Abstract
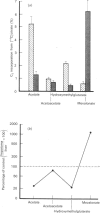
Cholesterol biosynthesis was characterized in cell-free post-mitochondrial supernatant systems prepared from both normal rat liver and Morris hepatoma 3924A. The rate of cholesterol synthesis per cell was 9-fold greater in the tumour system than in that from normal liver, and the tumour systems showed the loss of rate-limiting control at the hydroxymethylglutaryl-CoA reductase (HMGR)-catalysed step. The apparent absence of rate-limiting control over cell-free tumour cholesterogenesis was traced primarily to a discoordinate and dramatic increase in the amount of HMGR in the tumour relative to the liver system. Preliminary evidence for an altered control of the post-lanosterol portion of the pathway was also obtained with the tumour system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski W., Sokolowski W. HMG-CoA reductase activity in the microsomal fraction from human placenta in early and term pregnancy. Int J Biochem. 1984;16(9):1023–1026. doi: 10.1016/0020-711x(84)90120-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Limanek J. S. Regulation of cytosolic acetoacetyl coenzyme A thiolase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and mevalonate kinase by low density lipoprotein and by 25-hydroxycholesterol in Chinese hamster ovary cells. J Biol Chem. 1980 Aug 25;255(16):7787–7795. [PubMed] [Google Scholar]
- Chen H. W. Role of cholesterol metabolism in cell growth. Fed Proc. 1984 Jan;43(1):126–130. [PubMed] [Google Scholar]
- Coleman P. S., Lavietes B. B. Membrane cholesterol, tumorigenesis, and the biochemical phenotype of neoplasia. CRC Crit Rev Biochem. 1981;11(4):341–393. doi: 10.1080/10409238109104421. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Popják G., Fogelman A. M., Edmond J. Control of 3-hydroxy-3-methylglutaryl coenzyme A reductase by endogenously synthesized sterols in vitro and in vivo. J Biol Chem. 1977 Feb 10;252(3):1057–1063. [PubMed] [Google Scholar]
- Erickson S. K., Cooper A. D., Barnard G. F., Havel C. M., Watson J. A., Feingold K. R., Moser A. H., Hughes-Fulford M., Siperstein M. D. Regulation of cholesterol metabolism in a slow-growing hepatoma in vivo. Biochim Biophys Acta. 1988 May 22;960(2):131–138. doi: 10.1016/0005-2760(88)90058-6. [DOI] [PubMed] [Google Scholar]
- Faust J., Krieger M. Expression of specific high capacity mevalonate transport in a Chinese hamster cell variant. J Biol Chem. 1987 Feb 15;262(5):1996–2004. [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., Moser A. H., Siperstein M. D. Altered activation state of hydroxymethylglutaryl-coenzyme A reductase in liver tumors. Arch Biochem Biophys. 1983 Oct 1;226(1):231–241. doi: 10.1016/0003-9861(83)90289-8. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Björnsson O. G., Pullinger C. R. Evidence that changes in hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity are required partly to maintain a constant rate of sterol synthesis. J Biol Chem. 1984 Dec 10;259(23):14399–14405. [PubMed] [Google Scholar]
- Gregg R. G., Davidson M., Wilce P. A. Cholesterol synthesis and HMG CoA reductase activity during hepatocarcinogenesis in rats. Int J Biochem. 1986;18(4):389–393. doi: 10.1016/0020-711x(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Kaplan R. S., Morris H. P., Coleman P. S. Kinetic characteristics of citrate influx and efflux with mitochondria from Morris hepatomas 3924A and 16. Cancer Res. 1982 Nov;42(11):4399–4407. [PubMed] [Google Scholar]
- Kaplan R. S., Parlo R. A., Coleman P. S. Measurement of citrate transport in tumor mitochondria. Methods Enzymol. 1986;125:671–691. doi: 10.1016/s0076-6879(86)25055-7. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Brandt K. G., Rodwell V. W. 3-hydroxy-3-methylglutaryl-CoA reductase: solubilization in the presence of proteolytic inhibitors, partial purification, and reversible phosphorylation-dephosphorylation. Biochemistry. 1983 Jun 7;22(12):2784–2788. doi: 10.1021/bi00281a002. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langan T. J., Volpe J. J. Obligatory relationship between the sterol biosynthetic pathway and DNA synthesis and cellular proliferation in glial primary cultures. J Neurochem. 1986 Apr;46(4):1283–1291. doi: 10.1111/j.1471-4159.1986.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Salsgiver W. J. Levels of enzymes of energy metabolism are controlled by activity of cultured rat myotubes. Am J Physiol. 1983 May;244(5):C348–C355. doi: 10.1152/ajpcell.1983.244.5.C348. [DOI] [PubMed] [Google Scholar]
- Marco de la Calle C., Hwang W., Pullinger C. R., Gibbons G. F. A relationship between the activities of hepatic lanosterol 14 alpha-demethylase and 3-hydroxy-3-methylglutaryl-CoA reductase. Biochem J. 1988 Feb 15;250(1):33–39. doi: 10.1042/bj2500033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian M., Callaway K. A., Clarke C. F., Tanaka R. D., Greenspan M., Lusis A. J., Sparkes R. S., Mohandas T., Edmond J., Fogelman A. M. Regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A synthase and the chromosomal localization of the human gene. J Biol Chem. 1986 Dec 5;261(34):16249–16255. [PubMed] [Google Scholar]
- Ness G. C., Sample C. E., Smith M., Pendleton L. C., Eichler D. C. Characteristics of rat liver microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Biochem J. 1986 Jan 1;233(1):167–172. doi: 10.1042/bj2330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlo R. A., Coleman P. S. Continuous pyruvate carbon flux to newly synthesized cholesterol and the suppressed evolution of pyruvate-generated CO2 in tumors: further evidence for a persistent truncated Krebs cycle in hepatomas. Biochim Biophys Acta. 1986 Apr 29;886(2):169–176. doi: 10.1016/0167-4889(86)90134-5. [DOI] [PubMed] [Google Scholar]
- Parlo R. A., Coleman P. S. Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. The truncated Krebs cycle and other metabolic ramifications of mitochondrial membrane cholesterol. J Biol Chem. 1984 Aug 25;259(16):9997–10003. [PubMed] [Google Scholar]
- Quesney-Huneeus V., Galick H. A., Siperstein M. D., Erickson S. K., Spencer T. A., Nelson J. A. The dual role of mevalonate in the cell cycle. J Biol Chem. 1983 Jan 10;258(1):378–385. [PubMed] [Google Scholar]
- Reynolds G. A., Goldstein J. L., Brown M. S. Multiple mRNAs for 3-hydroxy-3-methylglutaryl coenzyme A reductase determined by multiple transcription initiation sites and intron splicing sites in the 5'-untranslated region. J Biol Chem. 1985 Aug 25;260(18):10369–10377. [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Sinensky M., Logel J. Defective macromolecule biosynthesis and cell-cycle progression in a mammalian cell starved for mevalonate. Proc Natl Acad Sci U S A. 1985 May;82(10):3257–3261. doi: 10.1073/pnas.82.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein M. D., Gyde A. M., Morris H. P. Loss of feedback control of hydroxymethylglutaryl coenzyme A reductase in hepatomas. Proc Natl Acad Sci U S A. 1971 Feb;68(2):315–317. doi: 10.1073/pnas.68.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein M. D. Role of cholesterogenesis and isoprenoid synthesis in DNA replication and cell growth. J Lipid Res. 1984 Dec 15;25(13):1462–1468. [PubMed] [Google Scholar]