Abstract
As many as one third of patients undergoing minimally invasive thoracic surgery and one half undergoing thoracotomy will have chronic pain, defined as pain lasting 2 to 3 months. There is limited information regarding predictors of chronic pain and even less is known about its impact on health-related quality of life, known as pain interference. Currently, there is a focus on decreased opioid prescribing after surgery. Interestingly, thoracic surgical patients are the least likely to be receiving opioids before surgery and have the highest rate of new persistent opioid use after surgery compared with other surgical cohorts. These studies of opioid use have identified important predictors of new persistent opioid use, but their findings are limited by failing to correlate opioid use with pain. The objectives of this invited review are to present the findings of pertinent studies of chronic pain and opioid use after thoracic surgery, “where we are,” and to discuss gaps in our knowledge of these topics and opportunities for research to fill those gaps, “where we need to go.”
This year approximately 230,000 people will be diagnosed with lung cancer, 40% of whom may undergo surgical resection.1 About half of those undergoing thoracotomy and one third undergoing video-assisted thoracic surgery (VATS) will have chronic pain,2,3 defined as pain lasting 2 to 3 months.4,5 There are limited data to predict which patients will have chronic pain and even less is known about its impact on health-related quality of life (HRQOL). In a study of predictors of pain after thoracic surgery, the only predictor of pain at 6 months was pain intensity during the first 3 postoperative days.3 In a post-hoc analysis, the only predictor of moderate to severe acute postoperative pain was the intensity of pain that the patient expected.6 Therefore, whereas most studies of chronic pain after thoracic surgery have focused on incidence and intensity, the role of pain interference and expectations for pain require further exploration.
Studies of pain after thoracic surgery have also examined the use of opioids after surgery.7–9 Importantly, these studies highlight that thoracic surgical patients are the least likely to be receiving opioids before surgery10 and have the highest rate of new persistent opioid use after surgery8 compared with other surgical cohorts. Fourteen percent of lung cancer resection patients continued to fill opioid prescriptions 90 to 180 days after surgery.7 Although these studies identified some predictors of new persistent opioid use, their findings are limited by failing to correlate opioid use with pain.7,9,11
Surgeons have responded to the opioid epidemic by developing prescribing guidelines.12–14 Although important from a public health perspective, guidelines discount individualization—the cornerstone of patient-centered care. In addition, these guidelines do not pertain to thoracic surgical patients and exclude patients with chronic pain, those on opioids before surgery, and those with postoperative complications. These may be the patients who need the most perioperative support to prevent and manage chronic pain.
Our aim is to provide a review of chronic pain and opioid use after thoracic surgery. We will discuss gaps in our knowledge of these topics and opportunities for research to fill those gaps. Our ultimate goals are to (1) develop a comprehensive, patient-centered approach to assessing pain after thoracic surgery, including intensity, quality (neuropathic vs nociceptive), and most importantly, how pain impacts function and HRQOL, known as pain interference; (2) identify predictors of pain interference and opioid use after thoracic surgery and determine whether opioid use correlates with pain interference; and (3) develop patient-centered education and support interventions that can be implemented before surgery to decrease the incidence of chronic pain and opioid use after thoracic surgery.
Chronic Pain After Thoracic Surgery
Chronic postsurgical pain is defined by the following criteria: (1) pain that developed after a surgical procedure; (2) pain of at least 2 months’ duration; (3) other causes of pain are excluded (for example, cancer recurrence, infection); and (4) also excluded is possibility of pain from a preexisting problem.4,15,16 A meta-analysis of chronic pain after thoracotomy reported an incidence of 57% at 3 months, 47% at 6 months, and 43% at 12 months.2 Only a few studies reported pain intensity with an average score of 30 ± 2, on a numeric rating scale of 0 to 100, at 3 months, 32 ± 7 at 6 months, and 26 ± 9 at 12 months after surgery. Moreover, the studies included in this meta-analysis spanned 3 decades, and the incidence of chronic pain did not change over time.2 In one study of 600 respondents who had undergone either thoracotomy or VATS ranging from 7 months to 7 years earlier, 45% had pain, including 45% of patients undergoing thoracotomy and 41% undergoing VATS.17 The results indicated that 8% reported their pain as severe and 60% noted improvement over time. Importantly, 39% were taking analgesics, 45% felt that their postsurgical pain was their worst medical problem, and 39% reported that it limited their daily activities.17
The first report to provide evidence of intense acute postoperative pain as a predictor of chronic pain was by Katz and colleagues18 in 1996 (Figure 1). This small landmark study aimed to identify predictors of chronic pain after thoracotomy in a small cohort of patients from a randomized trial of preemptive multimodal analgesia.18 Pain measures obtained within the first 2 days of surgery were compared between patients with (n = 12) and patients without (n = 11) pain one and a half years later. Early postoperative pain intensity was the only statistically significant predictor of chronic pain; pain intensity within the first 24 hours at rest and after movement were significantly greater among patients who had chronic pain.18 Interestingly, morphine consumption on postoperative days 1 and 2 were similar between the two groups, and the researchers hypothesized that either patients in the pain group were titrating morphine to avoid side effects or their pain may not have been as responsive to morphine, consistent with neuropathic pain.19 The researchers stated, “whether aggressively treating early postoperative pain will diminish the likelihood of long-term pain is not known, but it is a logical first step toward identifying factors responsible for the transition of acute, physiological pain to chronic, pathological pain.”18 In a recent prospective study of patients undergoing thoracic surgery, the only independent predictor of chronic pain at 6 months was the severity of acute postoperative pain during the first 3 days after surgery.3 These investigators conducted preoperative psychosocial and pain assessments as well as quantitative sensory testing (preoperative pain threshold to cold and pain magnitude to suprathreshold cold), and none of these factors was associated with the development of chronic pain.3
Figure 1.
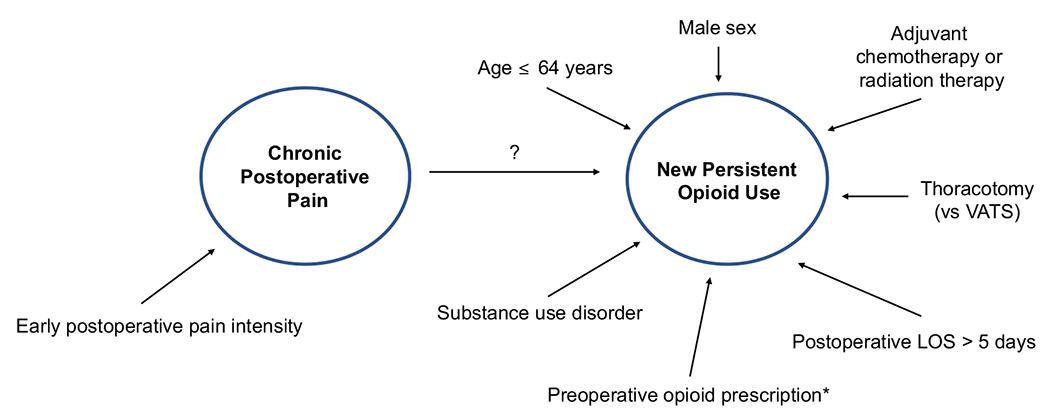
Risk factors for chronic postoperative pain and new persistent opioid use after thoracic surgery. *Opioid-naïve patients who filled opioid prescriptions in the 30 days before surgery. (LOS, length of stay; VATS, video-assisted thoracic surgery.)
Surgical approaches, thoracotomy vs VATS or robot-assisted thoracic surgery, have been compared to determine whether minimally invasive approaches are associated with less postoperative pain. A randomized trial comparing anterolateral thoracotomy with VATS demonstrated that the proportion of patients with clinically relevant pain (numeric rating score 3 or more) was significantly lower during the first 24 hours after VATS, 38% vs 63%.19 The VATS patients had decreased pain for the first 4 weeks, but beyond that there was a similar proportion of patients with moderate to severe pain (numeric rating score 3 or greater). The researchers acknowledge that anterolateral thoracotomy is likely less painful than posterolateral thoracotomy, although the two have never been directly compared, and speculated that the difference in postoperative pain between patients undergoing VATS vs thoracotomy may be even greater with posterolateral thoracotomy.19 A prospective study from Memorial Sloan Kettering Cancer Center found no difference in pain scores between patients undergoing VATS and thoracotomy at all time points (postoperative, and 4, 8, and 12 months).20
Neuropathic pain is common after thoracic surgery. It is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.”21 A gold standard approach to diagnosing neuropathic pain is lacking, but an algorithm consisting of history, physical examination findings, and confirmatory tests has been proposed to determine the level of certainty that pain is neuropathic.22 The lack of pathognomonic features of neuropathic pain make it challenging to definitively diagnose,22 but there are several validated screening tools. Neuropathic pain is characterized by moderate to severe pain, a prominent pain symptom (for example, burning, electric shocks) and significant numbness; sensory loss distal to the surgical scar is a feature of neuropathic pain.23 The prevalence of neuropathic pain in patients with chronic pain after thoracic surgery is approximately 25% to 50%.23,24 Pain intensity is significantly higher among patients having a neuropathic component compared with patients not having a neuropathic component.17,23 Moreover, neuropathic pain is associated with more analgesic use and the pain is more likely to limit daily activities.17 A subset of chronic pain patients with a neuropathic component indicated that pain was one of their worst medical problems, had a significant impact on quality of life, and led to sleep disorders.24 A predictor of neuropathic pain in persons with chronic pain after surgery is duration of chest tube drainage of 4 days or more.24
Intercostal nerve injury appears to be the most important factor leading to chronic neuropathic pain; however, the precise mechanism is still unknown.25 A study of intraoperative intercostal nerve conduction indicated two patterns of nerve injury.26 One was direct pressure on the intercostal nerve by the retractor, and the other was traction on the nerve. Interestingly, intercostal nerve damage detected at the time of operation was not associated with chronic pain or alterations in cutaneous sensation 3 months after surgery.26 In a study of patients undergoing thoracotomy or VATS with or without a metal retractor, VATS patients without retractor use had no change in current perception threshold values and no pain after 12 weeks.25 The other two groups had higher current perception threshold values 1 week after surgery, with pain in 70% at 12 weeks. There was a correlation between current perception threshold values and the intensity of pain 4 and 12 weeks after surgery.25 The investigators concluded that Aβ (large, myelinated fibers for touch and pressure sensation) and Aδ (small, myelinated fibers for sharp pain) fibers play a significant role in the development of intercostal nerve damage, whereas C (unmyelinated fibers for temperature and dull pain) fibers are not impacted by metal retractors and may not be responsible for this form of chronic pain.25
From a surgical standpoint, several techniques can be used to minimize the risk of postoperative neuropathic pain. One randomized trial indicated that mobilizing the intercostal muscle and its neurovascular bundle along the inferior edge of the fifth rib to avoid pressure from the retractor decreases postoperative pain.27 Patients in the intervention group also had a smaller decrease in lung function as measured by spirometry, used fewer analgesics, and were more likely to return to normal activity.27 Another technique to decrease pain is to close the chest using intracostal, rather than pericostal sutures.28,29 The mean pain scores of patients who had an intracostal closure were lower at 2 weeks and at 1, 2, and 3 months after surgery compared with patients who had a pericostal closure, and those in the latter group were more likely to use neuropathic pain descriptors.28
Although higher pain intensity at the time of surgery is almost always associated with higher rates of developing new chronic pain, it may not be the case that using more opioids in attempt to better control acute postoperative pain would reduce the rate of new postsurgical chronic pain. Patients with greater central nervous system pain amplification have higher postoperative pain intensity because of the hyperalgesia that accompanies this state.30–32 However, this problem should not be treated with opioids as this type of pain is inherently less responsive to opioids.32–34 Moreover, patients with centralized pain have high rates of sleep problems and mood disorders that place them at risk for transitioning from acute to new persistent opioid use.35,36
Health-Related Quality of Life
Despite numerous studies on the incidence and intensity of pain after thoracic surgery, there are few reports of the impact of pain on HRQOL. Health-related quality of life is defined as how well a person functions and his or her perceived well-being across the three domains of health: physical, social, and emotional.37 Although there are many studies of HRQOL after thoracic surgery, the focus is on the general impact of surgery on HRQOL, not specifically on the impact of pain on these domains. Commonly, HRQOL is evaluated using patient-reported outcome measures. A patient-reported outcome measure is any report of health status that comes directly from the patient without interpretation of the response by a clinician.38 Studies of HRQOL most often use the Medical Outcomes Short Form-36 (SF36)39,40 and the European Organization for Research and Treatment in Cancer (EORTC) core questionnaire (QLQ-C30)41 with an optional lung cancer module, the LC13.42 Although well-validated and useful for patients undergoing lung cancer surgery, neither is focused on pain and its impact on HRQOL after surgery. Indeed, the SF36 has two questions: intensity of pain; and extent pain interfered with normal work. The EORTC-QLQ-C30 has one question about pain, and the LC13 has four questions, namely, during the past week, (1) have you had pain in your chest; (2) have you had pain in your arm or shoulder; (3) have you had pain in other parts of your body; and (4) did you take any medicine for pain, and if yes, how much did it help?
A more comprehensive evaluation of pain after thoracic surgery is necessary to better understand the intensity and quality of pain, and more importantly, its impact on function and HRQOL. Only one study has specifically investigated the impact of chronic pain after thoracotomy on HRQOL.43 Assessment of HRQOL using the SF36 was compared between patients with and patients without postthoracotomy pain 3 months after surgery; patients with chronic pain had significantly decreased physical functioning and vitality despite the majority of patients reporting mild pain.43 The major drawback is that HRQOL comparisons were made between patients with and patients without pain rather than comparing baseline and postoperative pain and HRQOL for each patient. A patient-centered approach to evaluating these outcomes involves collecting longitudinal data, including baseline data before surgery, to determine the impact of pain on HRQOL for each patient.
Opioid Use Definitions
Several leading research groups that have characterized opioid use after surgery have used the same definitions to allow for comparisons between cohorts.7–9,44 Opioid-naïve is defined as no opioid prescription filled between 12 months and 31 days before surgery. New persistent opioid use is defined as an opioid-naïve patient who filled an opioid prescription attributed to surgery and filled at least one additional opioid prescription between 91 and 180 days after surgery. The 30-day window before surgery allows for opioid prescriptions filled immediately before surgery intended for postoperative use.
Preoperative Opioid Use
A large cross-sectional study demonstrated that nearly 1 of every 4 patients, 23%, presenting for surgery at an academic tertiary referral center was receiving opioids before elective surgery.10 Interestingly, preoperative opioid use was least common among patients undergoing thoracic procedures, 15%. Independent predictors of preoperative opioid use pertinent to patients undergoing thoracic surgery were tobacco use (current use, odds ratio [OR] 1.62; and former user, OR 1.32), depression (OR 1.22), American Society of Anesthesiologists score (OR 1.47), and Charlson Comorbidity Index (OR 1.29).10
Opioid-Naïve Patients
Among patients undergoing elective surgery, approximately 50% used opioids after discharge (one or more opioid prescriptions within 1 to 90 days after surgery) and only 3.1% had prolonged use (one or more opioid prescriptions within 1 to 90 days after surgery along with one or more within 91 to 180 days).8 Patients undergoing either open or minimally invasive thoracic surgery had the highest rate of prolonged opioid use, 8.5% and 6.3%, respectively.
Among patients undergoing lung cancer resection, 14% to 18% of opioid-naïve patients continued to fill opioid prescriptions 90 to 180 days after surgery.7,9,45 Three months after surgery, patients who had new persistent opioid use had a daily opioid dose equivalent to six tablets of hydrocodone 5 mg daily.9 Interestingly, this daily opioid dose was similar to patients who had used opioids chronically or intermittently before surgery, suggesting that patients with new persistent opioid use may transition to chronic opioid use.9
Risk Factors for New Persistent Opioid Use
Several risk factors for new persistent opioid use after thoracic surgery have been identified (Figure 1). Younger age is associated with an increased risk of prolonged opioid use.7,8 This agrees with prior work identifying younger age as a predictor of severe acute postoperative pain.46 Men are approximately 30% to 40% more likely to become new persistent opioid users after lung cancer resection.7,9 Patients with a substance abuse disorder are 1.3 times more likely to become new persistent opioid users.9 Not surprising, patients undergoing thoracotomy are at double the risk of new persistent opioid use compared with patients undergoing VATS.7
Patients undergoing curative intent surgery for cancer have a higher rate of new persistent opioid use than patients undergoing noncancer surgery, 10% vs 6% to 8%.9 Patients who have adjuvant therapy, either chemotherapy or radiation therapy, are two times more likely to be new persistent opioid users compared with patients who do not need adjuvant therapy and this is the strongest risk factor for persistent opioid use after lung cancer resection.7,9 The researchers hypothesized that psychological, emotional, and physical challenges associated with adjuvant therapy may exacerbate pain experienced by the patient after surgery. Interestingly, neoadjuvant therapy was not a risk factor for persistent opioid use after lung cancer resection.7
In a large cohort study of patients aged more than 65 years who were undergoing major elective surgery (including thoracic surgical patients), pulmonary disease (OR 1.53), heart failure (OR 1.32), and diabetes mellitus (OR 1.15) were independent predictors of prolonged opioid use.8 In addition, preoperative use of angiotensin-converting enzyme inhibitors (OR 1.26), a marker of heart failure, was independently associated with prolonged opioid use. Preoperative use of benzodiazepines and selective serotonin reuptake inhibitors was also an independent predictor of prolonged opioid use.8
Opioid Prescribing Patterns
Postoperative opioid prescribing practices of surgical teams are one contributor to new persistent opioid use. The proportion of prescriptions for patients undergoing surgery increased by 18%, and the mean oral morphine equivalent (OME) per prescription increased from 240 mg to 403 mg, from 2010 to 2016.47 In a systematic review of patients who underwent thoracic, orthopedic, obstetric, and general surgical operations, 67% to 92% of patients reported having unused opioids.48 There were some who did not fill the prescription (0% to 21%) whereas others filled it but did not take any opioids (7% to 14%). Overall, 42% to 71% of the pills prescribed after surgery went unused, with the top two reasons being adequate pain control and concern for side effects.48
In a retrospective study of outpatient general surgical operations, there was wide variation in the number of opioid pills prescribed both by operation and by provider.49 Follow-up surveys revealed that only 28.7% of the opioid pills prescribed were taken, indicating that 71.3% excess pills were prescribed.49 Similarly, in a statewide study of patients undergoing general and gynecologic operations, median consumption of opioids was 27% of the prescribed amount; patients were prescribed 150 OME (30 pills of hydrocodone/acetaminophen 5.325 mg), but used a median of 9 pills.50 Furthermore, 24% took no opioids after surgery, and only 22% used all pills. Most importantly, quantity of opioid prescribed had the strongest association with postoperative consumption: for every 10 additional opioid pills prescribed, patients used 5.3 more pills.50 Lastly, patients who had moderate pain in the week after surgery used an additional 45 OME (9 pills), and patients who had severe pain used an additional 82 OME (16 pills) compared with patients with no pain; patients with minimal pain used a similar amount.50
These data suggest that a dual approach consisting of improving pain control in the first week after surgery and tailoring prescription practices has the potential to decrease opioid use after surgery. Opioid naïve patients undergoing cardiothoracic surgery (coronary artery bypass graft, aortic valve replacement, and lobectomy) who were prescribed more than 465 mg OME at discharge were more likely to become new persistent opioid users.51 In a small, but informative survey of patients who had undergone thoracic surgery, 25% of patients took approximately half of the opioid pills prescribed, 32% took very few pills (5 or fewer), and 13% took none.52 Among patients who did not take all the prescribed pills, 70% had pain well controlled, 29% reported side effects, and 8% were concerned for becoming addicted.52 Ideally, thoracic surgeons should collect multicenter data on whether patients are using the opioids prescribed, and if not, why. Although there are emerging data on opioid prescribing patterns for patients undergoing general surgery, more research is necessary to better understand prescribing patterns for patients undergoing thoracic surgery.
Prescribing Guidelines
Attention is on clinicians as opioids are obtained by either prescription or diversion. Overall, opioid prescribing has increased dramatically: in 2012, 82.5 opioid prescriptions were written per every 100 persons, which had quadrupled compared with 1999.53 In a study of a prescription drug monitoring program in San Diego County, surgeons accounted for 10% of the total opioid prescriptions, and 91.6% of all prescriptions written by surgeons were for opioids.54 Clearly, there is room for improvement with regard to postoperative opioid prescribing. Several research groups are investigating the number of opioids needed by operation and are using this information to inform the development of prescribing guidelines.
Researchers in Michigan discovered that patients undergoing laparoscopic cholecystectomy received a median of 250 mg OME, but median use was 30 mg.14 They then used these data to develop a prescribing guideline that recommended 15 tablets hydrocodone/acetaminophen 5/325 mg (OME 75 mg) or 15 tablets oxycodone 5 mg (OME 112.5 mg) plus acetaminophen or ibuprofen as needed. After implementing these guidelines, the median amount of opioid prescribed decreased from 250 mg to 75 mg, with only 2.5% of patients requesting refills (similar to earlier requests); there was also no difference in median pain level during the first week after surgery.14
Similarly, researchers at Dartmouth educated surgeons on advising patients to use nonsteroidal antiinflammatory drugs and acetaminophen and provided recommendations on the number of opioid pills to prescribe according to general surgery operation.12 After educating the surgeons, the number of pills and variability in prescribing decreased significantly. They estimated that 6170 pills would have been prescribed to 224 patients before the educational intervention, but after, only 2932 pills were prescribed, a 53% decrease.12 Impressively, only 1 patient requested a prescription refill. This same group studied opioid use in patients undergoing inpatient general surgical operations and determined that 85% of patients’ outpatient opioid requirements would be met following a guideline based on the number of opioid pills (based on oxycodone 5 mg tablet) taken the day before discharge.13 For patients who used no opioids on the day before discharge, no prescription is needed; for patients who took 1 to 3 pills, 15 pills should be prescribed; and for patients who took 4 or more pills, 30 pills should be prescribed.13
These findings demonstrate that surgeons can have a significant impact on the national opioid epidemic by reducing the number of opioids that are prescribed. These studies developed prescribing guidelines based on the amount of opioid pills needed to satisfy approximately 80% of the patients.12,13 Further investigation is needed to determine whether a more patient-centered prescribing approach is feasible. For example, Hill and colleagues13 found that patients aged 60 years or more took fewer opioid pills than younger patients. Furthermore, all of these studies excluded patients with chronic pain already taking opioids before surgery and patients with postoperative complications. Additional research is also needed to determine how best to prescribe opioids to patients who may need them most. Also, minimal patient-reported outcome measures were collected and, therefore, details on pain control from the patient’s perspective are incompletely determined. Lastly, these studies only included patients undergoing general surgical operations; they serve as an example of research needed for thoracic surgery. Although new persistent opioid use after thoracic surgery is both a public health and an individual patient issue, additional work investigating both chronic post-surgical pain and new persistent opioid use is important to determine how these two primary outcomes of interest interact.
Preoperative Expectation Setting and Education
Setting realistic expectations before surgery and educating patients on postoperative pain and pain management have an impact on a patient’s postoperative recovery. Educational initiatives can change patient behavior, empower patients to actively participate in their own care, and improve clinical outcomes.55 In addition, patients who receive structured educational interventions are more likely to follow through with their recommended therapy compared with patients who receive unstructured education.56 These concepts are not new. In 1964, the New England Journal of Medicine published a randomized controlled trial of patients undergoing major abdominal surgery.57 The night before surgery, patients in the intervention arm were informed about postoperative pain including duration, severity, and management of it by the anesthetist. They were also taught breathing and mobility techniques to control postoperative pain. On postoperative day 0, there was no difference in narcotic use between the two groups, but on each of the next 5 postoperative days, patients in the intervention arm used half the amount of narcotics and went home an average of 2.7 days sooner than patients in the control group.57
Patients want more information about recovery after thoracic surgery and how to return to their baseline health after surgery.58 They often feel well informed before surgery but after discharge, feel less so regarding recovery and how to handle issues.58 There are discrepancies between the information health care professionals provide to patients before surgery and the information patients want.56 One study indicated that although pain and its management were discussed by most health care providers before surgery, patients indicated that the information was clear but not helpful.56 After discharge to home, patients wished that they had been better informed regarding pain before their surgery. That implies a disconnect in communication and expectations between surgeons and patients before surgery. One way to mitigate that is to solicit input from patients regarding what information they would find most relevant at different times throughout the perioperative period. For patient education to achieve the goal of empowering through knowledge and improving outcomes, information has to be perceived as relevant by the patient.56
Developing a preoperative intervention for expectation setting and education goes beyond simply providing information to patients and their caregivers. Identifying modifiable psychosocial factors that affect HRQOL is critical to the success of any preoperative intervention.59 Two such factors are social support and self-efficacy. Social support encompasses several types of support including instrumental, informational, emotional, and companionship. Self-efficacy reflects self-confidence in one’s ability to carry out a behavior necessary to reach a desired goal. Additional research is needed to determine to what degree these factors affect HRQOL outcomes after lung cancer resection, and if so, how to leverage them in the perioperative period. Moreover, it has been demonstrated that social support prompts self-efficacy.59 In other words, patients with social support are more likely to feel empowered to care for themselves.
A newer paradigm for perioperative education and expectation setting is patient centered and relies on patient activation, which is a “patient’s willingness and ability to take independent actions to manage their health and care.”60 An example of this is a self-management program. Traditional patient education provides information but self-management programs increase patient activation and self-efficacy.61 Internet-based self-management programs have demonstrated significantly reduced pain and improved physical functioning in patients with fibromyalgia compared with patients receiving standard care.62 A comprehensive perioperative self-management program was developed and piloted for patients undergoing lung cancer resection and their family caregivers.63 Knowledge of the operation and emotional quality of life was improved in the intervention group.63 Importantly, the investigators developed the intervention and refined it with input from patients and their familial caregivers.
In conclusion, chronic pain and new persistent opioid use after thoracic surgery are important current problems, with research into each of these but minimal studies focusing on how the two interact. Equally important, we need to know more about how chronic pain affects HRQOL and physical function after thoracic surgery. Additional research is needed to determine how best to set expectations and provide information regarding expected pain burden, multimodal analgesia, and appropriate opioid use and risks to patients before surgery. Patient education should be taken one step further. Surgeons should ensure that patients become more knowledgeable about expected recovery and how to manage pain so that they can better engage in their own care. Once we have the answers to these questions, we will better understand how to implement a patient-centered approach to perioperative care.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award KL2 TR001859. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. 2019; Available at: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed December 1, 2019.
- 2.Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15:887–897. [DOI] [PubMed] [Google Scholar]
- 3.Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126:938–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macrae W. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- 5.Werner M, Kongsgaard U. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113:1–4. [DOI] [PubMed] [Google Scholar]
- 6.Bayman EO, Parekh KR, Keech J, Larson N, Vander Weg M, Brennan TJ. Preoperative patient expectations of postoperative pain are associated with moderate to severe acute pain after VATS. Pain Med. 2019;20:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brescia AA, Harrington CA, Mazurek AA, et al. Factors associated with new persistent opioid usage after lung resection. Ann Thorac Surg. 2019;107:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke H, Soneji N, Ko DT. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ Open. 2014;1251:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35:4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilliard PE, Waljee J, Moser S, et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery. JAMA Surg. 2018;153:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larach DB, Sahara MJ, As-Sanie S, et al. Patient factors associated with opioid consumption in the month following major surgery [e-pub ahead of print]. Ann Surg. 10.1097/SLA.0000000000003509, accessed September 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth R. An educational intervention decreases opioid prescribing after general surgical operation. Ann Surg. 2018;267:468–472. [DOI] [PubMed] [Google Scholar]
- 13.Hill MV, Stucke RS, Barth R, Billmeier SE, Kelly JL. Guideline for discharge opioid prescriptions after inpatient general surgical procedures. J Am Coll Surg. 2018;226:996–1003. [DOI] [PubMed] [Google Scholar]
- 14.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg. 2018;153:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. [DOI] [PubMed] [Google Scholar]
- 16.Macrae W, Davies H. Chronic postsurgical pain. In: Crombie I, Linton S, Von Korff M, LeResche L, eds. Epidemiology of Pain. Seattle, WA: IASP Press; 1999:125–142. [Google Scholar]
- 17.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:800–805. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Jackson M, Kavanagh B, Sandler A. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–55. [DOI] [PubMed] [Google Scholar]
- 19.Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–844. [DOI] [PubMed] [Google Scholar]
- 20.Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen TS, Campbell JN, Cruccu G, et al. Neuropathic pain: redefinition and a grading system for clinical and research. Neurology. 2008;70:1630–1635. [DOI] [PubMed] [Google Scholar]
- 22.Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guastella V, Mick G, Soriano C, et al. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain. 2011;152:74–81. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One. 2014;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki T, Sakai T, Tsuchiya T, Yamasaki N. Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur J Cardiothorac Surg. 2011;39:1033–1039. [DOI] [PubMed] [Google Scholar]
- 26.Maguire MF, Latter JA, Mahajan R, Beggs FD, Duffy JP. A study exploring the role of intercostal nerve damage in chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:873–879. [DOI] [PubMed] [Google Scholar]
- 27.Cerfolio RJ, Bryant AS, Patel B, Bartolucci AA. Intercostal muscle flap reduces the pain of thoracotomy: a prospective randomized trial. J Thorac Cardiovasc Surg. 2003;130:987–993. [DOI] [PubMed] [Google Scholar]
- 28.Cerfolio RJ, Price TN, Bryant AS, Bass CS, Bartolucci AA. Intracostal sutures decrease the pain of thoracotomy. Ann Thorac Surg. 2003;76:407–412. [DOI] [PubMed] [Google Scholar]
- 29.Visagan R, Mccormack DJ, Shipolini AR, Jarral OA. Are intracostal sutures better than pericostal sutures for closing a thoracotomy? Interact Cardiovasc Thorac Surg. 2012;14:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brummett CM, Clauw DJ. Fibromyalgia: a primer for the anesthesia community. Curr Opin Anesthesiol. 2011;24:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty. Anesthesiology. 2013;119:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women. Anesthesiology. 2015;122:1103–1111. [DOI] [PubMed] [Google Scholar]
- 35.Hooten WM, Brummett CM, Sullivan MD, et al. A conceptual framework for understanding unintended prolonged opioid use. Mayo Clin Proc. 2017;92:1822–1830. [DOI] [PubMed] [Google Scholar]
- 36.Clauw D. Hijacking the endogenous opioid system to treat pain: who thought it would be so complicated? Pain. 2017;158:2283–2284. [DOI] [PubMed] [Google Scholar]
- 37.Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34:645–649. [DOI] [PubMed] [Google Scholar]
- 38.Food and Drug Administration. Guidance for industry use in medical product development to support labeling claims guidance for industry. Fed Regist. 2009;74:65132–65133. [Google Scholar]
- 39.Ware J, Sherbourne C. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 40.Scoggins JF, Patrick DL. The use of patient-reported outcomes instruments in registered clinical trials: evidence from clinicaltrials.gov. Contemp Clin Trials. 2009;30:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 42.Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer. 1994;30:635–642. [DOI] [PubMed] [Google Scholar]
- 43.Kinney MAO, Hooten WM, Cassivi SD, et al. Chronic postthoracotomy pain and health-related quality of life. Ann Thorac Surg. 2012;93:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2019;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson DB, Cata JP, Niu J, et al. Persistent opioid use is associated with worse survival after lobectomy for stage I non-small cell lung cancer. Pain. 2019;160:2365–2373. [DOI] [PubMed] [Google Scholar]
- 46.Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KGM. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–423. [DOI] [PubMed] [Google Scholar]
- 47.Larach DB, Waljee JF, Hu H-M, et al. Patterns of initial opioid prescribing to opioid-naive patients. Ann Surg. 2020;271:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bicket M, Long J, Pronovost P, Alexander G, Wu C. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill M, McMahon M, Stucke R, Barth R. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 50.Howard R, Fry B, Gunaseelan V, et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. 2019;154:e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown CR, Khurshan FN, Chen Z, Bavaria JE, Groenveld P, Desai ND. Development of opioid dependence after cardiothoracic surgery: are we to blame? Paper presented at: 99th Annual Meeting of the American Association for Thoracic Surgery. May 4-7, 2019; Toronto, Ontario, Canada. Available at: https://www.aats.org/aatsimis/AATSWeb/Association/Meetings/Annual_Meeting/99th_Annual_Meeting/AATS_99th_Annual_Meeting_Abstracts/2019-a-1050-AATS.ASPX. Accessed June 23, 2019. [Google Scholar]
- 52.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11:e014972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paulozzi LJ, Mack KA, Hockenberry JM. Variation among states in prescribing of opioid pain relievers and benzodiazepines—United States 2012. J Safety Res. 2014;51:125–129. [DOI] [PubMed] [Google Scholar]
- 54.Lev R, Lee O, Petro S, et al. Who is prescribing controlled medications to patients who die of prescription drug abuse? Am J Emerg Med. 2016;34:30–35. [DOI] [PubMed] [Google Scholar]
- 55.Soffin EM, Waldman SA, Stack RJ, Liguori GA. An evidence-based approach to the prescription opioid epidemic in orthopedic surgery. Anesth Analg. 2017;125:1704–1713. [DOI] [PubMed] [Google Scholar]
- 56.King J, Chamberland P, Rawji A, et al. Patient educational needs of patients undergoing surgery for lung cancer. J Cancer Educ. 2014;29:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egbert L, Battit G, Welch C, Bartlett M. Reduction of postoperative pain by encouragement and instruction of patients. N Engl J Med. 1964;270:825–827. [DOI] [PubMed] [Google Scholar]
- 58.Oswald N, Hardman J, Kerr A, et al. Patients want more information after surgery: a prospective audit of satisfaction with perioperative information in lung cancer surgery. J Cardiothorac Surg. 2018;13:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banik A, Luszczynska A, Pawlowska I, Cieslak R, Knoll N, Scholz U. Enabling, not cultivating: received social support and self-efficacy explain quality of life after lung cancer surgery. Ann Behav Med. 2017;15:1–12. [DOI] [PubMed] [Google Scholar]
- 60.Hibbard BJH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff. 2013;32:207–214. [DOI] [PubMed] [Google Scholar]
- 61.Sun V, Kim JY, Raz DJ, et al. Preparing cancer patients and family caregivers for lung surgery: development of a multimedia self-management intervention. J Cancer Educ. 2018;33:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DJ. Internet-enhanced management of fibromyalgia: a randomized controlled trial. Pain. 2010;151:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun V, Raz DJ, Ruel N, et al. A Multimedia self-management intervention to prepare cancer patients and family caregivers for lung surgery and postoperative recovery. Clin Lung Cancer. 2017;18:e151–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]


