Abstract
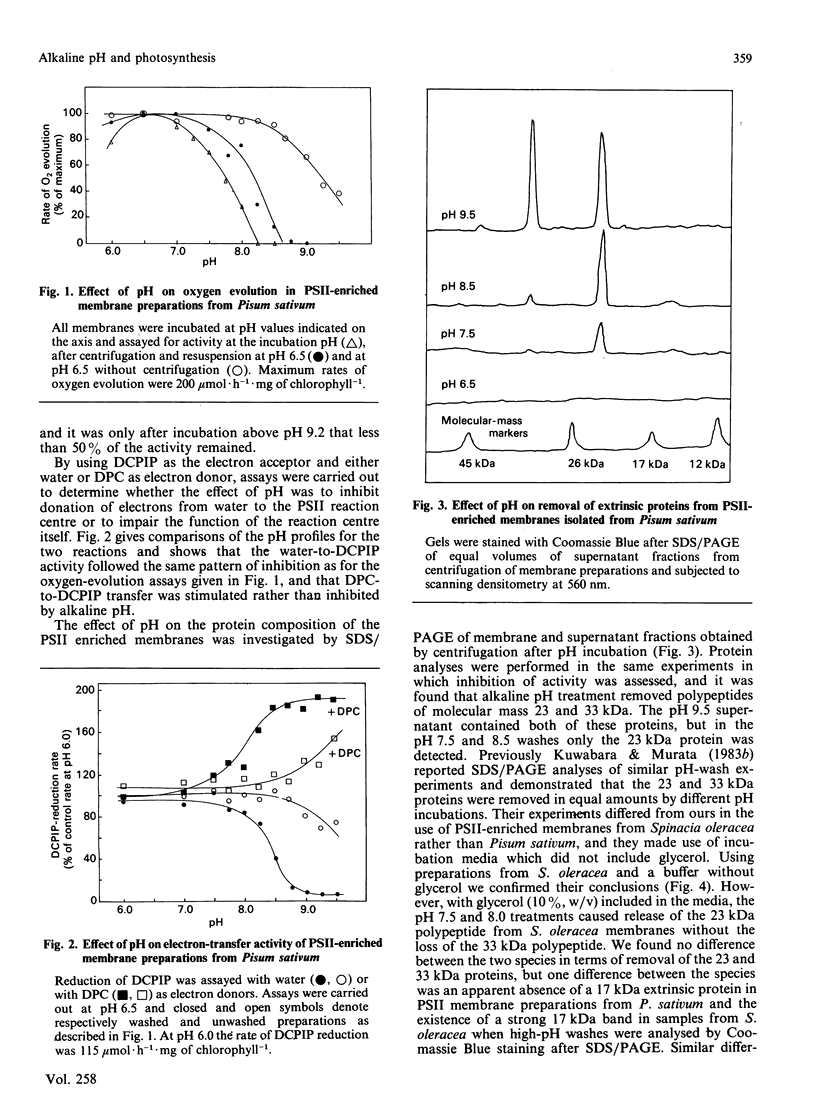
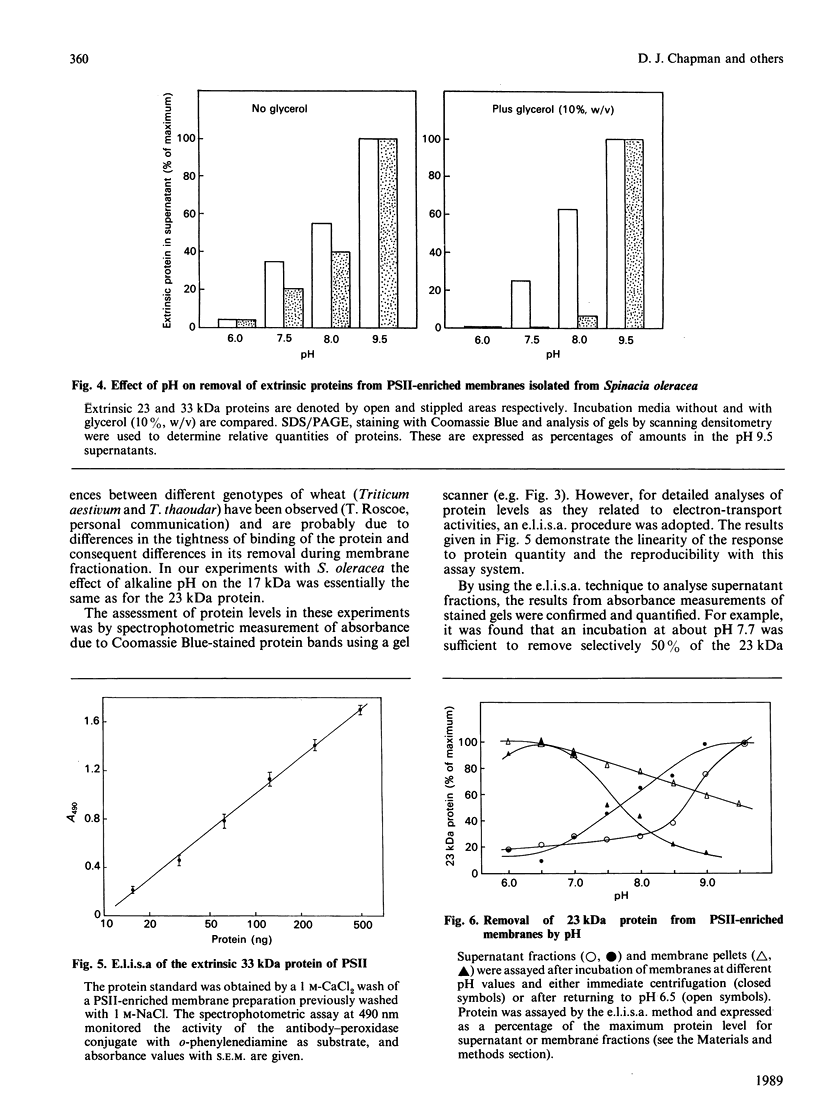
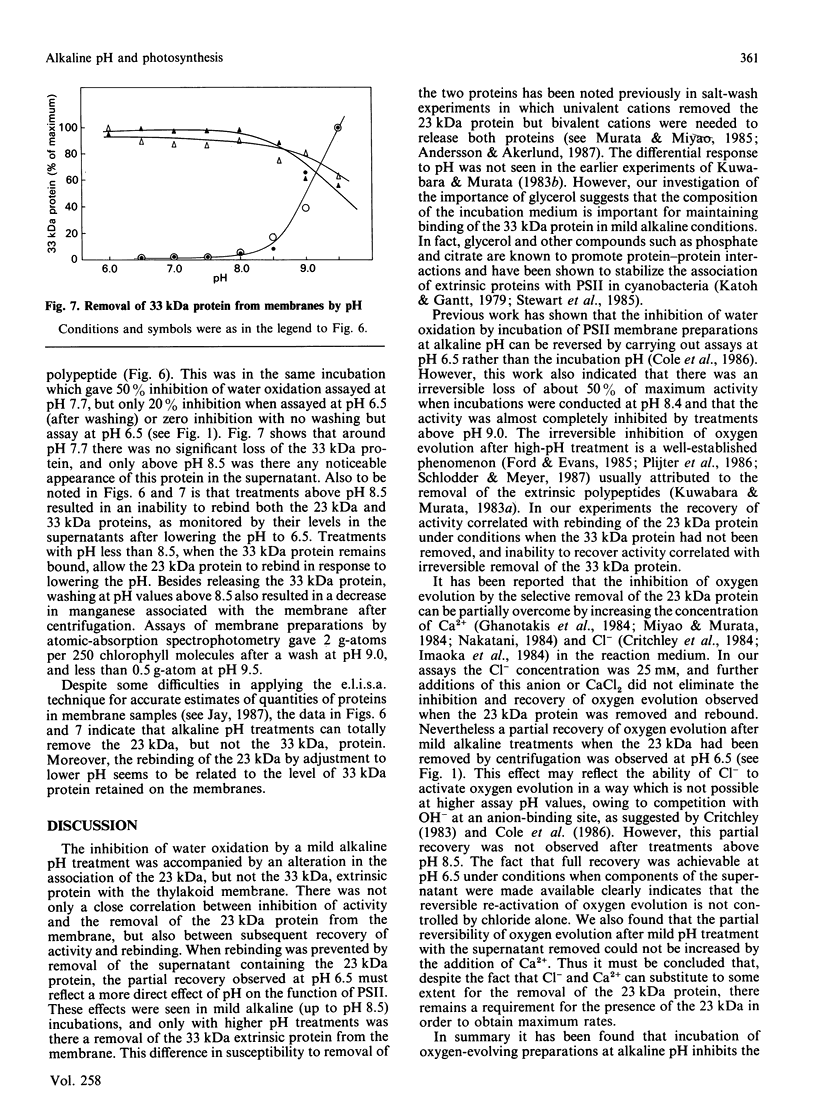
Incubation of a membrane preparation enriched in Photosystem Two (PSII) at alkaline pH inhibited the water-splitting reactions in two distinct steps. Up to pH 8.5 the inhibition was reversible, whereas at higher alkalinities it was irreversible. It was shown that the reversible phase correlated with loss and rebinding of the 23 kDa extrinsic polypeptide. However, after mild alkaline treatments a partial recovery was possible without the binding of the 23 kDa polypeptide when the assay was at the optimal pH of 6.5 and in a medium containing excess Cl-. The irreversible phase was found to be closely linked with the removal of the 33 kDa extrinsic protein of PSII. Treatments with pH values above 8.5 not only caused the 33 kDa protein to be displaced from the PSII-enriched membranes, but also resulted in an irreversible modification of the binding sites such that the extrinsic 33 kDa protein could not reassociate with PSII when the pH was lowered to 6.5. The results obtained with these more extreme alkaline pH treatments support the notion that the 23 kDa protein cannot bind to PSII unless the 33 kDa protein is already bound. The differential effect of pH on the removal of the 23 kDa and 33 kDa proteins contrasted with the data of Kuwabara & Murata [(1983) Plant Cell Physiol. 24, 741-747], but this discrepancy was accounted for by the use of glycerol in the incubation media.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briantais J. M., Vernotte C., Lavergne J., Arntzen C. J. Identification of S2 as the sensitive state to alkaline photoinactivation of photosystem II in chloroplasts. Biochim Biophys Acta. 1977 Jul 7;461(1):61–74. doi: 10.1016/0005-2728(77)90069-x. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Cohen W. S., Bertsch W. Inhibition of photosystem II by uncouplers at alkaline pH and its reversal by artificial electron donors. Biochim Biophys Acta. 1975 Jan 31;376(1):97–104. doi: 10.1016/0005-2728(75)90208-x. [DOI] [PubMed] [Google Scholar]
- Cole J., Boska M., Blough N. V., Sauer K. Reversible and irreversible effects of alkaline pH on Photosystem II electron-transfer reactions. Biochim Biophys Acta. 1986 Jan 28;848(1):41–47. doi: 10.1016/0005-2728(86)90158-1. [DOI] [PubMed] [Google Scholar]
- Katoh T., Gantt E. Photosynthetic vesicles with bound phycobilisomes from Anabaena variabilis. Biochim Biophys Acta. 1979 Jun 5;546(3):383–393. doi: 10.1016/0005-2728(79)90075-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y. Photosynthetic oxygen evolution does not require the participation of polypeptides of 16 and 24 kilodaltons. Biochem Biophys Res Commun. 1984 Apr 16;120(1):299–304. doi: 10.1016/0006-291x(84)91448-7. [DOI] [PubMed] [Google Scholar]


