free medium did not display GT-binding activity. Also, MNNG treatment did not provoke GT binding under phosphate-depleted conditions (Fig. 4B). The specificity of the MutSα oligonucleotide complex was verified by competition experiments (data not shown) and by the addition of anti-MSH6 antibody causing a supershift, which is shown in Figure 4C. It should be noted that the lower band detectable in Figure 4B represents a non-specific complex which is frequently observed (the lane representing the unbound DNA substrate is not shown). We also wish to indicate that, as demonstrated before, the nuclear levels of MSH2 and MSH6 protein (Fig. 3) and even the amount of co-immunoprecipitated nuclear MutSα (Fig. 4A) were unaltered in cells cultivated in normal and phosphate-free medium, respectively. Therefore, loss of GT-binding activity in cells cultivated in phosphate-free medium (as shown in Fig. 4B) cannot be explained as due to a lower amount of MutSα present in the nuclear extract, which could theoretically have arisen from impaired nuclear transport or reduced MSH2–MSH6 heterodimer formation. Therefore, the most likely explanation for the reduced GT-binding activity of extracts of cells cultivated under phosphate-free conditions is lack of MutSα phosphorylation. Overall, the data show that MSH2 and MSH6 phosphorylation is involved in regulation of the basal and MNNG-induced mismatch-binding activity of MutSα.
Dephosphorylation of MutSα results in abrogation of GT-binding activity
To further prove the role of CKII/PKC-mediated phosphorylation of MSH2 and MSH6 in GT binding by MutSα, experiments were conducted using either kinase inhibitors or treatment with phosphatases. To check the effect of kinase inhibitors, HeLa-MR cells were cultivated for 36 h in phosphate-free medium, the protein kinase inhibitor H7 or quercetin was added to the medium and cells were incubated for a further 30 min. Thereafter the phosphate-free medium was replaced by normal medium not containing or containing protein kinase inhibitors and the cells were incubated for another 4 h. Nuclear extracts of these cells were prepared and subjected to EMSA. The autoradiographic detection of MutSα bound to GT mispaired oligonucleotides (marked by arrow) revealed that only phosphorylated MutSα is able to bind to GT mismatches, whereas abolition of phosphorylation of MutSα by inhibition of PKC or CKII significantly alleviated GT-binding activity. Interestingly, the simultaneous inhibition of both kinases, PKC and CKII, by co-treatment of cells with H7 and quercetin completely abrogated GT-binding activity (Fig. 5A). The lower band again represents a non-specific band; the unbound DNA substrate is not shown.
Figure 5.
Effect of phosphorylation/dephosphorylation of MutSα on GT-binding activity. (A) HeLa-MR cells were cultivated in phosphate-depleted medium and pretreated with the protein kinase inhibitor H7 or quercetin. Thereafter, phosphate-free medium was replaced with normal medium containing the protein kinase inhibitors and the cells were incubated for a further 2 h. Nuclear extracts were prepared and subjected to EMSA. The specific band is labelled by an arrow. The lower band appearing in all lanes is non-specific. (B) HeLa-MR cells were incubated for 2 h with 25 µM MNNG to increase the amount of nuclear MutSα and nuclear extracts for EMSA were prepared. Cell extract protein was incubated with CIP, λ-PPase or SAP or, as a control, with heat-denatured phosphatases (labelled i) or dephosphorylation buffer only and subjected to EMSA using a GC- (control) or GT-containing oligonucleotide. The specific binding complex is indicated by an arrow and the radioactively labelled substrate is marked by an asterik. (C) Recombinant MutSα was incubated with CIP or SAP or, as a control, with heat-inactivated CIP (labelled i) and subjected to EMSA using a GC- (control) or GT-containing oligonucleotide.
To analyse the effect of phosphatases on GT-binding activity of MutSα, nuclear extracts of HeLa-MR cells were incubated with different phosphatases (in order to dephosphorylate MSH2/MSH6) and subjected to EMSA following incubation with GT oligonucleotide. Incubation of nuclear extracts with shrimp alkaline phosphatase (SAP) or CIP completely abrogateded GT-binding activity of MutSα, whereas incubation with heat-inactivated CIP or dephosphorylation buffer alone did not affect MutSα GT-binding activity (Fig. 5B). We would like to indicate that in these EMSA experiments the non-specific band frequently observed (Fig. 5A) did not occur, which is likely because of different incubation conditions (preincubation of the nuclear extract for 30 min at 37°C in CIP/SAP buffer). In this experiment the unbound DNA substrate is shown to demonstrate that the oligonucleotide was not degraded under SAP and CIP conditions (Fig. 5B, left panel). It is important to note that disappearance of the specific MutSα–GT oligonucleotide complex in the EMSA experiment upon phosphatase treatment (labelled by an arrow) is not because of loss of the radioactively labelled substrate. The oligonucleotides were labelled with [α-32P]dCTP using Klenow enzyme and, therefore, cannot be dephosphorylated by SAP or CIP. In Figure 5B (right panel) an additional EMSA experiment is shown, demonstrating that dephosphorylation of MutSα by CIP and SAP and also by λ-PPase results in loss of GT-binding activity.
One could argue that phosphatase treatment of cell extracts exerts an effect not only on MSH2/MSH6 but also on other proteins that could be involved in MSH2/MSH6 band shift formation. To disprove this argument, the binding properties of purified recombinant MutSα (which was produced in Sf9 cells; 31) was analysed. Treatment of recombinant MutSα with SAP and CIP for 30 min clearly abrogated its GT-binding ability (Fig. 5C). This further supports the conclusion that MutSα is subject to phosphorylation and that dephosphorylation of MutSα results in loss of GT-binding activity.
DISCUSSION
Whereas the DNA targets for binding of MMR proteins and the enzymology of MMR have been largely elucidated in recent years, only limited data are available concerning the regulation of MMR. This work aimed at elucidating whether MMR proteins are regulated by post-translational modification. We have demonstrated that MSH2 and MSH6 are subject to modification by phosphorylation, regulating mismatch binding of MutSα and nuclear translocation. Phosphorylation in vitro of recombinant MSH6 and MSH2 by CKII and PKC was shown to occur. The proteins were not phosphorylated by PKA, indicating the specificity of the in vitro phosphorylation reaction. Phosphorylation of MSH6 and MSH2 was also observed under in vivo conditions, by immonoprecipitation of radioactively labelled proteins from cell extracts. Phosphorylation of MSH2 and MSH6 increases the GT-binding activity of MutSα, which was demonstrated by the finding that HeLa cells cultivated in phosphate-free medium, which reduced the phosphorylation level of MutSα, showed a significant reduction in GT-binding activity. Under these conditions MSH2 and MSH6 proteins were still detectable in unchanged amounts, as shown in co-precipitation experiments, present as a complex. Furthermore, protein kinase inhibitors such as H7 and quercetin reduced GT-binding activity of MutSα and treatment of cell extract protein with phosphatases such as CIP, SAP and λ-PPase resulted in complete loss of GT-binding activity. Similar results were obtained by incubation of recombinant MutSα with phosphatases, which clearly abrogated the GT-binding activity of MutSα. It should be noted that recombinant human MutSα protein was produced in Sf9 cells (31) where it was obviously phosphorylated by a kinase such as CKII, which was recently identified in these cells (35). Taken together, the effects of both kinase inhibitors and phosphatases strongly suggest that phosphorylation of MutSα increases mismatch-binding capacity of the protein complex.
The sites of phosphorylation involved in increasing the GT-binding activity of MutSα are difficult to predict because of the high number of potential kinase targets in both MSH2 and MSH6. Thus, in MSH2 a total of five potential PKC and 18 CKII sites were found by computer-assisted search (not shown). In the MSH6 protein even more potential phosphorylation sites (15 PKC and 29 CKII sites) are present. None of these potential phosphorylation sites is located in a known functional domain, i.e. neither in the ATP-binding domain nor the helix–turn–helix loop domain required for DNA binding (36). One potential PKC site (positions 33–35) is located in the N-terminal domain (comprising amino acids 19–57) of MSH2, which is supposed to interact with DNA mismatches (37). The role of these potential phosphorylation sites will be elucidated in the future.
It has been shown that binding of ATP to MutSα and hydrolysis of ATP to ADP is essential for mismatch repair (31,35,38). One could speculate that phosphorylation of MSH2 and MSH6, as observed in radioactivity assays, is the result of binding of radioactively labelled ATP to the ATP-binding site of MutSα (39). This, however, is highly unlikely because phosphorylation was alleviated in the presence of the protein kinase inhibitor quercetin (data not shown) and was reduced by phosphatase treatment. Also, ATP binding reduces the GT-binding activity of MutSα (38–40), whereas the opposite was found to be true for the phosphorylated proteins, which showed enhanced GT-binding capacity.
Phosphorylation of MutSα could also have an impact on the stability of the protein complex and nuclear translocation. As shown by western blot experiments, the amount of MSH2 and MSH6 in cells incubated in phosphate-free medium was unaffected, although the phosphorylation level was clearly reduced. This indicates that phosphorylation does not affect the stability of the proteins. However, MNNG-induced nuclear translocation of MutSα was found to be abrogated in cells in which phosphorylation of MutSα did not occur. This suggests that phosphorylation of MutSα is required for MNNG-induced translocation of the protein complex into the nucleus. Nuclear translocation of MutSα could theoretically be mediated by actively increasing nuclear transport of the phosphorylated proteins or, in a more indirect way, by increasing the level of binding of MutSα to O6-MeG/C and O6-MeG/T lesions (20). This would reduce the pool of free MutSα in the nucleus and could provide the signal for increased nuclear transport. It should be noted that nuclear translocation of MutSα in the absence of DNA damage appears not to be affected by the phosphorylation level of the proteins because comparable amounts of MSH2 were found in the nucleus of non-treated and phosphate-depleted cells.
Phosphorylation both in vitro and in vivo was found to be more efficient for MSH6, which contains more potential phosphorylation sites than MSH2. The difference in phosphorylation level of both proteins could be of particular interest with regard to the nuclear transport of MutSα. Because of their large size, MSH2 (102 kDa) and MSH6 (160 kDa) cannot enter the nucleus by diffusion. Therefore, nuclear translocation of MutSα very likely requires nuclear localisation signal (NLS)-mediated transport. Since only MSH6 and not MSH2 contains a potential NLS (as revealed by computer search), MSH6 seems to be required for translocation of MSH2 into the nucleus (20). An attractive speculation would thus be that phosphorylation of MSH6 not only affects mismatch binding but is also involved in regulation of nuclear translocation of MutSα.
As demonstrated above, phosphorylation of MutSα was mediated by PKC and CKII. Both kinases were previously shown to be regulated by growth factors and tumour promoters (41). Also, the DNA repair enzymes apurinic endonuclease (APE, alias Ref-1) and MGMT were reported to be subject to phosphorylation by CKII (26–28). It therefore seems that CKII is involved in the regulation not only of proteins involved in cell proliferation and differentiation (42,43), but also in DNA repair. Importantly, various stress signalling agents have been shown to stimulate CKII activity (44). Moreover, CKII as well as PKC are variably expressed in tumours (45,46). Therefore, induction and differential basal expression of CKII and PKC could have an impact on the regulation of a large set of defense proteins, including MMR and other DNA repair functions, both in tumours and normal tissues and upon exposure of cells to genotoxic stress.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Prof. J. Jiricny and Dr P. Dufner (Zürich) for the generous gift of recombinant MutSα protein. This work was supported by Deutsche Forschungsgemeinschaft (SFB 519/B4).
REFERENCES
- 1.Modrich P. and Lahue,R. (1996) Mismatch repair in replication fidelity, genetic recombination and cancer biology. Annu. Rev. Biochem., 65, 101–133. [DOI] [PubMed] [Google Scholar]
- 2.Umar A. and Kunkel,T.A. (1996) DNA-replication fidelity, mismatch repair and genome instability in cancer cells. Eur. J. Biochem., 238, 297–307. [DOI] [PubMed] [Google Scholar]
- 3.Fishel R., Lescoe,M.K., Rao,M.R.S., Coperland,N.G., Jenkins,N.A., Garber,J., Kane,M. and Kolodner,R. (1993) The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell, 75, 1027–1038 [DOI] [PubMed] [Google Scholar]
- 4.Palombo F., Gallinari,P., Iaccario,I., Lettieri,T., Hughes,M., D’Arrigo,A., Truong,O., Hsuan,J.J. and Jiricny,J. (1995) GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science, 268, 1912–1914. [DOI] [PubMed] [Google Scholar]
- 5.Duckett D., Drummond,J.T., Murchie,A.I.H., Reardon,J.T., Sancar,A., Lilley,D.M.J. and Modrich,P. (1996) Human MutSα recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine or the cisplatin d(GpG) adduct. Proc. Natl Acad. Sci. USA, 93, 6443–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada M., O’Regan,E., Brown,R. and Karran,P. (1997) Selective recognition of a cisplatin adduct by human mismatch repair proteins. Nucleic Acids Res., 25, 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Lawrence,C.W. Li,G.-M. and Hays,J.B. (1999) Specific binding of human MSH2-MSH6 mismatch-repair protein heterodimers to DNA incorporating thymine- or uracil-containing UV light photoproducts opposite mismatched bases. J. Biol. Chem., 274, 16894–16900. [DOI] [PubMed] [Google Scholar]
- 8.Wu J., Gu,L., Wang,H., Geacintov,N.E. and Li,G.-M. (1999) Mismatch repair processing of carcinogen-DNA adducts triggers apoptosis. Mol. Cell. Biol., 19, 8292–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G.-M., Wang,H. and Romano,L.J. (1996) Human MutSα specifically binds to DNA containing aminofluorene and acetylaminofluorene adducts. J. Biol. Chem., 271, 24084–24088. [PubMed] [Google Scholar]
- 10.Galloway S.M., Greenwood,S.K., Hill,R.B., Bradt,C.I. and Bean,C.L. (1995) A role for mismatch repair in production of chromosome aberrations by methylating agents in human cells. Mutat. Res., 346, 231–245. [DOI] [PubMed] [Google Scholar]
- 11.Hickman M.J. and Samson,L.D. (1999) Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc. Natl Acad. Sci. USA, 96, 10764–10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaina B., Ziouta,A., Ochs,K. and Coquerelle,T. (1997) Chromosomal instability, reproductive cell death and apoptosis induced by O6-methylguanine in Mex–, Mex+ and methylation-tolerant mismatch repair compromised cells: facts and models. Mutat. Res., 381, 227–341. [DOI] [PubMed] [Google Scholar]
- 13.Ochs K. and Kaina,B. (2000) Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res., 60, 5815–5824. [PubMed] [Google Scholar]
- 14.Branch P., Aquilina,G., Bignami,M. and Karran,P. (1993) Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature, 362, 652–654. [DOI] [PubMed] [Google Scholar]
- 15.Aaltonen L.A., Peltomäki,P., Leach,F.S., Sistonen,P., Pylkkänen,L., Mecklin,J.P., Järvinen,H., Powell,S.M., Jen,J., Hamilton,S.R., Petersen,G.M., Kinzler,K.W., Vogelstein,B. and de la Chapelle,A. (1994) Clues to the pathogenesis of familial colorectal cancer. Science, 260, 812–816. [DOI] [PubMed] [Google Scholar]
- 16.Branch P., Hampson,R. and Karran,P. (1995) DNA mismatch binding defects, DNA damage tolerance and mutator phenotypes in human colorectal carcinoma cell lines. Cancer Res., 55, 2304–2309. [PubMed] [Google Scholar]
- 17.Drummond J.T., Anthoney,A., Brown,R. and Modrich,P. (1996) Cisplatin and adriamycin resistance are associated with MutLα and mismatch repair deficiency in an ovarian tumor cell line. J. Biol. Chem., 271, 19645–19648. [DOI] [PubMed] [Google Scholar]
- 18.Hampson R., Humbert,O., Macpherson,P., Aquilina,G. and Karran,P. (1997) Mismatch repair defects and O6-methylguanine-DNA methyltransferase expression in acquired resistance to methylating agents in human cells. J. Biol. Chem., 272, 28596–28606. [DOI] [PubMed] [Google Scholar]
- 19.Belloni M., Uberti,D., Rizzibi,R., Jiricny,J. and Memo,M. (1999) Induction of two DNA mismatch repair proteins, MSH2 and MSH6 in differentiated human neuroblastoma SH-SY5Y cells exposed to doxorubicin. J. Neurochem., 72, 974–979. [DOI] [PubMed] [Google Scholar]
- 20.Christmann M. and Kaina,B. (2000) Nuclear translocation of mismatch repair proteins MSH2 and MSH6 as a response of cells to alkylating agents. J. Biol. Chem., 275, 36256–36262. [DOI] [PubMed] [Google Scholar]
- 21.Desclozeaux M., Poulat,F., de Santa-Barbara,P., Capony,J.P., Turowski,P., Jay,P., Mejean,C., Moniot,B., Boizet,B. and Berta,P. (1998) Phosphorylation of an N-terminal motif enhances DNA-binding activity of the human SRY protein. J. Biol. Chem., 273, 7988–7995. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto Y., Yoshida,M., Semba,K. and Hunter,T. (1997) Inhibition of the DNA-binding and transcriptional repression activity of the Wilms’ tumor gene product, WT1, by cAMP-dependent protein kinase-mediated phosphorylation of Ser-365 and Ser-393 in the zinc finger domain. Oncogene, 15, 2001–2012. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S. and Baltimore,D. (1990) Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature, 344, 678–682. [DOI] [PubMed] [Google Scholar]
- 24.Gould K.L., Woodgett,J.R., Cooper,J.A., Buss,J.E., Shalloway,D. and Hunter,T. (1985) Protein kinase C phosphorylates pp60src at a novel site. Cell, 42, 849–857. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y., Raychaudhuri,B., Gurney,A., Campbell,C.E. and Williams,B.R. (1996) Regulation of WT1 by phosphorylation: inhibition of DNA binding, alteration of transcriptional activity and cellular translocation. EMBO J., 15, 5606–5615. [PMC free article] [PubMed] [Google Scholar]
- 26.Srivenugopal K.S., Mullapudi,S.R., Shou,J., Hazra,T.K. and Ali-Osman,F. (2000) Protein phosphorylation is a regulatory mechanism for O6-alkylguanine-DNA alkyltransferase in human brain tumor cells. Cancer Res., 60, 282–287. [PubMed] [Google Scholar]
- 27.Fritz G. and Kaina,B. (1999) Phosphorylation of the DNA repair protein APE/Ref-1 by CKII affects redox regulation of AP-1. Oncogene, 18, 1033–1040. [DOI] [PubMed] [Google Scholar]
- 28.Yacoub A., Kelley,M.R. and Deutsch,W.A. (1997) The DNA repair activity of human redox/repair protein APE/Ref-1 is inactivated by phosphorylation. Cancer Res., 57, 5457–5459. [PubMed] [Google Scholar]
- 29.Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O.H., Rosebrough,N.J., Farr,A.L. and Randall,R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193, 265–275. [PubMed] [Google Scholar]
- 31.Iaccarino I., Marra,G., Palombo,F. and Jiricny,J. (1998) hMSH2 and hMSH6 play distinct roles in mismatch binding and contribute differently to the ATPase activity of hMutSα. EMBO J., 17, 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prigent C., Lasko,D.D., Kodama,K., Woodgett,J.R. and Lindahl,T. (1992) Activation of mammalian DNA ligase I through phosphorylation by casein kinase II. EMBO J., 11, 2925–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann K., Bucher,P., Falquet,L. and Bairosh,A. (1999) The PROSITE database, its status in 1999. Nucleic Acids Res., 27, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laks M.S., Harrison,J.J., Schwoch,G. and Jungmann,R.A. (1981) Modulation of nuclear protein kinase activity and phosphorylation of histone H1 subspecies during the prereplicative phase of rat liver regeneration. J. Biol. Chem., 256, 8775–8785. [PubMed] [Google Scholar]
- 35.Steplewski A., Ebel,W., Planey,S.L., Alnemri,E.S., Robertson,N.M. and Litwack,G. (2000) Phosphorylation of the insect immunophilin FKBP46 by the Spodoptera frugiperda homolog of casein kinase II. Gene, 246, 169–178. [DOI] [PubMed] [Google Scholar]
- 36.Alani E., Sokolsky,T., Studamire,B., Miret,J.J. and Lahue,R.S. (1997) Genetic and biochemical analysis of Msh2p-Msh6p: role of ATP hydrolysis and Msh2p-Msh6p subunit interactions in mismatch base pair recognition. Mol. Cell. Biol., 17, 2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culligan K.M., Meyer-Gauen,G., Lyons-Weiler,J. and Hays,J.B. (2000) Evolutionary origin, diversification and specialization of eukaryotic MutS homolog mismatch repair proteins. Nucleic Acids Res., 28, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackwell L.J., Martik,D., Bjornson,K.P., Bjornson,E.S. and Modrich,P. (1998) Nucleotide-promoted release of hMutSα from heteroduplex DNA is consistent with an ATP-dependent translocation mechanism. J. Biol. Chem., 273, 32055–32062. [DOI] [PubMed] [Google Scholar]
- 39.Gradia S., Acharya,S. and Fishel,F. (1997) The human mismatch recognition complex hMSH2-hMSH6 functions as a novel molecular switch. Cell, 91, 995–1005. [DOI] [PubMed] [Google Scholar]
- 40.Matton N., Simonetti,J. and Williams,K. (2000) Identification of mismatch repair protein complexes in HeLa nuclear extracts and their interaction with heteroduplex DNA. J. Biol. Chem., 275, 17808–12813. [DOI] [PubMed] [Google Scholar]
- 41.Ackerman P., Glover,C.V. and Osheroff,N. (1990) Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc. Natl Acad. Sci. USA, 87, 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinna L.A. and Meggio,F. (1997) Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell Cycle Res., 3, 77–97. [DOI] [PubMed] [Google Scholar]
- 43.Guerra B. and Issinger,O.G. (1999) Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis, 20, 391–408. [DOI] [PubMed] [Google Scholar]
- 44.Sayed M., Kim,S.O., Salh,B.S., Issinger,O.G. and Pelech,S.L. (2000) Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J. Biol. Chem., 275, 16569–16573. [DOI] [PubMed] [Google Scholar]
- 45.Kuranami M., Powell,C.T., Hug,H., Zeng,Z., Cohen,A.M. and Guillem,J.G. (1995) Differential expression of protein kinase C isoforms in human colorectal cancers. J. Surg. Res., 58, 233–239. [DOI] [PubMed] [Google Scholar]
- 46.Stalter G., Siemer,S., Becht,E., Ziegler,M., Remberger,K. and Issinger,O.G. (1994) Asymmetric expression of protein kinase CK2 subunits in human kidney tumors. Biochem. Biophys. Res. Commun., 202, 141–147. [DOI] [PubMed] [Google Scholar]