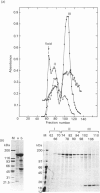
Abstract
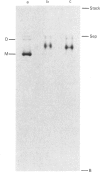
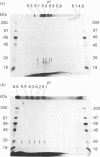
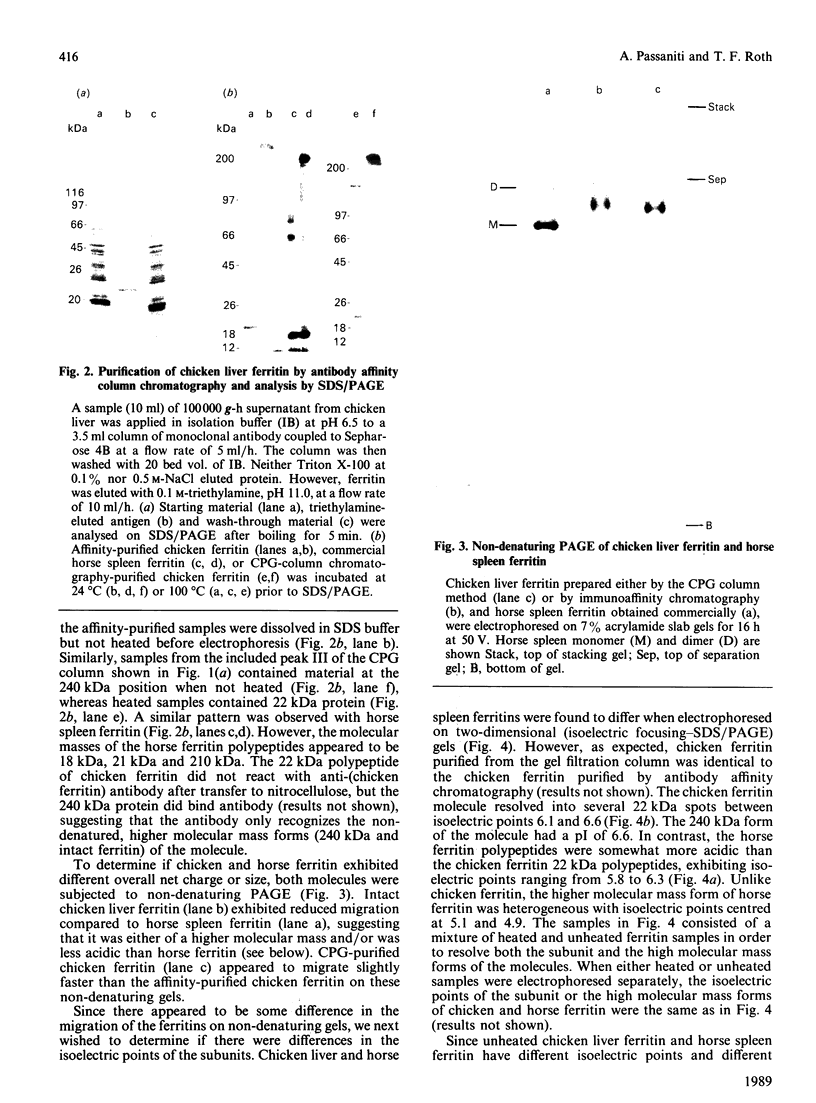
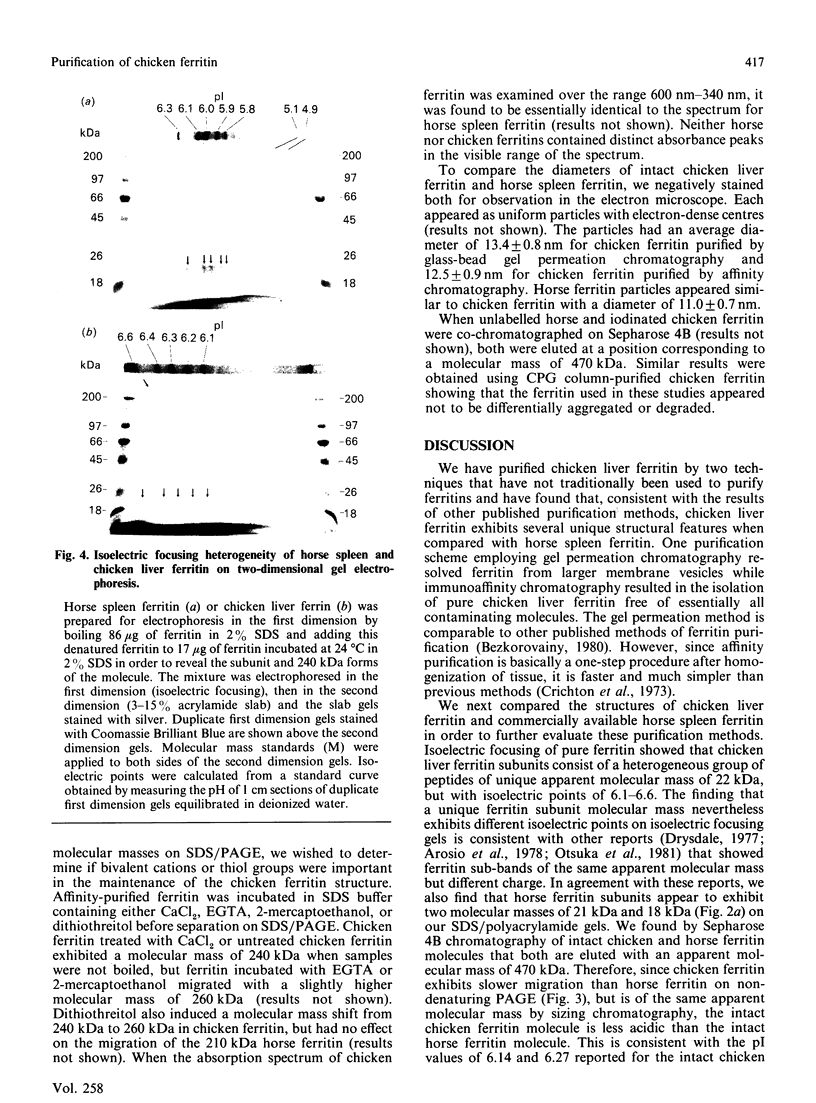
Ferritin was purified from chicken liver by two different methods: gel filtration on controlled-pore glass beads, and immunoaffinity chromatography employing a chicken ferritin-specific monoclonal antibody that did not cross-react with horse spleen ferritin. This antibody recognizes intact ferritin and an oligomeric 240 kDa form of the molecule after protein transfer to nitrocellulose, but not the 22 kDa chicken ferritin subunit. Chicken liver ferritin purified by these methods exhibited reduced migration on non-denaturing polyacrylamide gels compared with horse spleen ferritin. These results were consistent with the difference in calculated isoelectric points of chicken and horse ferritin subunits. By two-dimensional gel electrophoresis, chicken ferritin 22 kDa subunits exhibited isoelectric points from 6.1 to 6.6 whereas horse spleen ferritin subunits exhibited isoelectric points of 5.8-6.3. The 240 kDa form of the chicken ferritin molecule had an isoelectric point of 6.6 whereas the 210 kDa form of the horse ferritin molecule had isoelectric points of 5.1 and 4.9. Intact chicken liver ferritin particles were 13.4 +/- 0.8 nm (controlled-pore glass-purified) and 12.5 +/- 0.9 nm (affinity-purified) in diameter when viewed by electron microscopy. Horse spleen ferritin consisted of slightly smaller particles with an average diameter of 11.0 +/- 0.7 nm. However, ferritin from chicken liver and horse spleen co-migrated with an apparent molecular mass of 470 kDa when analysed by Sepharose 4B gel filtration chromatography. These results indicate that, consistent with results from other published purification methods, the chicken ferritin purified by the methods reported here exhibits both structural similarities to, and differences from, horse spleen ferritin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Treffry A., Harrison P. M. The location of antigenic sites on ferritin molecules. FEBS Lett. 1984 Oct 1;175(2):333–336. doi: 10.1016/0014-5793(84)80763-2. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. The catalytic activity of horse spleen apoferritin. Preliminary kinetic studies and the effect of chemical modification. Biochem J. 1973 Jun;133(2):301–309. doi: 10.1042/bj1330301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. J., Martín M., García J. R., Recio J. M. Radioinmunoensayo de feritina plasmática de Gallus domesticus L. Rev Esp Fisiol. 1981 Dec;37(4):447–454. [PubMed] [Google Scholar]
- Calvo J. J., Martín M., Recio J. M. Correlación entre los niveles de ferritina plasmática y del hierro de hígado y bazo en pollo. Rev Esp Fisiol. 1982 Mar;38(1):79–82. [PubMed] [Google Scholar]
- Crichton R. R., Eason R., Barclay A., Bryce C. F. The subunit structure of horse spleen apoferritin; the molecular weight of the oligomer and its stability to dissociation by dilution. Biochem J. 1973 Apr;131(4):855–857. doi: 10.1042/bj1310855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. N., Easterbrook K. Ferritin in the fungus Phycomyces. J Cell Biol. 1971 Jan;48(1):15–28. doi: 10.1083/jcb.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dognin J., Crichton R. R. Mobilisation of iron from ferritin fractions of defined iron content by biological reductants. FEBS Lett. 1975 Jun 15;54(2):234–236. doi: 10.1016/0014-5793(75)80081-0. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W. Ferritin phenotypes: structure and metabolism. Ciba Found Symp. 1976 Dec 7;(51):41–67. doi: 10.1002/9780470720325.ch3. [DOI] [PubMed] [Google Scholar]
- FINE J. M., HARRIS G. ELECTROPHORETIC AND IMMUNOLOGICAL STUDIES OF HORSE AND HUMAN FERRITIN. Clin Chim Acta. 1963 Sep;8:794–798. doi: 10.1016/0009-8981(63)90148-7. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Bayne E. K. Multiple forms of (Na+ + K+)-ATPase in the chicken. Selective detection of the major nerve, skeletal muscle, and kidney form by a monoclonal antibody. J Biol Chem. 1983 Mar 25;258(6):3926–3935. [PubMed] [Google Scholar]
- González del Barrio P., Martin Mateo M. C. Comparative study of ferritins from dove Columba oena and chicken Gallus domesticus livers. Comp Biochem Physiol B. 1983;76(3):567–568. doi: 10.1016/0305-0491(83)90294-8. [DOI] [PubMed] [Google Scholar]
- HYDE B. B., HODGE A. J., KAHN A., BIRNSTIEL M. L. STUDIES ON PHYTOFERRITIN. I. IDENTIFICATION AND LOCALIZATION. J Ultrastruct Res. 1963 Oct;59:248–258. doi: 10.1016/s0022-5320(63)80005-2. [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Passaniti A., Woods J. W., Daiss J. L., Roth T. F. Tubulin as a molecular component of coated vesicles. J Cell Biol. 1983 Oct;97(4):1191–1199. doi: 10.1083/jcb.97.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Nandi P. K., Irace G., Van Jaarsveld P. P., Lippoldt R. E., Edelhoch H. Instability of coated vesicles in concentrated sucrose solutions. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5881–5885. doi: 10.1073/pnas.79.19.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Otsuka S., Maruyama H., Listowsky I. Structure, assembly, conformation, and immunological properties of the two subunit classes of ferritin. Biochemistry. 1981 Sep 1;20(18):5226–5232. doi: 10.1021/bi00521a020. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Perdew G. H., Schaup H. W., Selivonchick D. P. The use of a zwitterionic detergent in two-dimensional gel electrophoresis of trout liver microsomes. Anal Biochem. 1983 Dec;135(2):453–455. doi: 10.1016/0003-2697(83)90711-x. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Kelly R. B. Identification of minor components of coated vesicles by use of permeation chromatography. J Cell Biol. 1981 Nov;91(2 Pt 1):385–391. doi: 10.1083/jcb.91.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter G. W. The iron-loaded cell--the cytopathology of iron storage. A review. Am J Pathol. 1978 May;91(2):362–404. [PMC free article] [PubMed] [Google Scholar]
- Santos Benito F. F., Martin Mateo M. C. Isolation, purification and characterization of spleen ferritin of Gallus domesticus L. Comp Biochem Physiol B. 1983;74(3):643–645. doi: 10.1016/0305-0491(83)90243-2. [DOI] [PubMed] [Google Scholar]
- Shinjyo S. [Isolation and properties of ferritin from chicken (Gallus domesticus, broiler) spleen]. Seikagaku. 1973 Jun;45(6):289–295. [PubMed] [Google Scholar]
- Stefanini S., Chiancone E., Arosio P., Finazzi-Agrò A., Antonini E. Structural heterogeneity and subunit composition of horse ferritins. Biochemistry. 1982 May 11;21(10):2293–2299. doi: 10.1021/bi00539a004. [DOI] [PubMed] [Google Scholar]
- TOWE K. M., LOWENSTAM H. A., NESSON M. H. INVERTEBRATE FERRITIN: OCCURRENCE IN MOLLUSCA. Science. 1963 Oct 4;142(3588):63–64. doi: 10.1126/science.142.3588.63. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijk H. G., Van Noort W. L. Isolation of rat transferrin using CNBr-activated sepharose 4B. J Clin Chem Clin Biochem. 1976 Oct;14(10):475–478. doi: 10.1515/cclm.1976.14.1-12.475. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Roth T. F. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4394–4398. doi: 10.1073/pnas.75.9.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamiri I., Mason J. Electrophoresis of ferritins. Nature. 1968 Jan 20;217(5125):258–259. doi: 10.1038/217258a0. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]