Abstract
Dietary specializations in animals lead to adaptations in morphology, anatomy and physiology. Neotropical bats, with their high taxonomic and trophic diversity, offer a unique perspective on diet-driven evolutionary adaptations. Here we assess the metabolic response to different dietary sugars among wild-caught bats. We found that insectivorous bats had a pronounced metabolic response to trehalose, whereas bats with nectar and fruit-based diets showed significantly higher blood glucose levels in response to glucose and sucrose, reaching levels over 750 mg dl−1. The genomic analysis of 22 focal species and two outgroup species identified positive selection for the digestive enzyme trehalase in insect eaters, while sucrase–isomaltase showed selection in lineages with omnivorous and nectar diets. By examining anatomical and cellular features of the small intestine, we discovered that dietary sugar proportion strongly impacted numerous digestive traits, providing valuable insight into the physiological implications of molecular adaptations. Using hybridization chain reaction (HCR) RNA fluorescence in situ hybridization, we observed unusually high expression in the glucose transporter gene Slc2a2 in nectar bats, while fruit bats increased levels of Slc5a1 and Slc2a5. Overall, this study highlights the intricate interplay between molecular, morphological and physiological aspects of diet evolution, offering new insights into the mechanisms of dietary diversification and sugar assimilation in mammals.
Subject terms: Ecology, Genetics
An analysis of Neotropical bats with different diets reveals molecular and physiological mechanisms of dietary diversification and sugar assimilation.
Main
Organisms adapt to their environments to improve their chances of survival and reproductive success. Throughout animal evolution, diet is a major environmental input that influences adaptations in nutrient acquisition, such as feeding morphology and behaviour1–8. Changes in metabolism underlie these diet-related evolutionary variations, affecting the ability of cells and tissues to sense and respond to altered nutrient availability. This ability is crucial for maintaining energy homoeostasis and overall health9–13.
Glucose homoeostasis is a tightly regulated biochemical pathway that maintains circulating glucose levels within a narrow physiological range for cellular function and organismal energy. Blood glucose levels fluctuate throughout the day, are easily measured and relate to lifestyle and diet14. This pathway is expected to have a critical role in metabolic adaptations15. However, wildlife studies investigating these adaptations are scarce and include few taxa16–20. Existing research is primarily focused on accessible lab populations of wild animals21–25 or restricted within the context of metabolic disorders26,27.
Natural systems exhibiting a wide diversity of diets, such as in bats, provide a unique opportunity to investigate diet-related evolutionary changes. Bats have diversified from an insect-heavy ancestral diet to nutritional sources including fruit, nectar, meat and fish, among others28,29. The dietary shifts among bats reflect the evolutionary changes across major clades of mammals30,31, such as herbivores, omnivores and carnivores (that is, orders Artiodactyla, Primates and Carnivora). Therefore, investigating their adaptive radiation is expected to provide new perspectives on the diversification of metabolic traits and emphasize the molecular basis of these adaptations.
In this study, we explore the metabolic adaptations involved in dietary changes over evolutionary time. We observe adaptation to the glucose homoeostasis pathway across 29 bat species with different diets, using three dietary sugars—trehalose (found in insects’ hemolymph), sucrose and glucose (both found in fruits and nectar). We demonstrate that the metabolic phenotype is mediated by four different adaptations to digestive morphology, including intestinal length, exposed villi and enterocyte and microvilli number. We have discovered genetic traits linked to maximal extraction of glucose energy from nutritional resources associated with diet. These include positive selection on genes encoding the digestive enzymes TREH, trehalase (Treh), and SI, sucrase–isomaltase (SI), as well as glucose transporters GLUTs, solute carrier family 2 members 1–5 (Slc2a1–5). We investigated the effects of these amino acid substitutions on protein function through structural comparisons. Additionally, we observed a change in the expression of genes encoding transporters SLC2A2 (Slc2a2 gene), SCL2A5 (Slc2a5 gene) and SLC5A1 (Slc5a1 gene) in response to an acute glucose meal within enterocytes along the brush border of the small intestine. The emerging picture illustrates the sophisticated adaptability of absorptive villi, highlighting the evolution of traits and mechanisms that enable species to utilize a broader range of nutrient resources compared with those found ancestrally. Overall, this study advances our understanding of how metabolic evolution contributes to species diversification.
Results and discussion
In vivo physiology
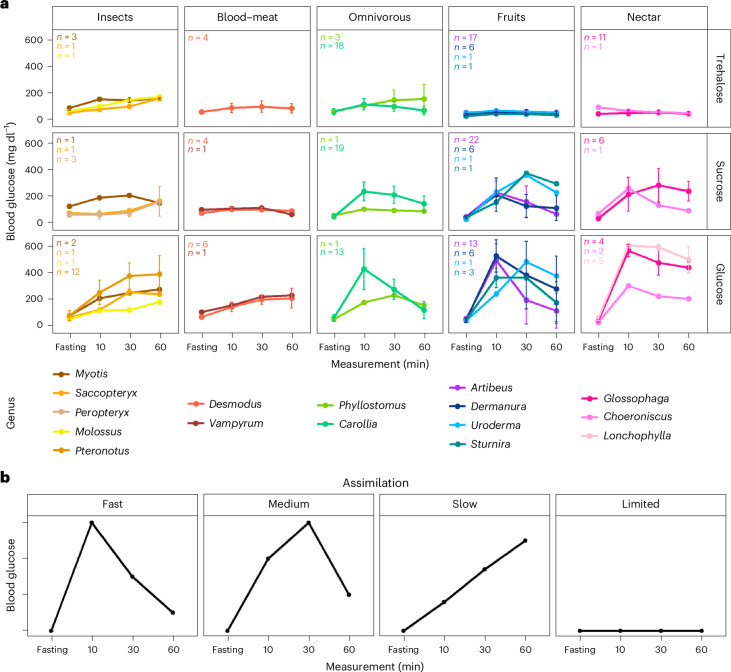
Bats provide a unique perspective on metabolic adaptations due to their high energetic demands and diverse diets. They have specialized from an ancestor with an insect-heavy diet to fruit, nectar, meat, blood and other food sources28. To assess metabolic adaptations related to dietary diversification, we focused on the glucose homoeostasis pathway, which regulates glucose energy between meals and is crucial for health and survival. We performed in vivo oral glucose tolerance tests on 199 wild-caught bats across 29 species (Extended Data Table 1) to assess their ability to process three dietary sugars32,33: trehalose (formed by two glucose molecules), sucrose (formed by glucose and fructose) and glucose. Wild-caught bats were fasted for 10–12 h, and blood glucose levels were measured before and after consuming a single sugar meal (5.4 g kg−1(ref. 22)). Measurements were taken at 10, 30 and 60 min post-ingestion, revealing distinct patterns in the rise and fall of blood glucose levels between dietary guilds (Fig. 1a). To better quantify physiological responses, we classified them into four assimilation patterns, ‘fast’, ‘medium’, ‘slow’ and ‘limited’ (Fig. 1b), on the basis of the speed and extent of assimilation (see ‘General patterns of sugar assimilation curves’ section in Methods).
Extended Data Table 1.
Glucose tolerance test with different sugars

Species included in the in vivo physiology essay. A. lit: Artibeus lituratus, A. pla: Artibeus planirostris, A. aeq: Artibeus aequatorialis, D. pha: Dermanura phaeotis, U. con: Uroderma convexum, U. bak: Uroderma bakeri, P. hel: Platyrrhinus helleri, S. gia: Sturnira giannae, S. lud: Sturnira ludovici, S. lui: Sturnira luisi, S. par: Sturnira parvidens, G. sp.: Glossophaga sp., C. god: Choeroniscu godmani, L. con: Lonchophylla concava, L. rob: Lonchophylla robusta, C. cas: carollia castanea, C. per: Carollia perspicillata, C. bre: Carollia brevicauda, P. dis: Phyllostomus discolor, P. has: Phyllostomus hastatus, V. spe: Vampyrum spectrum, D. rot: Desmodus rotundus, G. cre: Gardnerycteris crenulatum, M. cau: Myotis caucensis, M. alb: Myotis albescens, M. mol: Molossus molossus, S. bil: Saccopteryx billineata, P. kap: Peropteryx kappleri.
Fig. 1. Glucose tolerance tests for three different dietary sugars.
a, Average assimilation curves for trehalose, sucrose and glucose among Neotropical bats with different food preferences: insects, blood or meat, mixed (omnivorous), fruits and nectar. The sample size varied among genera from 1 to 52 individuals (Extended Data Table 1). The data are presented as mean values ± standard deviation for a sample size greater than three individuals. b, General curves to describe the temporal pattern of sugar assimilation.
In insectivorous and omnivorous bats, we observed trehalose assimilation with blood glucose levels reaching up to 160 mg dl−1with a ‘slow’ tolerance curve indicating glucose absorption continuing until 60 min. In contrast, bats with nectar and fruit diets exhibited a ‘limited’ ability to assimilate trehalose, showing blood glucose levels remaining within a narrow range and only rising to 60 mg dl−1. These findings align with existing evidence that the trehalase gene (Treh) is non-functional in non-insectivorous mammals, including bats, and functional in omnivores34,35, such as Carollia and Phyllostomus. Interestingly, vampire bats showed a slight rise and fall in blood glucose levels following trehalose consumption, reaching levels of 90 mg dl−1. We propose that the minor elevation in blood glucose levels in non-insectivorous bats may be linked to gut microbiome activity36,37, as trace amounts of trehalase were found in their gut34.
When tested for sucrose, insect-feeding bats showed a gradual increase in blood glucose levels 30 min post-ingestion, except for Myotis, which showed a rapid rise to 200 mg dl−1 within 10 min, followed by a decline to 140 mg dl−1 at 60 min, indicating a ‘medium’ sucrose assimilation. This suggests that Myotis might be capable of sucrose digestion more efficiently than trehalose despite its classification as an insect feeder. As expected, vampire bats showed ‘limited’ sucrose assimilation, maintaining blood glucose levels below 96 mg dl−1 throughout the experiment. Among omnivores, Carollia levels peaked at 10 min (200 mg dl−1) and gradually declined, whereas Phyllostomus had a lower peak at 10 min (100 mg dl−1) and slowly decreased. These patterns reflect the varying degrees of omnivory, where Carollia mainly feed on piper fruits and some insects, while Phyllostomus consume insects, fruits, small vertebrates, flowers, nectar and pollen38,39. Fruit and nectar bats exhibited two major responses to sucrose: (1) ‘fast’ assimilation with a rapid rise in blood glucose (200 mg dl−1) within 10 min and rapid decrease to basal levels after 60 min for Artibeus, Dermanura and Choeroniscus and (2) ‘medium’ assimilation with a slow increase, reaching a maximum after 30 min (300 mg dl−1) for Sturnira, Uroderma and Glossophaga. These findings highlight notable variations in sucrose assimilation across and within dietary categories, suggesting diversified disaccharide assimilation mechanisms and potential seasonal impacts, meriting further study, especially for omnivorous bats.
When tested for glucose ingestion, we observed higher blood glucose levels across all bat species compared with trehalose and sucrose feedings, due to direct intestinal absorption of monosaccharides, unlike disaccharides, which require prior hydrolysis30. Bats with insect, meat and blood diets showed a gradual rise in blood glucose levels, staying under 300 mg dl−1, except in Pteronotus (> 350 mg dl−1) but still indicating a ‘slow’ assimilation pattern. Among omnivorous bats, Carollia peaked at 423 mg dl−1 at 10 min before rapidly declining by 60 min, while Phyllostomus reached a peak of 225 mg dl−1 at 30 min and declined by 60 min. Fruit bats, such as Artibeus and Dermanura, exhibited the most extreme rise and fall of blood glucose levels, reaching 600 mg dl−1 within 10 min and dropping to 100 mg dl−1 in some individuals after 60 min. This ‘fast’ assimilation curve is probably due to heightened insulin sensitivity in these species40. Other fruit bats showed high glucose levels within 30 min, with Uroderma reaching 476 mg dl−1 and Sturnira reaching 357 mg dl−1, followed by a subsequent decrease. In nectarivores, we observed ‘fast’ assimilation curves, with Glossophaga and Lonchophylla reaching levels above the detection limit of 600 mg dl−1 (GlucoQuick G30a) at 10 min and Choeroniscus reaching 300 mg dl−1 at the 10-min timepoint, probably influenced by varying plant and nectar preferences41,42. In contrast, captive Glossophaga soricina individuals reached a peak of 360 mg dl−1 after 30 min when fed with a similar single dose and did not exceed 470 mg dl−1, even with higher doses (9 g kg−1)22. Nectar bats from Glossophaginae and Lonchophyllinae subfamilies showed slower declines in blood glucose levels compared with fruit bats, aligning with studies suggesting glucose regulation through exercise rather than insulin response22,43–46. These findings highlight how sugar assimilation capability in Neotropical bats reflects their natural food preferences, raising questions about their ability to manage blood glucose fluctuations.
To evaluate the impact of evolutionary history on sugar assimilation, we employed Pagel’s λ (ref. 47) to measure the phylogenetic signal for each assimilation pattern with the area under the glucose tolerance test curve as a proxy. We obtained λ values of 0.99 for sucrose assimilation (P < 0.05) (Extended Data Fig. 2). However, glucose and trehalose did not show a phylogenetic signal. These results suggest that phylogenetic relationships are probably playing an important role in determining sucrose assimilation but not in the monosaccharide or trehalose assimilation, which is probably why we see most variation among species in response to glucose and the higher response to trehalose in insectivorous and omnivorous bats even when they are not closely related. This observation is consistent with a prior study that examined traits related to digestion and found that the gut surface area, body mass and digestion is not constrained by phylogeny48. Additionally, we fitted a Bayesian multi-level phylogenetic model to compare adaptations in glucose assimilation while controlling for phylogenetic relatedness. We found differences in the assimilation pattern of glucose, sucrose and trehalose between species with high- and low-sugar diets. However, the omnivorous bats, with the three sugars present in their diets, showed similar glucose and sucrose assimilation patterns to bats with rich glucose and sucrose diets (fruit and nectar bats) and similar trehalose assimilation patterns to bats with rich trehalose diets (insectivorous). This indicates a strong association between assimilation patterns and dietary preferences.
Extended Data Fig. 2. Alphafold protein predictions and foldseek comparisons.
Ribbon representation of AlphaFold modeled proteins, viewed in the plane of the membrane, from positive selected genes. The human reference protein structure has functional protein features highlighted using PyMOL for protein orientation as follows for 1) SI: P-type 1 and 2 are colored in magenta, the binding sites for sugar are colored in green, and the known mutations that affect function are colored in red (a-b); and 2) GLUTs: transmembrane (TM) 1 and 4 in the N-terminal domain are colored in cyan and light cyan, respectively; TM 7 and 10 in the C-terminal bundle are colored in cyan and light cyan, respectively; intracellular domain helices (ICH) unique to the sugar transporters are shown in yellow (c-e); single amino acid change (R238C) in the human GLUT5 model relates to colon cancer (Warburg effect, Tate et al. 2019). For each GLUT transporter shown, the C-terminal transmembrane (TM) bundle is located on the left and the N-terminal TM bundle is located on the right. The C- and N- terminal TM bundles transport glucose with a rocker-switch-type movement1. TM7 and TM10 support a gated-pore mechanism for glucose binding and release. The intracellular helices (ICH) domain provides stabilization for conformational changes. Foldseek structural comparisons between pairs of species for a-b) SI; c) GLUT2; d) GLUT3; and e) GLUT5. Each protein comparison is based on genes undergoing positive selection and is phylogenetically informed (f). Observed structural changes are summarized as a TM-score (TM-score = 1 is a perfect structural match). Arrows highlight structural changes in SI that relate to enzymatic function and in GLUTs where glucose binds extracellularly (ext) and where structural changes might affect substrate transport (int). (a) Folkseek comparisons for sucrase-isomaltase (SI) of vesper bats in the genus Myotis and Eptesicus. We observe global differences among Mytois species in the sucrase subunit’s P-type 2 domain, as well as the isomaltase subunit P-type 1 domain. SI activity relies on attachment to the gastrointestinal mucosa’s plasma membrane, facilitated by the isomaltase subunit’s P-type 1 domain. Further, changes along the alpha and beta strands of both subunits have been known to cause dissociation of SI from the membrane and a single amino acid change in the second helical domain of the isomaltase subunit is sufficient to cause dissociation of SI from the membrane and the inability to process sucrose. It is possible that the changes in Myotis, compared to other insect-feeding bats like Eptesicus, provides the ability to process sugar, preventing them from having intestinal malabsorption and intestinal distress from undigested sugar. Greater differences in Myotis SI structure may reflect their ability to assimilate sucrose. (b) Folkseek comparisons for SI structure for Myotis, P. parnellii, P. hastatus, and A. caudifer. For the sugar-eating phyllostomid bats, we observe localized changes to the isomaltase subunit’s P-type 1 domain, which is in line with the fact that ingesting high-sugar sources is expected to increase sugar metabolism proteins, particularly SI expression. (c) We detected positive selection in Slc2a2 in the ancestral branch leading to nectarivores as well as in M. harrisonii. Compared to the outgroup species P. parnellii, we observe differences predicted to be along the extracellular N-terminal between TM bundles 4 and 5, which is usually highly conserved among sugar transporters. We also predicted differences along the C-terminal end of the ICH5 domain, which undergoes post-translational modification for stability along the cell surface of pancreatic beta cells. d) GLUT3 predicted structure and Foldseek comparisons between nectar bat A. caudifer (gray) and P. parnellii (red); vampire bat D. rotundus (gray) and P. parnellii (red), and D. rotundus (gray) and A. caudifer (red). The amino acid substitutions are located along extracellular C-terminal transmembrane (TM) 10, where glucose binds, as well as within the plasma membrane, where it interacts with TM7 to coordinate glucose transport. We also identified changes at the intracellular side of the C-terminal domain, possibly affecting the degree of opening during glucose transport. This implies that the GLUT3 transporter may undergo modifications to glucose delivery to the brain’s neurons that may contribute to protection against their heightened susceptibility to fasting. e) Folkseek comparisons for GLUT5 predictions between Myotis species, the genus Myotis (gray) and Eptesicus (red), and the fruit bat S. hondurensis (gray) and insect bat M. blainvillei (red). We observe differences along the intracellular helices (ICH) domain 2-3, which anchor the conformational changes needed to transport glucose/fructose.
Molecular adaptations related to sugar assimilation
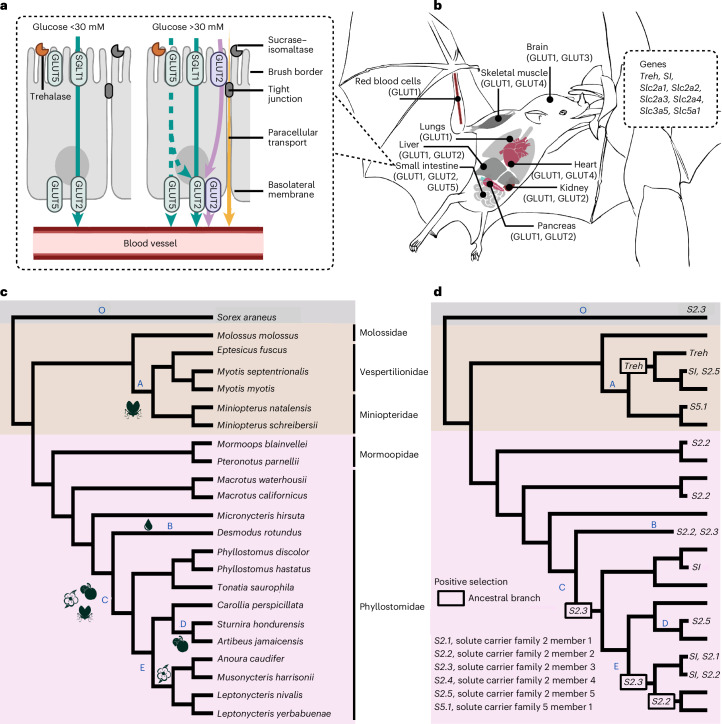
To identify molecular adaptations underlying the association between sugar assimilation and different diets, we tested genes involved in sugar digestion and transport for positive selection. We analysed genes encoding for the digestive enzyme TREH, trehalase (Treh), responsible for breaking down trehalose into glucose, and SI, sucrase–isomaltase (SI), which converts sucrose to glucose and fructose (Fig. 2a). We also examined genes encoding GLUTs, glucose transporters, involved in the absorption of these sugars from the intestinal lumen into the blood (Fig. 2a), including sodium/glucose co-transporter (Slc5a1), solute carrier family 2 member 2 (Slc2a2) and solute carrier family 2 member 5 (Slc2a5). Interestingly, while SLC2A5 functions as a transporter for fructose, it can also transport glucose49,50. Furthermore, transporters have crucial roles in directing circulating blood glucose into cells to fulfil tissue-specific energy demands51 (Fig. 2b). These genes include Slc2a1, predominantly found in red blood cells and astrocytes52; Slc2a2, enriched in the intestine, liver, hypothalamus and pancreatic beta cells53; Slc2a3, mainly in neurons54; Slc2a4, expressed in insulin-sensitive tissues such as cardiac muscle, skeletal muscle and fat55; and Slc2a5, abundant in microglia, liver, kidney and testis49.
Fig. 2. Molecular basis of sugar assimilation.
a, Dietary sugar assimilation begins in the small intestine, along the brush border of enterocytes, where dietary enzymes TREH, trehalase, and SI, sucrase–isomaltase, are located. Glucose transporters (SLC2A2/GLUT2 and SLC2A5/GLUT5), sodium-glucose co-transporter (SGLT1) and paracellular transport determine the rate of glucose absorption into the bloodstream under low (<30 mM) and high (>30 mM) glucose concentrations. While GLUT5 is primarily a fructose transporter, it has the capacity to transport glucose1,2. b, Glucose transporters move glucose from the bloodstream into specific tissues. The genes include: Treh, SI, Slc2a1, Slc2a2, Slc2a3, Slc2a4, Slc2a5 and Slc5a1. c, Species topology and foraging data follow Rojas et al. 2018. Insect-feeding branch leading to vesper and Miniopterus bats (A), blood-feeding (B), ancestral branch towards omnivores (C), obligate fruit eating (D) and nectarivore ancestral branch (E). d, Exploratory positive selection tests were performed using aBSREL with Holm–Bonferroni correction. Duodenal-enriched genes (P < 0.01) are shown across ancestral and extant Neotropical bats, using shrew as an outgroup (O).
We included 22 bats represented in our in vivo data (Fig. 2c), along with two outgroup species (Extended Data Table 3). Using adaptive branch-site random effects likelihood56 (aBSREL) with HyPhy57,58, we performed unbiased branch-site tests to identify episodic positive selection on orthologous gene sequences (Extended Data Table 4). While positive selection suggests advantageous genetic changes, it remains challenging to relate selection signatures to functional adaptation. In contrast, proteins possess distinct three-dimensional configurations intricately associated with their functions. Therefore, we modelled proteins from genes showing signatures of positive selection using Alphafold59 and performed phylogenetically informed pairwise comparisons with Foldseek60. We interpret the predicted structural changes in the protein based on known experimental evidence to suggest potential functional alterations resulting from positive selection in gene sequences.
Extended Data Table 3.
Exploratory aBSREL results
A) aBSREL (adaptive Branch-Site Random Effects Likelihood) tests whether a proportion of sites have evolved under positive selection along each branch in the phylogeny. Baseline model refers to MG94xREV baseline model that infers a single omega rate per branch. Full adaptive model infers an optimized number of omega rate categories per branch. After aBSREL fits the full adaptive model, the Likelihood Ratio Test (LRT) is performed at each branch and compares the full model to a null model where branches are not allowed to have rate classes of ω > 1. B) Detailed aBSREL results in bats. The results are from exploratory analysis where all branches are tested for positive selection. In this scenario, p-values at each branch must be corrected for multiple testing (using the Holm-Bonferroni correction). The Likelihood Ratio Test (LRT) is performed at each branch and compares the full model to a null model where branches are not allowed to have rate classes of ω > 1. Branches and nodes under selection correspond to Fig. 2d.
Extended Data Table 4.
Average blood glucose and gene expression changes
Change in blood glucose levels and change in gene expression 10-minutes after eating a 20% glucose solution. The fold change in gene expression was calculated from the mean log2 expression as follows: (μt=10/μt=0)-1. Each bat was fed a single dose of glucose (5.4 mg/kg body weight) after fasting (t = 0). Samples in bold were used for HCR FISH (Extended Data Fig. 4), while others were sampled non-lethally. The change in gene expression were calculated from the following values found in Extended Data Fig. 4: Carollia perspicillata average log2 gene expression for Slc5a1 μt=0 = 3.62 and μt=10 = 7.40; Slc2a2 μt=0 = 5.24 and μt=10 = 7.69; Slc2a5 μt=0 = 3.31 and μt=10 = 6.98. Glossophaga soricina average log2 gene expression for Slc5a1 μt=0 = 3.06 and μt=10 = 6.91; Slc2a2 μt=0 = 11.65 and μt=10 = 12.93; Slc2a5 μt=0 = 2.73 and μt=10 = 6.22. Pteronotus parnellii average log2 gene expression for Slc5a1 μt=0 = 2.61 and μt=10 = 7.35; Slc2a2 μt=0 = 4.15 and μt=10 = 8.58; Slc2a5 μt=0 = 1.95 and μt=10 = 7.03. Anoura geoffroyi average log2 gene expression for Slc5a1 μt=0 = 4.63 and μt=10 = 4.55; Slc2a2 μt=0 = 8.76 and μt=10 = 7.64; Slc2a5 μt=0 = 1.85 and μt=10 = 5.02. Phyllostomus discolor average log2 gene expression for Slc5a1 μt=10 = 5.75; Slc2a2 μt=10 = 8.14; Slc2a5 μt=10=no signal. Artibeus jamaicensis average log2 gene expression for Slc5a1 μt=0 = 5.58 and μt=10 = 6.12; Slc2a2 μt=0 = 5.13 and μt=10 = 6.93; Slc2a5 μt=0 = 5.32 and μt=10 = 6.99. Micronycteris minuta average log2 gene expression for Slc5a1 μt=60 = 3.84; Slc2a2 μt=60 = 5.94; Slc2a5 μt=60=no signal. Games-Howell pairwise comparisons with holm-bonferroni p-adjustments were performed using the ggstatsplot function in R. *pholm-adj<0.001; **pholm-adj<1e-6.
We identified positive selection signatures in the trehalase gene (Treh) along the ancestral branch of vespertilionids (Fig. 2d, branch A) and the Eptesicus branch, indicating possible adaptations to insectivorous diets. Surprisingly, the sucrase–isomaltase gene (SI) and transporter gene Slc2a5 showed selection in the vespertilionid lineage Myotis. Comparisons of predicted protein structures revealed alterations to functional domains (Extended Data Fig. 5), which are known to affect sucrose tolerance and sugar absorption61,62. Perhaps these molecular changes contribute to the heightened sucrose assimilation observed in Myotis (Fig. 1a).
Extended Data Fig. 5. HCR data.
HCR RNA-FISH data for Slc5a1 (A), Slc2a5 (B), and Slc2a2 (C). Image data for fluorescence intensity was log2 transformed for comparisons between time points within a species and across species. Two to three sections per individual with good morphology were chosen for study. For each section, 5-10 cells were isolated for analysis. Fluorescence signals were quantified using threshold-based segmentation and spot detection (n = 17-55 cells per treatment per species) in FIJI. We performed Games-Howell pairwise comparisons using the ggstatsplot function in R, with holm-bonferroni p-adjustments for multiple comparisons. Within each boxplot, a red dot denotes mean values, horizontal black lines denote median values, the boxes represent the range from the 25th to the 75th percentile of each group’s value distribution, while the vertical lines indicate the most extreme values within 1.5 times the interquartile range from the 25th and 75th percentiles of each group. Mean values are listed in Extended Data Table 5. Comparisons within species (t = 0 vs. t = 10) with significant expression level differences are bracketed at the bottom of each boxplot graph as follows: AGt0-t10 Slc2a2 (padj=4.11e-04), AJt0-t10 Slc2a5 (padj=1.66e-03), AJt0-t10 Slc2a2 (padj=2.82e-04), CPt0-t10 Slc5a1 (padj=0.0001), CPt0-t10 Slc2a5 (padj=5.27e-08), CPt0-t10 Slc2a2 (padj=0.001), GSt0-t10 Slc5a1 (padj=4.60e-10), GSt0-t10 Slc2a5 (padj=2.80e-09), PPt0-t10 Slc5a1 (padj=7.50e-10), PPt0-t10 Slc2a5 (padj=4.13e-09), PPt0-t10 Slc2a2 (padj=0.001).Comparisons between species at each respective time point are shown at the top of each graph, where only significant data (padj < 0.001) are shown as follows: Slc5a1 (A) AGt0-GSt0 (padj=7.24e-03), AGt0-PPt0 (padj=5.91e-05), AJt0-GSt0 (padj=1.03e-03), AJt0-PPt0 (padj=0.001), AJt0-CPt0 (padj=5.67e-07), AGt10-AJt10 (padj=1.01e-04), AGt10-CPt10 (padj=4.70e-08), AGt10-GSt10 (padj=2.59e-08). AGt10-CPt10 (padj=4.74e-05); Slc2a5 (B) AGt0-AJt0 (padj=6.83e-03), AJt0-PPt0 (padj=5.60e-07), AGt10-AJt10 (padj=1.10e-04), AGt10-CPt10 (padj=1.19e-05), AGt10-PPt10 (padj=1.19e-05), CPt10-GSt10 (padj=1.91e-05), GSt10-PPt10 (padj=1.13e-06); Slc2a2 (C) AGt0-GSt0 (padj=3.38e-03), AGt0-PPt0 (p = 4.75e-11), AGt0-AJt0 (padj=3.19e-11), AGt0-CPt0 (padj=6.70e-06), AGt10-GSt10 (padj=3.58e-06), AGt10-PPt10 (padj=8.38e-03), GSt10-PDt10 (padj=0.001), AJt10-PPt10 (padj=8.07e-05), GSt10-PPt10 (padj=3.49e-10). The following species represent each dietary guild: insects, Ptenonotus parnellii (PP, n = 4) and Micronycteris minuta (Mm n = 1); omnivore, Carollia perspicillata (CP, n = 4); fruit, Artibeus jamaicensis (AJ, n = 2); nectar, Glossophaga soricina (GS, n = 4) and Anoura geoffroyi (AG, n = 4). Phyllostomus discolor (PD, n = 1), is an omnivorous bat that has a large portion of nectar in their diet. Only technical replicates are available for species with n = 1.
We also found positive selection on the SI gene in omnivore Phyllostomus and the nectar bats Musonycteris harrisonii and Anoura caudifer (Fig. 2d) accompanied by structural changes to the sucrase domain of the predicted SI structure (Extended Data Fig. 5). While Phyllostomus (Fig. 2c, branch C) showed limited glucose assimilation from digested sucrose (Fig. 1a), they have the potential for elevated sucrase activity and sucrose assimilation34. This suggests that the sucrase domain may facilitate dietary expansion to omnivory in Phyllostomus, allowing them to respond to seasonal changes in food availability. In nectar bats, it is expected for SI expression to increase34,63, which increases blood glucose levels after consuming sucrose (Fig. 1a).
For glucose transporters, the vampire bat Desmodus rotundus (Fig. 2d, branch B), the ancestral branch leading to the Mexican nectar bat genus Leptonycteris and M. harrisonii (Fig. 2d, branch E) showed signatures of selection in gene Slc2a2. The vampire bat is renowned for its proficiency in running to hunt, and the Mexican nectar bats are known to undergo seasonal migrations to find flowering plants64, which are metabolically expensive behaviours65,66. It is possible that the signatures of selection on Slc2a2 might reveal functional adaptations related to assimilating glucose (Fig. 1a). When comparing predicted structures of GLUT2 (Extended Data Fig. 5), we observed changes in the ICH5 domain. This domain is post-translationally modified in the pancreatic beta cells to stabilize glucose sensing67. The observed domain change may possibly contribute to vampire bats and nectar bats having low levels of pancreatic beta cell release of insulin in response to glucose46,68, conserving glucose energy for exercise.
We detected positive selection in the neuron-enriched Slc2a3 gene (Fig. 2d) in the vampire bat D. rotundus (Fig. 2c, branch B), the ancestral branch leading to plant eating (Fig. 2c, branch C) and in the ancestral branch leading to nectar eating (Fig. 2c, branch E). The brain predominantly uses glucose as its main energy source, facilitated by the GLUT3 transporter known for its strong glucose affinity, especially during instances of low blood glucose levels, such as during fasting69. Positive selection on Slc2a3 might relate to the evolution of expanded foraging variety and may have functional consequences in brain metabolism, as suggested by others70,71. The extent and location of structural change of GLUT3 is similar in the vampire bat and the nectar bat, relative to their closest outgroup relative P. parnellii (Extended Data Fig. 5). Possibly, these predicted structural changes in known binding site and transmembrane transport domains reflect similar functional changes to GLUT3 in these groups. From previous studies, we know vampire bats and nectar bats are unable to meet their metabolic demands with glucose released from liver glycogen stores, leading to a higher fasting intolerance68,72, which may have resulted in positive selection for GLUT3 to protect the brain against such metabolic stress.
In the ancestral branch leading to fruit-eating species, we observed no significant molecular adaptations, corroborating a recent study on ancestral molecular evolution in Neotropical bats73. However, we observed a potential molecular adaptation related to glucose and fructose absorption in the genus Sturnira (Fig. 2c, branch D). We detected positive selection on Slc2a5 together with structural changes in SLC2A5 in at least two domains known to affect nutrient transport (Extended Data Fig. 5) that might explain the distinctive in vivo blood glucose levels observed in Sturnira after sucrose ingestion (368 mg dl−1), a pattern not observed in other fruit bats (Fig. 1a).
Intestine anatomy
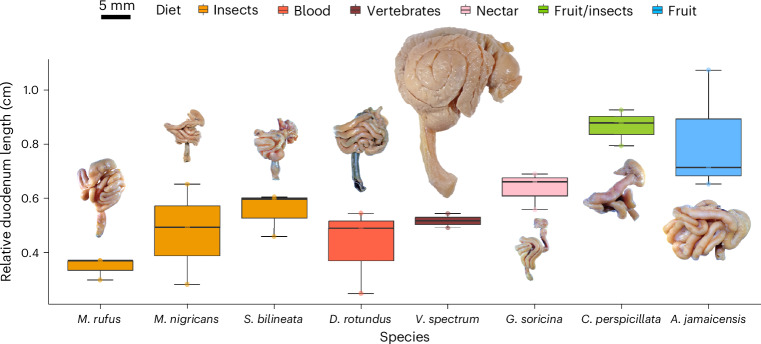
We next investigated anatomical features of the small intestine, particularly the duodenum, where most glucose absorption occurs74–76. Consequently, the absorption through the duodenum represents the immediate pathway that glucose follows to reach the blood before reaching the jejunum or ileum. Since glucose assimilation did not show a phylogenetic signal, intestinal traits are more likely to be related to diet than to evolutionary relationships. In comparison with humans, bats lack circular folds in their small intestine77, a finding that was confirmed in our analysis. We also observed striking differences in the intestinal length among bat species (Fig. 3), consistent with historical observations78. Notably, we found a correlation between duodenum length and the proportion of dietary sugar. Bats with rich-sugar diets exhibited longer duodenum, suggesting anatomical adaptations to deal with increased sugars and plant material, such as fibre, as they shifted from insectivory to omnivory/frugivory. However, nectar bats are not constantly consuming high fibre levels, increased amino acids or increased fatty acid quantities through their diets79. Instead, their diet mainly consists of water and a high concentration of sugar80. Thus, we posit that simple sugars play a significant role in the shift in duodenum length.
Fig. 3. Relative length of duodenum associated with gastrointestinal tract morphology from eight bat species with different diets.
Duodenum length relative to torso length in each species (shoulders to rump). S. bilineata, insectivorous; M. nigricans, insectivorous; M. rufus, insectivorous; D. rotundus, haematophagous; V. spectrum, carnivorous; C. perspicillata, omnivorous; A. jamaicensis, frugivorous; G. soricina, nectar-eating bat. Three biological replicates per species are reported, except for V. spectrum with two individuals. In the boxplots, the centre line represents the median, the limits or hinge are the first and third quartiles (the 25th and 75th percentiles), the whiskers extend to the largest and smallest value no further than 1.5× interquartile range) and the points represent the individual data. In general, species with fruit and nectar diets tend to have longer duodenum than the blood, meat and insect-eating species.
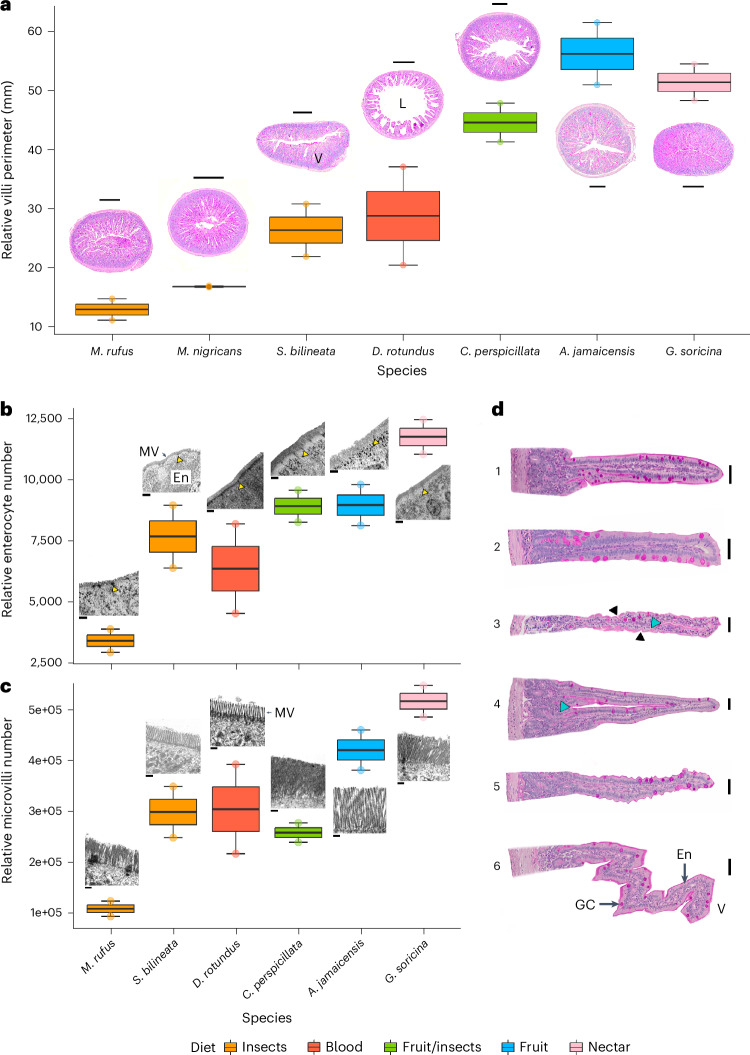
The intestine, with its specialized villi structures projecting into the gut lumen, plays a crucial role in the absorption of nutrients such as sugars, amino acids, fatty acids and vitamins81,82. These villi consist of specialized epithelial cells (enterocytes) and goblet cells83 and vary in shape among bats (Fig. 4). Among the seven bat species examined, we observed common finger-like villi (Fig. 4d, no. 1 and no. 2), previously described pyramidal villi (Fig. 4d, no. 4) and zig-zag villi (Fig. 4d, no. 6)77,84,85. Additionally, we found villi shapes (Fig. 4d, no. 3 and no. 5) that, to our knowledge, have not been reported before. Across the duodenum and beginning of the jejunum, insectivorous bats exhibited finger-like villi, some of which had simple entrances along the border (Fig. 4d, black triangles). Glossophaga exhibited entrances and sometimes an additional ‘gap’ in the middle of the finger-like villi body (Fig. 4d, no. 3, blue triangle). Pyramidal-shaped villi (Fig. 4d, no. 4) were found in Carollia, Artibeus and some Myotis individuals, while zig-zag villi (Fig. 4d, no. 6) were mainly found in Artibeus and Carollia, with rare occurrences in Myotis. Glossophaga also displayed pyramidal and zig-zag villi but with more pronounced entrances along the entire length of the villi. We identified a novel villi type (Fig. 4d, no. 5), that we referred to as ‘ruffled’, in Carollia and Myotis, characterized by multiple crests and troughs along its perimeter. The villi were scaled to the same size and their perimeter was measured (Extended Data Fig. 3). We found that while finger-like villi showed a perimeter of 339.56 µm, zig-zag villi were 931.99 µm and pyramidal villi with a gap in the middle were 1124.73 µm. The diverse villi morphologies observed suggest functional adaptations aimed at increasing the absorptive area in bats’ intestines.
Fig. 4. Histology of duodenum of bats with different diets.
a, Relative villi perimeter across the duodenum related to cross sections for each species. b, Relative number of enterocytes along the duodenum related to TEM of the enterocytes and microvilli; c. Relative number of microvilli along the duodenum related to TEM of microvilli. d, Different types of villi in bats; no. 1 and no. 2, finger-like villi; no. 3, finger-like villi with entrances (black triangle) and gap (blue triangle); no. 4, arch-like villi with gap (blue triangle); no. 5, new villi ‘ruffled’; and no. 6, zig-zag villi (nos. 1, 4 and 5 were extracted from C. perspicillata; no. 2 from S. bilineata; no. 3 from G. soricina; and no. 6 from A. jamaicensis). The species include: M. rufus, S. bilineata, D. rotundus, C. perspicillata, A. jamaicensis and G. soricina. Periodic acid–Schiff staining was performed for a and d. Scale bars, 250 µm (a), 2 µm (b), 0.4 µm (c), 20 µm (nos. 1–3 and 5, d) and 25 µm (nos. 4 and 6, d). The yellow triangles in b are pointing at tight junctions between enterocytes (En). MV, microvilli; V, villi; L, lumen; GC, goblet cells. Measurements were taken in different sections of the duodenum and are reported from one individual per species with two technical replicates for the histology section. For TEM, we had one individual and five technical replicates for enterocyte width and microvilli number, and the extrapolation to the whole duodenum was done for the two technical replicates from the histology sections. In the boxplots, the centre line represents the median, the limits or hinge are the first and third quartiles (the 25th and 75th percentiles), the whiskers extend to the largest and smallest value no further than 1.5× interquartile range and the points represent the individual data.
Extended Data Fig. 3. Measurements from museum samples.

a) Measures extracted from the intestine cross sections. 1. Villi-lumen area (VLA); 2. Sample area (SA); 3. Villi perimeter in the sampled area (VPSA); 4. Lumen area (LA); 5. Cross section perimeter (CSP). The measurements for the VPSA were taken twice with similar results. b) Intestinal segments selected for analysis. The duodenum is the first section of the intestine from the end of the stomach-pylorus to the duodenojejunal flexure. The jejunum is the next proximal ~2/3 of the small intestine until the presence of peyer’s patches, which marks the ileum, through histology.
Interestingly, the insectivorous Myotis exhibited higher sucrose assimilation compared with other insectivorous bats. We found evidence of positive selection for genes encoding SI and SLC2A5 as well as predicted changes to protein structure in the Myotis lineage. Additionally, we observed similar villi shapes in fruit, nectar and omnivorous bats. Myotis was traditionally considered a strict insectivore; however, Novaes et al. (2015) reported fruit consumption in a species of this genus86. This finding, along with predation on insects that feed on nectar, may be linked to similar trait evolution observed in bats with sugar-rich diets. Further ecophysiological investigations are necessary within this genus and within phyllostomidae insectivorous species that might also consume plant material.
After adjusting for body size, we found a greater exposed surface area of villi in the duodenum for bats with sugar-rich diets (Artibeus, Glossophaga and Carollia) compared with insectivorous and vampire bats (Fig. 4a). The variation in duodenum villi exposure, combined with the in vivo physiology data suggests that the increased absorptive area in the duodenum of bats with sugar-rich diets may contribute to enhanced glucose absorption (Fig. 1a). A similar pattern has been observed in the proximal intestine of the distantly related frugivorous megabat Epomophorus wahlbergi87,88.
Using transmission electron microscopy (TEM), we visualized the enterocytes and their microvilli in bats. We measured individual enterocytes, defined as the distance between tight junctions, and quantified the number of microvilli per enterocyte. Our analysis showed that bats with sugar-rich diets, particularly Glossophaga and Artibeus, had a higher number of enterocytes (Fig. 4b) and microvilli (Fig. 4c) compared with insect and vampire bats, especially compared with Molossus. The microvilli pattern observed in Neotropical bats aligns with discoveries in Paleotropical bats, despite their divergence in the early Eocene. Paleotropical insect eaters have smaller and fewer microvilli, while Paleotropical fruit bats have longer and more abundant microvilli87,88. The increased number of enterocytes suggests enhanced paracellular absorption between intestinal cells, as previously found in studies89–92, contributing to the rise in blood glucose levels (Fig. 1a). In addition, the increase in microvilli provides greater space for glucose transporters and digestive enzymes along the brush border93. These results indicate that bats have varying intestinal absorption capabilities, which broadly relate to diet. For example, in nectar and fruit-eating bats, the numerous microvilli could increase the density of SI and apical transporters (SLC5A1, SLC2A2 and SLC2A5), thus facilitating the absorption of glucose, resulting in increased blood glucose levels to different degrees within the same diet type (Fig. 1a). This hypothesis would fit with observations made on the increased levels of the sucrase enzyme expression in nectar and fruit-eating bats34.
Molecular mechanism of glucose absorption by transporters
We investigated the molecular activity underlying transcellular glucose absorption following a glucose meal and its impact on plasma glucose levels. We focused on the key molecular components of glucose absorption: Na+-dependent glucose co-transporter, SGLT1 (Slc5a1 gene), the apical solute carrier family member 2 glucose transporter, SLC2A2/GLUT2 (Slc2a2 gene) and SLC2A5/GLUT5 (Slc2a5 gene) (Fig. 2a) across seven bat species. The postprandial regulation of SI is not directly stimulated by glucose, as confirmed by quantitative polymerase chain reaction (qPCR) (Extended Data Fig. 4); therefore, we did not investigate this further. Additionally, non-insect-feeding bats lack a functional trehalase enzyme35, precluding further examination in these species.
Extended Data Fig. 4. qPCR summary.
(a) Primer sequences. (b) RT-qPCR heatmap of normalized expression (ΔCt) relative to housekeeping gene Gapdh for nutrient transporter genes Slc2a2, Slc5a1 and digestive enzyme gene SI. Five species representing four dietary guilds are shown [insect, Ptenonotus parnellii (Pp, nt=10 = 3, nt=0 = 3); omnivore, Carollia perspicillata (Cp, nt=10 = 2, nt=0 = 3); fruit, Artibeus jamaicensis (Aj, nt=10 = 2, nt=0 = 1); nectar, Anoura geoffroyi (Ag, nt=10 = 4, nt=0 = 3) and Glossophaga soricina (Gs, nt=10 = 3, nt=0 = 1)]. Samples are ordered by hierarchical clustering using Euclidean distance and the colors assigned to the clusters correspond to the dietary guild. (c) Logfold expression change (ΔΔCt) of Slc2a2, Slc5a1 and SI at t = 10 relative to t = 0. (d) No significant differences in logfold expression change were determined between t = 10 and t = 0 individuals for species by one-way ANOVA (Slc2a2; p = 0.178, Slc5a1; p = 0.663, SI; p = .0568).
We employed multiplexed, single-molecule hybridization chain reaction (HCR) RNA fluorescence in situ hybridization (FISH) (Fig. 5a) on fasted bats (t = 0) and bats that were fed (t = 10) a single dose of glucose (5.4 g kg−1(ref. 22) body weight). We note here that we were unable to examine protein localization at the apical membrane or within vesicles due to epitope disruption caused by prolonged methanol storage. We investigated two species within each dietary category (Fig. 5b–i): Pteronotus parnellii (n = 4) and Micronycteris minuta (n = 1) for insect-eating bats, Carollia perspicillata (n = 4) and Phyllostomus discolor (n = 1) for omnivorous bats and G. soricina (n = 4) and Anoura geoffroyi (n = 4) for nectar-feeding bats. Additionally, we examined one fruit-eating bat species, Artibeus jamaicensis (n = 2). For these experiments, we also used a more specialized glucometer (AlphaTRAK 2.0) and obtained blood glucose readings of more than 750 mg dl−1 (Extended Data Table 4). These levels are, to our knowledge, the highest documented for any wild species feeding on a single sugar dose of glucose solution. While we had limited biological replicates for some species (<2), we were still able to observe a notable pattern in gene expression at the level of the enterocyte. Insectivorous and omnivorous bats showed a comparable gene expression response to glucose. However, bats with a diet rich in sugar displayed markedly distinct patterns. Nectar bats (Fig. 5h,i)) consistently displayed a high Slc2a2 signal in both fasting and fed states, whereas the fruit bat A. jamaicensis exhibited the highest Slc2a5 signal (Fig. 5c).
Fig. 5. HCR RNA-FISH in the duodenum of bats from different diets.
a, RNA expression is shown for bats fed a single dose (5.4 mg kg−1body weight) of glucose. We performed HCR RNA-FISH with probe sets targeting messenger RNA (mRNA) for genes Slc5a1, Slc2a2 and Slc2a5 on fixed paraffin-embedded bat intestinal tissue and quantified the RNA fluorescent signal per enterocyte. An example composite image of the duodenum with all probes labelled with anatomical features is shown. The apical epithelial layer is shown with a fine-dotted line, and the microvilli brush border is shown with a dashed line; goblet cells are denoted by ‘G’. Scale, 10 µm. b–i, 40× overview images of the intestinal villi for two omnivorous, two insectivorous, one frugivorous and two nectarivorous species. Scale, 50 µm. Negative control with no probes (b), fruit (A. jamaicensis (n = 2)) (c), insect (M. minuta* (n = 1) (d) and P. parnellii (n = 4) (e)), omnivore (C. perspicillata (n = 4) (f) and P. discolor (n = 1) (g)), nectar (G. soricina (n = 4) (h) and A. geoffroyi (n = 4) (i) at t = 10 or *t = 60. b′–i′, Enlarged view of enterocytes along the villi. Scale bar, 10 µm. HCR probe sets are shown in cyan (Slc5a1), magenta (Slc2a2) and red (Slc2a5). DAPI (yellow) labels the cell nuclei. Bright, uniform and large circular spots (white) are background noise from amplifiers.
Next, we quantified the fluorescent signal intensity of each HCR probe within individual enterocytes (nent) along the mid-length of the villi (Fig. 6). Additionally, we performed qPCR with reverse transcription (RT–qPCR) to observe broad patterns in RNA expression in the duodenum (Extended Data Fig. 4 and Extended Data Table 5). After administering dietary glucose, the molecular response patterns closely resembled those observed in mouse studies94; although, variations were evident among bat species (Extended Data Fig. 5). In the insectivorous bat, P. parnellii, enterocytes responded with a log2 fold change of 1.8 (nent = 40; adjusted P (Padj) = 7.50 × 10−10) in Slc5a1 expression, 1.1 (nent = 40; Padj = 0.001) in Slc2a2 expression and 2.6 (nent = 37; Padj = 4.13 × 10−9) in Slc2a5 expression, accompanied by an average rise of 148.2 mg dl−1 in their blood glucose levels (n = 7; Extended Data Table 4). Comparatively, the insect bat M. minuta had an average change of 174.7 mg dl−1 in blood glucose (n = 3; Extended Data Table 4), indicating increased expression in molecular activity. Meanwhile, P. discolor (omnivorous) demonstrated a blood glucose level change of 500 mg dl−1 from fasting levels (n = 5; Extended Data Table 4). It is worth noting that the expression level in response to glucose (t = 10) in this species is lower for Slc5a1 (nent = 18; Extended Data Fig. 5a) compared with that observed in P. parnellii and the same for Slc2a2 (nent = 19; Extended Data Fig. 5c) compared with P. panellii. The insect-eating, short-tailed fruit bat, C. perspicillata, exhibited an increase of 440.6 mg dl−1 in blood glucose levels (n = 7; Extended Data Table 4), a log2 fold change of 1.05 (nent = 75; Padj = 0.0001) in Slc5a1 expression, 0.468 (nent = 72; Padj = 0.001) in Slc2a2 expression and 1.11 (nent = 74; Padj = 5.27 × 10−8) in Slc2a5 (Extended Data Table 4), which is comparatively lower expression change than the insectivorous P. parnellii (Extended Data Fig. 5). RT–qPCR data further highlights that P. parnellii exhibits a broader change in glucose transporter activity in response to glucose compared with these more specialized phyllostomids (Extended Data Fig. 4). These data, summarized in Fig. 6, suggest that the dramatic rise in blood glucose levels in P. discolor and C. perspicillata can be primarily attributed to paracellular absorption.
Fig. 6. Parsimony-based evolutionary inferences of blood glucose levels in relation to gene expression.
Average species blood glucose levels across 60 min (AUC) and change in gene expression 10 min after eating a 20% glucose solution. Most dietary guilds are represented by two species: insect, P. parnelli and M. minuta; fruit, A. jamaicensis; nectar, A. geoffroyi and G. soricina. P. discolor and C. perspicillata are omnivores incorporating large proportions of nectar or fruits, respectively, in their diet. Right: results of the single-cell image analysis taken from a minimum of 20 cells at fasted (t = 0) and fed (t = 10), presented as ridgeline density plots, coloured by the HCR probe and set as follows: Slc5a1 (cyan), Slc2a5 (yellow) and Slc2a2 (magenta). The log2 values of fluorescent intensity are displayed for t = 0 in a lighter shade and t = 10 in a darker shade. Games–Howell pairwise comparisons with Holm–Bonferroni P adjustments were used to evaluate differences in gene expression across species (Extended Data Fig. 5). Shared gene expression responses (no notable differences) are taken as evidence for inheritance from a common ancestor. Significant gene expression differences among species (nent, P < 0.001) and blood glucose levels at 10-min post-feeding (Extended Data Table 4) are noted as apomorphies on the tree as follows: a: a decrease in Slc5a1 in M. minuta (nent = 23) and blood glucose levels 200 mg dl−1 (n = 3), b: an increase in Slc2a5 expression in A. jamaicensis (nent = 43) and blood glucose levels 600 mg dl−1 (n = 6), c: a decrease in Slc2a5 expression and an increase in Slc2a2 gene expression in A. geoffroyi (nent = 76) and G. soricina (nent = 95), with an increase in blood glucose (>600 mg dl−1) and d: the highest expression of Slc2a2 and the highest average reported blood glucose levels in G. soricina (n = 7, >750 mg dl−1).
Extended Data Table 5.
qPCR in triplicate
(A) RT-qPCR samples ran in triplicate against each target gene. Resulting Ct values from QuantStudio 7 Pro Real-time qPCR platform were averaged and ΔCt values were generated from the change between each target gene compared to GAPDH and visualized using heatmap (Extended Data 7). (B) Within each species tested, t = 10 ΔCt values were compared against each t = 0 ΔCt value. Taken as a grouped averages, as in this array, ΔΔCt of gene expression changes between the species were compared and visualized using violin plot (Extended Data 7).
The fruit-eating bat A. jamaicensis increased blood glucose levels by 483 mg dl−1 upon eating sugar (n = 6; Extended Data Table 4) but only displayed a log2 fold change of 0.006 in Slc5a1 (nent = 43; Padj = 0.0001), 0.350 in Slc2a2 (nent = 43; Padj = 2.82 × 10−4) and 0.294 in Slc2a5 (nent = 43; Padj = 1.66 × 10−3) (Extended Data Fig. 5). While expression changes were minimal, the initial fasting levels of Slc5a1 and Slc2a5 were significantly higher than those in P. parnellii (Padj = 0.001 and Padj = 5.60 × 10−7) and C. perspicillata (Padj = 5.67 × 10−7 and Padj < 0.003) (Extended Data Fig. 5), indicating that gene regulatory changes, in addition to paracellular absorption, may explain some of the rapid increase of blood glucose levels during the first 10 min interval of A. jamaicensis (Fig. 6). The nectar specialists, A. geoffroyi and G. soricina, showed the highest blood glucose change of 639.4 mg dl−1 (n = 7) and 659.2 mg dl−1 (n = 7), respectively (Extended Data Table 4). These two species showed significantly elevated expression in Slc2a2 (nAg = 37, nGs = 40; Padj = 4.7 × 10−11) at fasting (Extended Data Fig. 5c) and the lowest log2 fold change in Slc2a2, −0.123 (nent = 76) and 0.109 (nent = 95), respectively (Extended Data Table 4). Both nectar bat species also had lower expression (nAg = 61, nGs = 93; Padj = 1.19 × 10−5) of Slc2a5 compared with P. panellii (Extended Data Fig. 5b). These findings suggest a gain of function regulatory change in Slc2a2 within enterocytes in relation to the rapid rise of blood glucose levels (Fig. 6).
When considering the broad patterns of blood glucose levels concerning the expression of intestinal transporters along the microvilli (Fig. 2a), we observe molecular apomorphies in transporter gene expression (Fig. 6). These differences may contribute to the diverse blood glucose patterns observed in the insectivore M. minuta (Fig. 6a), the frugivore A. jamaicensis (Fig. 6b) and the nectar bats (Fig. 6c,d). To relate how much and how long blood glucose levels remain elevated after the consumption of a glucose solution, we used a summary measure of the overall glucose response, the area under the curve (AUC) (Fig. 6). Relative to the outgroup P. parnellii, a higher or lower AUC indicates a change in glucose assimilation. For example, Slc5a1 expression is lower in M. minuta (t = 60), and we observe an AUC30–60 of 6,705 (Fig. 6a), in contrast to P. parnellii, which has higher expression of Slc5a1 and an AUC30–60 of 9,630, indicating a diminished transfer of glucose through Slc5a1 in M. minuta from the intestinal lumen to the bloodstream. Comparatively, the expression levels of transporter genes for P. discolor (n = 5; AUC0–10 of 3,312) and C. perspicillata (n = 7; AUC0–10 of 2,828) were lower to those of P. parnellii (AUC0–10 of 1,368). Since the expression in each enterocyte is lower in P. discolor and C. perspicillata, the rise in blood glucose levels is probably the result of an increase in the number of enterocytes made possible by an elongation of the duodenum (Fig. 3) and an expansion of the villi perimeter (Fig. 4a), which allows for more paracellular absorption89–92. On the contrary, A. jamaicensis enterocytes have an increase in Slc5a1 (nent = 43) and Slc2a5 (nent = 43) expression (Extended Data Fig. 5) and an AUC0-10 of 2973 (n = 6; Extended Data Table 4). The increased expression of glucose transporters may contribute to the rise in blood glucose levels, given that A. jamaicensis exhibits similar enterocyte numbers as C. perspicillata (Fig. 4b). The regulatory changes may be an apomorphic molecular change in the genus Artibeus (Fig. 6b) or it may be a plesiomorphic change in the subfamily Stenordermatidae, a specialized group of fruit-eating bats. It is worth mentioning that Sturnira’s molecular adaptations to SLC2A5 (Fig. 2 and Extended Data Fig. 5e) might have been facilitated by the consistent usage of this molecular transport system. While fruit-eating bats have increased Slc2a5, nectar bats have decreased it, instead depending extensively on Slc2a2 to increase blood glucose levels (Fig. 6c,d). The changes in the timing of Slc2a2 and the amount of Slc2a2 and Slc2a5 expression probably reflect molecular plesiomorphies in the nectar bat subfamily Glossophaginae (Fig. 6c). A possible molecular apomorphy is represented by the excessively elevated levels of Slc2a2 within the genus Glossophaga (Fig. 6d). Correspondingly, A. geoffroyi (AUC0–10 of 3,842) and G. soricina (AUC0–10 of 3,636) have extremely high blood glucose levels (average >700 mg dl−1; Extended Data Table 4) compared with other bats.
This study demonstrates the first case of persistent intestinal Slc2a2 expression found in a non-pathologic state in mammals. A comparable observation has been reported in the ruby-throated hummingbird, providing a notable example of convergent evolution95. The increase in Slc2a2 expression also exemplifies repeated evolution towards a diet with a high sugar proportion, a molecular trait observed in Paleotropical fruit bats such as Rousettus leschenaultia and Cynopterus sphinx96, which have maximum blood glucose levels between 430 and 490 mg dl−1. These paleotropical species, along with neotropical bats such as Carollia, Artibeus and Anoura, were found to possess an 11-base pair deletion in the proximal promoter of the Slc2a2 gene96, predicted to disrupt the transcriptional repressor ZNF354C, thereby enhancing gene activity. The authors found an increase in the expression of liver Slc2a2 in paleotropical fruit bats96. While we did not observe a prominent increase in Slc2a2 expression at the level of the enterocyte in neotropical fruit bats, it might be increased in the liver cells. This alteration might explain their ability to rapidly clear blood glucose (Fig. 1a) and their increased capacity to store liver glycogen compared with nectar bats72,97. Future studies on tissue-specific gene regulation are needed to uncover mechanisms of evolutionary change across species.
Given the high glucose concentration present in nectar and their extremely high postprandial glucose levels, our study suggests that the distinctive expression pattern of Slc2a2 is unique to nectarivorous bats (Fig. 6c). The permanent presence of apical Slc2a2 might result from defective insulin action, as insulin typically triggers the internalization of GLUT2 (Slc2a2 gene) to slow down sugar uptake and prevent high blood glucose levels after a sugar meal98. This is in line with the fact that nectar-feeding bats need to absorb high quantities of glucose to fuel their energetic requirements for hovering flight43,99,100 and the hypothesis about additional mechanisms, complementary to insulin, to regulate glucose homoeostasis22,44,45. The magnitude of postprandial hyperglycaemia observed in nectar bats provides strong evidence that the adaptation towards nectar feeding has primed the duodenum for immediate and enhanced dietary sugar absorption, combining paracellular absorption with molecular absorption. The molecular metabolic adaptation is further supported by the permanent apical expression of Slc2a2 associated with obesity and diabetes, conditions characterized by elevated blood glucose levels, insulin resistance and an increase in villous surface area101–103. Therefore, the long-term dietary adaptation to excess sugar consumption required the modification of the fundamental regulation of glucose absorption at the enterocyte level involving transporter trafficking in addition to the well-known paracellular absorption mechanism.
Conclusion
In our investigation into the metabolic adaptations across more than 29 bat species with diverse diets, we found higher assimilation of glucose and sucrose in nectarivorous, frugivorous and certain omnivorous bats, whereas insectivorous and omnivorous bats exhibited greater trehalose assimilation. Intriguingly, no insectivorous bat showed sugar absorption and assimilation as rapid and extensive as bats with sugar-rich diets. The observed variations in metabolic phenotypes are intricately linked to distinct adaptations in digestive morphology, including alterations in intestinal length, exposed villi and microvilli. Bats with sugar-rich diets exhibited a longer duodenum and higher numbers of enterocytes and microvilli along the initial section of the small intestine. These features suggest an enhanced capability for glucose absorption in bats with sugar-rich diets. Moreover, our study identifies key genetic traits associated with efficiently extracting maximal glucose energy from diet. Positive selection is evident in genes encoding for sucrase–isomaltase and glucose transporters in nectar and omnivorous bats and on a fructose transporter in a fruit bat. Structural comparisons of these proteins further elucidate the impact of amino acid substitutions on their functional roles, which may change enzymatic reaction speed or the affinity of glucose to transporters. Notably, our investigation extends beyond genetic traits to explore shifts in gene expression within single enterocytes along the brush border of the duodenum. This detailed examination of transporters SLC2A2/GLUT2 (Slc2a2), SLC2A5/GLUT5 (Slc2a5) and SLC5A1/SGLT1 (Slc5a1) unveils a nuanced interplay in response to dietary glucose. Across most bat species examined, there is a conserved expression response of sugar assimilation genes to glucose per enterocyte, so gut morphology appears to be the primary driver for glucose assimilation differences. As bats must limit gut size due to the constraints imposed by flight, their guts’ microanatomy gets modified. It could also be that the structural changes we have documented make each transporter more efficient while keeping gene expression the same. However, the continuous expression of Slc2a2 encoding for glucose transporter GLUT2 was exclusive to nectar bats, indicative of enhanced glucose receptivity. This interplay between physiology and ecology over evolutionary time illuminates the intricate adaptive mechanisms that underlie diet evolution in bats.
Methods
Our research complies with all relevant ethical regulations. The study protocol was approved by the Institute Board Committee Scientific Advisory Panel and the Institutional Animal Care and Use Committee at the Stowers Institute for Medical Research. The fieldwork was made under the permit of the National Authority of Environmental licences and the Ministry of Environment and Sustainable Development of Colombia, Resolution 1070, 28 August 2015, a Special Game licence from the Republic of Trinidad and Tobago Wildlife Section, Forestry Division, Ministry of Agriculture, Land and Marine Resources (2022) and permit R-014-2022-OT-Conagebio from the Costa Rica National Commission for Biodiversity Management Division, Ministry of Environment and Energy (2022).
Field work
Bats were captured between June 2019 and December 2020 in 11 localities of the dry tropical forest ecosystem in the department of Valle del Cauca, Colombia, some within the Dry Tropical Project from the Institute for Research and Preservation of the Cultural and Natural Heritage of Valle del Cauca. To catch species with different food preferences (frugivores, insectivores, nectarivores, omnivores and haematophages), we opened mist nets between 18:00 and 24:00. In some instances, bats were manually captured in their refuge. Each individual was taxonomically identified, and their weight, age, sex, reproductive status and diet type were recorded. We also include species captured in Trinidad and Tobago as well as Costa Rica under an approved Institutional Animal Care and Use Committee from the Stowers Institute for Medical Research. We acknowledge that the bat’s diet is more a continuum rather than static categories; however, for analysing the data, we grouped species according to their food preference and morphological adaptations for the consumption of the food resources78,104–106. In this way, the species of the genera Artibeus, Dermanura, Uroderma and Sturnira are considered frugivores; Carollia and Phyllostomus omnivores; Glossophaga, Choeroniscus and Lonchophylla nectarivores; Desmodus haematophagous; Vampyrum a carnivore; and Saccopteryx, Peropteryx, Myotis, Molossus and Pteronotus insectivorous. We include in vivo physiology data for 79 individuals with a preference for fruits, 55 omnivorous bats, 23 with a preference for nectar, 27 for insects, 14 for blood and 2 carnivorous bats (Extended Data Table 1). Juvenile individuals and pregnant or lactating females were excluded from the study due to high energy requirements and physiological changes during these stages, compared with non-pregnant or non-lactating adult individuals107,108.
Extended Data Fig. 1. Phylogenetic signal.
a) Comparison of assimilation curves for glucose, sucrose, and trehalose solutions among Neotropical bats with different food preferences: high sugar in glucose and sucrose graphs refer to frugivorous and nectarivorous bats, while low sugar refers to insectivorous, carnivorous and hematophagous bats; high sugar in the trehalose graph refers to insectivorous bats and low sugar refers to the rest of the dietary categories; omnivores have their own category because they have diets where the three sugars are present in relatively high proportions. The Bayesian multilevel phylogenetic model estimated blood glucose levels for each time point and for each group, data are presented as mean values +/− SEM; we included the taxa for which we had data for each individual sugar (Supplemental Table 1). For glucose assimilation we included 22 species, for sucrose assimilation 18 species and for trehalose assimilation 19 species. b) Table. Phylogenetic signal (Pagel’s λ) evaluated for the assimilation proxy (corrected area under the curve) of glucose, sucrose and trehalose in Neotropical bats.
Oral glucose tolerance tests
After identification, bats were fed a 20% sugar solution and subsequently subjected to a period of fasting for 10–12 h. After fasting, each bat was fed a bolus of sugar (5.4 g kg−1of body weight) as previously established by Kelm et al.22. Individual bats were only fed one type of sugar (glucose, sucrose or trehalose). To determine blood glucose levels, a drop of blood was drawn from the forearm with a 30G lancet before the sugar bolus and 10, 30 and 60 min post-feeding. The blood was immediately measured using a GlucoQuick G30a glucometer (Diabetrics) with a 20–600 mg dl−1 range. The individuals corresponding to Lonchophylla and Pteronotus were captured in Costa Rica and Trinidad and Tobago in 2022, respectively (Extended Data Table 4). Their blood glucose levels were measured with an AlphaTRAK 2.0 glucometer (Zoetis) with a range of 20–750 mg dl−1; however, the measurements above 600 mg dl−1 were treated as 600 measurements to match the rest of the data previously obtained with the more limited range glucometer.
The bats individually remained in cloth bags between readings. Finally, the bats were tagged, fed and released. In total, blood glucose was measured for 199 individuals from 29 species and five families: Phyllostomidae, Mormoopidae, Emballonuridae, Vespertilionidae and Molossidae (Extended Data Table 1).
General patterns of sugar assimilation curves
We proposed general curves (Fig. 1b) to describe the temporal pattern of assimilation of different sugars in Neotropical bats based on a 1-h glucose tolerance test. The glucose curves were classified according to the speed of sugar assimilation to facilitate the understanding of the behaviour of the curves. The ‘fast’ assimilation curve shows a peak of blood glucose levels only after 10 min of sugar ingestion, followed by a decrease in blood glucose levels. The ‘medium’ assimilation curve shows a peak of blood glucose levels 30 min after sugar ingestion followed by a decrease in blood glucose levels. The ‘slow’ assimilation curve shows a continuous increase in blood glucose levels until the final timepoint, with the maximum levels 60 min after sugar ingestion. Finally, the ‘limited’ assimilation curve shows blood glucose levels with little variation throughout the different timepoints of the test.
Statistical analysis
Blood glucose levels of all bats measured were grouped by species and plotted in R (Fig. 1). Afterward, we evaluated the phylogenetic signal for the assimilation of the various sugars by the Pagel λ index47. We measured assimilation as the area under the glucose tolerance curve corrected, that is, setting the base of the curve at the minimal blood glucose level recorded for the average measurements of each genera. Additionally, to test for the interactive effect of timepoints and food preferences on the assimilation of sugars, by considering various individuals per species and the non-independence due to shared evolutionary history among taxa, we implemented a Bayesian multi-level phylogenetic model in the brms R package109. This multi-level model includes a varying intercept over species (using an indicator variable for species) and a covariance matrix to specify the lack of phylogenetic independence, allowing the analysis of hierarchical biological data while incorporating evolutionary relationships among species. For statistical inference, we compared 95% credible intervals from the models between timepoints and food preference combinations. We calculated the phylogenetic correlations among species from an ultrametric tree using the vcv function of the ape R package110. For phylogenetic analyses, we used the species-level mammal phylogeny of Upham et al. (2019) (http://vertlife.org/phylosubsets/), so we downloaded a credible set of 10,000 trees for all taxa we have data on for sugar assimilation and computed the consensus tree with the averageTree function of the phytools R package111. We performed the analysis using R112 4.3.1.
Morphology and histology of the gastrointestinal tract
We extracted the gastrointestinal tract from individuals preserved in 70% ethanol from species obtained in previous studies on bats31 obtained under the Wildlife Section, Forestry Division, Ministry of Agriculture, Land and Marine Resources (Republic of Trinidad and Tobago) permit number 1737 (2014). The species incorporated in this section were G. soricina, A. jamaicensis, C. perspicillata, Molossus rufus and Saccopteryx bilineata. Additionally, we included two species obtained from the University of Kansas (KU) Biodiversity Institute and Natural History Museum: Myotis nigricans (KU 134846) and D. rotundus (KU 100374) and one species obtained from the American Museum of Natural History (AMNH): Vampyrum spectrum (M-267445, 272936). Finally we added more individuals to the analysis from the AMNH (A. jamaicensis 7473; C. perspicillata 7458, 175795; M. rufus 178675, 178672; S. bilineata 184693, 149982; M. nigricans 175725, 175724; D. rotundus 175406, 239943). We measured the length of the duodenum by imaging the unfolded, stretched intestine of each species with a Canon EOS Rebel E7i. Then, we determined the length through the Fiji platform113—Image J 1.53f51—and compared relative intestine length as the gut length divided by torso length (shoulders to rump length). We used this measure to control body size due to our focus on the intestine and its location, without adding measures related to the length of the rostrum or the tail, which can vary across bat species and families.
We investigated the duodenum because it is the main segment of the small intestine responsible for glucose absorption, and in vertebrates, the amount of absorbed glucose decreases as it reaches the next intestinal segments74,75. The duodenum is the first section of the intestine from the end of the stomach–pylorus to the duodenojejunal flexure114. The duodenum was recognized by its greater diameter compared with the jejunum, the intestine segment after the duodenum. The jejunum was also investigated through histology; however, its length could not be assessed due to the external similarity between the end of the jejunum and the start of the ileum (Extended Data Fig. 3).
For histological comparisons, we cut 0.5–1 cm sections of the duodenum. The gut tissues were embedded in paraffin, serially cross sectioned to 10 µm and stained with periodic acid–Schiff. All tissues were imaged using an Olympus slide scanner equipped with a 20× objective and exported as a TIFF image. Two sections with good morphology per individual were chosen for study. We measured the area of villi and lumen (VLA) in each cross section, and then, we delimited 1/10 of VLA to measure the well-preserved villi perimeter (VPSA) in this sample area (SA). We also measured the lumen area (LA) to calculate the total villi perimeter along the cross section. Then, we corrected the measurement for size differences among species by considering the cross-section perimeter (CSP) from the different species. Finally, we extrapolated the final value from villi exposed in the whole cross section, corrected by size, to the relative duodenum length, as shown in equation (1) (measures summarized in Extended Data Fig. 3a):
| 1 |
Summary statistics were visualized with boxplots in Figs. 3 and 4. Descriptive comparisons among species were based on species averages.
TEM
To look closer at the enterocytes and microvilli, we used TEM. We sectioned 0.5–1.0 cm of duodenum from some of the species mentioned above (Fig. 3). The samples were rehydrated and fixed in 50 mM sodium cacodylate (pH 7.4) containing 2.5% paraformaldehyde and 2% glutaraldehyde. The tissue segments were then post-fixed with 2% OsO4 for 2 h, washed and stained with 1% aqueous uranyl acetate overnight. After dehydration with a gradient of ethanol, the samples were infiltrated and embedded into Epon resin. Ultrathin (80 nm) sections were cut with a Leica UC7 Ultramicrotome, collected onto slot copper grids and stained with 4% uranyl acetate in 70% methanol and Sato’s triple lead solution. Sections were imaged using a Field Emission Instruments (FEI) transmission electron microscope at 80 kV using the DigitalMicrograph software.
To calculate the number of enterocytes in the duodenum, we measured the enterocyte width of five different cells from each species, and the average was extrapolated to the relative length of the duodenum by dividing the villi surface found in the duodenum by the enterocyte width average. In addition, for calculating the number of microvilli in the duodenum, we counted the number of microvilli in each of the five different enterocytes where we measured the width, and then, we multiplied the average microvilli value by the number of enterocytes. All the measurements were made with the Fiji platform—Image J 1.53f51.
Genomics
Genome assemblies for 22 Chiroptera species, which included representatives from each of the dietary groups, were downloaded from online sources (Extended Data Table 3a). Transcriptomes and proteomes for 16 species were obtained from the National Center for Biotechnology Information (NCBI) and Ensembl databases (Extended Data Table 3b). After filtering out genomic contigs less than 10 kb, we generated gene models using MAKER (v3.01.03) using the 16 transcriptomes and proteomes (plus SwissProt/Uniprot v2021_03) as ‘EST evidence’ and ‘Protein Homology Evidence’. RepeatMasker (open-4.0.7 with DB version 20170127) was used by MAKER to identify mammalian repeats. Links to the versions of all publicly available data (genome assemblies, transcriptomes and proteomes) used by MAKER can be accessed on SIMRbase. Our SIMR bat models were assigned names derived from Uniprot115 best hit with an e-value ≤1 × 10−5. SIMR bat models are available for download and for browsing at https://simrbase.stowers.org/bats/data. Genome browsers were built using Jbrowse116 and SIMRbase was built using Tripal117.
Ortholog assignment and candidate gene identification
To relate the blood glucose tolerance assay to evolution, we identified genes involved in sugar assimilation, specifically from the gut absorption into the bloodstream. A short list of eight genes were identified as having biased function and expression in the duodenum using mouse ENCODE (NCBI Bioproject PRJNA66167) and human HPA (NCBI Bioproject PRJEB4337). Orthologs were assigned to the SIMR bat models with standalone Orthologous Matrix (OMA) (version 2.4.2) using three species included in the OMA FASTA database (April 2021), Homo sapiens (HUMAN), Pteropus vampyrus (PTEVA) and Myotis lucifugus (MYOLU). A gene is considered an ortholog if it is found in the same OMA ortholog group as a HUMAN candidate gene with appropriate HGNC identifiers (Extended Data Table 3).
Phylogenetic analysis of sugar related genes
Whole genome alignments of single-copy orthologs trimmed with trimAl v1.4.rev15 (ref. 118) from 22 SIMR bat models and two outgroups (Extended Data Table 3) and were used to generate a phylogenomic tree119 using RaxML version 8.1.15 (PROTFAMMAUTO model). Our molecular phylogeny, which matches published species topologies73,120–122, includes a broad taxonomic sampling, incorporating Miniopteridae, Vespertilionidae and Molossidae (Fig. 2, branch A). The resulting phylogeny was used for all exploratory selection tests of candidate genes along all branches (Extended Data Table 4). The tested DNA sequences were from single-copy orthologs found using OMA. For selection tests, we used the codon-based method measuring non-synonymous (dN) to synonymous nucleotide (dS) substitutions (the dN/dS metric, ω) with the tool HyPhy (hyphy.org). To identify branches under positive selection, aBSREL56 was used.
Structural phylogenetics using Alphafold and Foldseek
Alphafold is a large scale language model used to impute protein structures from sequence59. We used Alphafold v2, which is available at https://github.com/google-deepmind/alphafold. With the protein structure, we then performed structural alignments with Foldseek60, which can be downloaded at https://github.com/steineggerlab/foldseek. The metric for assessing the topological similarity of protein structure is the TM-score, which has the value in (0,1), where 1 represents a perfect match between two structures.
HCR RNA-FISH
Additional glucose tolerance tests (Extended Data Table 4) were performed on P. parnellii (n = 7) and M. minuta (n = 3, insect diet), C. perspicillata (n = 7, piper–insect diet), A. jamaicensis (n = 6, fig-fruit diet), A. geoffroyi (n = 7) and G. soricina (n = 7, nectar diet) and P. discolor (n = 5, insect–fruit–nectar diet) in April 2022 and April 2023 with permission from the Republic of Trinidad and Tobago. To determine blood glucose levels, a drop of blood was drawn from the forearm with a 30G lancet before the sugar bolus and 10 min post-feeding. The blood was immediately measured using an AlphaTRAK 2.0 glucometer (Zoetis) with a range of 20–750 mg dl−1 calibrated for cats (health range 120–300 mg dl−1). Finally, the bats were euthanized (n = 2–4 per species), and the tissue was dissected, fixed and stored in 4% paraformaldehyde.
Fluorescently labelled HCR hairpins targeting SGLT1 (Slc5a1), GLUT2 (Slc2a2) and GLUT5 (Slc2a5) in fasted and fed bats were purchased from Molecular Technologies, and thrid generation HCR RNA-FISH was performed on 10 µm paraffin sections of the duodenum. Slides were deparaffinized and microwave antigen retrieval was applied at 95 °C for 15 min. A hydrophobic barrier was created using ImmEdge Hydrophobic Barrier PAP Pen (Vector laboratories) around the sections on a slide, and bleaching solution (3% H2O2, 5% formamide, 0.5× SSC) was applied for 30 min under strong light-emitting diode light. Tissue sections were permeabilized with proteinase K (20 ug ml−1) for 12 min at room temperature, washed with PBS five times, incubated in hybridization buffer for 30 min at 37 °C, and incubated in hybridization buffer containing probes at 37 °C for 16 h. Tissue sections were washed five times with the wash buffer for 5 min each, then two times in the 5× SSCT (5× SSC + 0.1% Tween 20). Fluorescently labelled HCR amplifiers were snap-cooled by heating at 95 °C for 90 s, cooling to room temperature for 30 min under dark conditions and added to the amplification buffer. Tissue sections were incubated in the amplification buffer for 30 min at room temperature and incubated in an amplification buffer containing amplifiers overnight at room temperature in a humid chamber under dark conditions. Slides were washed four times in 5× SSCT for 5 min each, stained with 4,6-diamidino-2-phenylindole (DAPI, 10ug ml−1) in 5× SSCT for 30 min and then washed two times in 5× SSCT. Prolong gold (Invitrogen) was mounted, and slides were stored at 4 °C until imaging.
Image analysis
Images were taken on a Nikon Ti Eclipse with CSU-W1 spinning disk confocal microscope at 40× magnification. Image data for fluorescence intensity were log2 transformed for comparisons between timepoints within a species and across species. Two to three sections per individual with good morphology were chosen for study. For each section, five to ten cells were isolated for analysis. Fluorescence signals were quantified using threshold-based segmentation and spot detection (n = 17–55 cells per treatment per species) in FIJI. We performed Games–Howell pairwise comparisons using the ‘ggstatsplot’ function in R, with Holm–Bonferroni P adjustments for multiple comparisons (Extended Data Fig. 5).
qPCR
Total RNA was isolated from 3–5 mg flash frozen duodenum tissue from Ptenonotus parnellii (nt=10 = 3, nt=0 = 3), C. perspicillata (nt=10 = 2, nt=0 = 3), A. jamaicensis (nt=10 = 2, nt=0 = 1) A. geoffroyi (nt=10 = 4, nt=0 = 3) and G. soricina (nt=10 = 3, nt=0 = 1). RNA was extracted using Promega Maxwell RSC simplyRNA Tissue Kit. Quantification was performed using Qubit RNA High Sensitivity Assay Kit and quality was assessed using a 1% agarose gel stained with ethidium bromide. Downstream complementary DNA synthesis was performed using Applied Biosystems High-Capacity RNA-to-cDNA Kit using a programmed thermal cycler at 37 °C for 60 min, followed by a 5 min incubation at 95 °C, before cooling to 4 °C. The specific primer sequences used for target genes sglt1 Slc5a1/sglt1, Slc2a2/glut2, sucrase–isomaltase (SI) and reference gene gapdh are shown in Extended Data Fig. 4 and were ordered from Integrated DNA Technologies (IDT). Primer pair validation and experimentation were performed using QuantStudio 7 Pro Real-time qPCR platform and software. The data generated from qPCR were analysed using the 2−ΔΔCt method123 to assess variance in relative fold gene expression between Slc5a1/sglt1, si and Slc2a2/glut2 against the housekeeping gene gapdh. For heat map visualization of normalized gene expression (ΔCt), the R package ‘pheatmap’ version 1.0.12 was used. Clusters are based on ‘Euclidean’ distance measures, also performed on the R package ‘pheatmap’. Statistical analysis on fold gene expression changes (ΔΔCt) at t = 10 relative to t = 0 between species was evaluated with a one-way analysis of variance in GraphPad Prism 10.2.0 (Extended Data Table 5).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Original analysis generated by Hyphy in .json format per gene.
Html links to predicted proteins.
Unprocessed qPCR data.
Multiple raw image files within folder.
Source data
Raw data for intestinal measurements
Average glucose tolerance test (GTT) values from 0, 10, 30 and 60 used for AUC.
Values for average glucose tolerance test (GTT) per species.
Data used for calculations.
HCR source data per enterocyte.
Acknowledgements
A portion of the fieldwork was carried out within the framework of the project ‘Contribución a la conservación del Bosque seco Tropical del Valle del Cauca’ under the CVC permit no. 1122 of 2018, from the Institute for Research and Preservation of the Cultural and Natural Heritage of Valle del Cauca, whom we thank, along with the support of J.O. Ortíz, R. Rivera and A.Bernal and the field assistance from Therios (Cali, Colombia), C. Calvache, S. Tabares, A. Chito and O. Cuellar. Thanks to M. Eifler at the KU Biodiversity Institute and Natural History Museum, N. Simmons and M. Surovy from the AMNH for facilitating access and research of museum specimens. We thank the scientists at the Stowers Institute for Medical Research, S. Malloy, H. Wilson, M. Frangello, C. Maddera and X. Zhao, for help in processing and imaging histological and electron microscopy sections, as well as D. Bradford for providing training on RT–qPCR instrumentation and data analysis. We also thank J. A. Riascos for the bat illustration in the graphical abstract; D. Narang, R. Smith and S. Mahadeo for their support in Trinidad; F. Reid and J. Jamison for their support in Costa Rica; S. Sanatana for guidance in phylogenetic comparative methods analysis; and A. Sadier and L. Yohe for comments that improved this manuscript. Finally, we thank the Rohner lab members for creating a stimulating scientific environment and for their support during the manuscript writing. Funding for the project was provided by the Stowers Institute and National Institutes of Health (NIH) grant no. 1DP2AG071466-01 to N.R., the National Science Foundation (NSF) Postdoctoral Research Fellowships in Biology no. 2109717 (2021–2022) to J.C., the Burroughs Wellcome Fund (BWF) Postdoctoral Diversity Enrichment Program (G-1022339) to J.C. and the Howard Hughes Medical Institute (HHMI) Hanna H. Gray Fellows Program (GT15991) to J.C. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIH, NSF, BWF or HHMI.
Extended data
Extended Data Table 2.
Bat genomes
A) Twenty-two bat genomes were assembled and annotated for selection tests. Species were selected to best match the diversity of the in vivo physiology data. Two outgroup genomes, human and shrew, were used to polarize the evolutionary changes in bats. B) In addition to the twenty-two Neotropical bat genomes, we obtained an additional 24 genomes from all available bat species for structural comparisons of proteins with Foldseek.
Author contributions
A.B.-R. and J.C. designed the study. A.B.-R., J.C. and V.P. designed the figures. A.B.-R. performed the in vivo physiology experiments as an undergraduate thesis project (2019–2020) directed by O.E.M.-G. A.B.-R. and O.E.M.-G. designed the in vivo physiology experiments and performed the statistical analyses. A.B.-R. and J.C. performed additional glucose tolerance tests and obtained gut tissue for molecular analyses in April 2022. A.B.-R. and J.C. dissected the gastrointestinal tract of museum bats and performed morphological data interpretation. K.Y. performed the TEM experiments and imaging. A.B.-R. made the gut anatomy measurements and comparisons. S.M.C.R. produced gene models across the assembled genomes, assigned orthologs, built webpages and genome browsers and generated the phylogenomic tree. J.R. and J.C. performed selection tests. J.R. performed Alphafold and Foldseek analysis. J.C., D.T. and Y.W. performed the HCR experiments, J.C. and V.P. performed HCR imaging, and J.C. performed HCR analysis. V.P. and P.M.-S. extracted RNA from the duodenum, and P.M.-S. performed qPCR. A.B.-R., J.C. and N.R. co-wrote the manuscript.
Peer review
Peer review information
Nature Ecology & Evolution thanks Zixia Huang, Ekaterina Osipova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Original data underlying this manuscript can be publically accessed from the Stowers Original Data Repository at http://www.stowers.org/research/publications/LIBPB-2406. Original genome data can be found in Extended Data Table 3. Our Genome browsers are publically available here: https://simrbase.stowers.org/bats_sugar_assimilation. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jasmin Camacho, Andrea Bernal-Rivera.
Contributor Information
Jasmin Camacho, Email: jcamacho@stowers.org.
Oscar E. Murillo-García, Email: oscar.murillo@correounivalle.edu.co
Nicolas Rohner, Email: nro@stowers.org.
Extended data
is available for this paper at 10.1038/s41559-024-02485-7.
Supplementary information
The online version contains supplementary material available at 10.1038/s41559-024-02485-7.
References
- 1.Pauli, J. N., Peery, M. Z., Fountain, E. D. & Karasov, W. H. Arboreal folivores limit their energetic output, all the way to slothfulness. Am. Nat.188, 196–204 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Tokita, M., Yano, W., James, H. F. & Abzhanov, A. Cranial shape evolution in adaptive radiations of birds: comparative morphometrics of Darwin’s finches and Hawaiian honeycreepers. Philos. Trans. R. Soc. B372, 20150481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camacho, J. et al. Peramorphosis, an evolutionary developmental mechanism in Neotropical bat skull diversity. Dev. Dyn.248, 1129–1143 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Morris, Z. S., Vliet, K. A., Abzhanov, A. & Pierce, S. E. Heterochronic shifts and conserved embryonic shape underlie crocodylian craniofacial disparity and convergence. Proc. R. Soc. B286, 20182389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons, K., Dugon, M. M. & Healy, K. Diet breadth mediates the prey specificity of venom potency in snakes. Toxins12, 74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobreva, M. P., Camacho, J. & Abzhanov, A. Time to synchronize our clocks: connecting developmental mechanisms and evolutionary consequences of heterochrony. J. Exp. Zoolog. B338, 87–106 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Yohe, L. R. et al. Ecological constraints on highly evolvable olfactory receptor genes and morphology in Neotropical bats. Evolution76, 2347–2360 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadier, A. et al. Bat teeth illuminate the diversification of mammalian tooth classes. Nat. Commun.14, 4687 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deas, J. B., Blondel, L. & Extavour, C. G. Ancestral and offspring nutrition interact to affect life-history traits in Drosophila melanogaster. Proc. R. Soc. B286, 20182778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyama, T., Texada, M. J., Halberg, K. A. & Rewitz, K. Metabolism and growth adaptation to environmental conditions in Drosophila. Cell. Mol. Life Sci.77, 4523–4551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karasov, W. H. & Caviedes-Vidal, E. Adaptation of intestinal epithelial hydrolysis and absorption of dietary carbohydrate and protein in mammals and birds. Comp. Biochem. Physiol. A253, 110860 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Riddle, M. R. et al. Genetic mapping of metabolic traits in the blind Mexican cavefish reveals sex-dependent quantitative trait loci associated with cave adaptation. BMC Ecol. Evol.21, 1–22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong, S. et al. Enhanced lipogenesis through Pparγ helps cavefish adapt to food scarcity. Curr. Biol.32, 2272–2280.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisaka, S. et al. Diet, genetics, and the gut microbiome drive dynamic changes in plasma metabolites. Cell Rep.22, 3072–3086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karasov, W. H. & Douglas, A. E. Comparative digestive physiology. Compr. Physiol.3, 741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa, D. P. & Ortiz, C. L. Blood chemistry homeostasis during prolonged fasting in the northern elephant seal. Am. J. Physiol. Regul. Integr. Comp. Physiol.242, R591–R595 (1982). [DOI] [PubMed] [Google Scholar]
- 17.Tomasek, O., Bobek, L., Kralova, T., Adamkova, M. & Albrecht, T. Fuel for the pace of life: baseline blood glucose concentration co‐evolves with life‐history traits in songbirds. Funct. Ecol.33, 239–249 (2019). [Google Scholar]
- 18.Sparkman, A. M. et al. Convergence in reduced body size, head size, and blood glucose in three island reptiles. Ecol. Evol.8, 6169–6182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett, K. A., Turner, L. M., Millward, S., Moss, S. E. & Hall, A. J. Obtaining accurate glucose measurements from wild animals under field conditions: comparing a hand held glucometer with a standard laboratory technique in grey seals. Conserv. Physiol.5, cox013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxton, M. W. et al. Serum plays an important role in reprogramming the seasonal transcriptional profile of brown bear adipocytes. iScience25, 105084 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oriel, R. C., Wiley, C. D., Dewey, M. J. & Vrana, P. B. Adaptive genetic variation, stress and glucose regulation. Dis. Model. Mech.1, 255–263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelm, D. H., Simon, R., Kuhlow, D., Voigt, C. C. & Ristow, M. High activity enables life on a high-sugar diet: blood glucose regulation in nectar-feeding bats. Proc. R. Soc. B278, 3490–3496 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riddle, M. R. et al. Insulin resistance in cavefish as an adaptation to a nutrient-limited environment. Nature555, 647–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reznick, J., Park, T. J. & Lewin, G. R. in The Extraordinary Biology of the Naked Mole-Rat (eds Buffenstein, R., Park, T. J. & Holmes, M. M.) 271–286 (Springer, 2021).
- 25.Gao, X. et al. The function of glucose metabolism in embryonic diapause of annual killifish. Comp. Biochem. Physiol. Part D42, 100965 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Chouchani, E. T. & Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab.1, 189–200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bundgaard, A. et al. Metabolic adaptations during extreme anoxia in the turtle heart and their implications for ischemia-reperfusion injury. Sci Rep.9, 2850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuweiler, G. The Biology of Bats (Oxford Univ. Press, 2000).
- 29.Arbour, J. H., Curtis, A. A. & Santana, S. E. Signatures of echolocation and dietary ecology in the adaptive evolution of skull shape in bats. Nat. Commun.10, 2036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price, S. A., Hopkins, S. S., Smith, K. K. & Roth, V. L. Tempo of trophic evolution and its impact on mammalian diversification. Proc. Natl Acad. Sci USA109, 7008–7012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camacho, J. et al. Differential cellular proliferation underlies heterochronic generation of cranial diversity in phyllostomid bats. EvoDevo11, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt, G. R. & Kalf, G. F. The chemistry of insect hemolymph. J. Gen. Physiol.40, 833–847 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker, H. G., Baker, I. & Hodges, S. A. Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica30, 559–586 (1998). [Google Scholar]
- 34.Schondube, J. E., Herrera-M, L. G. & Martínez Del Rio, C. Diet and the evolution of digestion and renal function in phyllostomid bats. Zoology104, 59–73 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Jiao, H. et al. Trehalase gene as a molecular signature of dietary diversification in mammals. Mol. Biol. Evol.36, 2171–2183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zepeda Mendoza, M. L. et al. Hologenomic adaptations underlying the evolution of sanguivory in the common vampire bat. Nat. Ecol. Evol.2, 659–668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingala, M. R. et al. You are more than what you eat: potentially adaptive enrichment of microbiome functions across bat dietary niches. Anim. Microbiome3, 82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos, M., Aguirre, L. F., Vázquez, L. B. & Ortega, J. Phyllostomus hastatus. Mamm. Species10.1644/0.722.1 (2003). 10.1644/0.722.1 [DOI] [Google Scholar]
- 39.York, H. A. & Billings, S. A. Stable-isotope analysis of diets of short-tailed fruit bats (Chiroptera: Phyllostomidae: Carollia). J. Mammal.90, 1469–1477 (2009). [Google Scholar]
- 40.Protzek, A. O. P. et al. Insulin and glucose sensitivity, insulin secretion and β-cell distribution in endocrine pancreas of the fruit bat Artibeus lituratus. J. Comp. Physiol. B180, 627–634 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Ayala-Berdon, J., Rodríguez-Peña, N., García Leal, C., Stoner, K. E. & Schondube, J. E. Sugar gustatory thresholds and sugar selection in two species of Neotropical nectar-eating bats. Comp. Biochem. Physiol. A164, 307–313 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Ortega-García, S. & Saldaña-Vázquez, R. A. Synthesis of knowledge of the plant diet of nectar-feeding bats of Mexico. Therya13, 335–343 (2022). [Google Scholar]
- 43.Welch, K. C. Jr, Herrera M, L. G. & Suarez, R. K. Dietary sugar as a direct fuel for flight in the nectarivorous bat Glossophaga soricina. J. Exp. Biol.211, 310–316 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Welch, K. C. & Chen, C. C. Sugar flux through the flight muscles of hovering vertebrate nectarivores: a review. J. Comp. Physiol. B184, 945–959 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Peng, X. et al. Flight is the key to postprandial blood glucose balance in the fruit bats Eonycteris spelaea and Cynopterus sphinx. Ecol. Evol.7, 8804–8811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro, D. L. J. et al. Insulin and glucose regulation at rest and during flight in a Neotropical nectar-feeding bat. Mamm. Biol.101, 987–996 (2021). [Google Scholar]
- 47.Pagel, M. Inferring the historical patterns of biological evolution. Nature401, 877–884 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Saldaña‐Vázquez, R. A., Ruiz‐Sanchez, E., Herrera‐Alsina, L. & Schondube, J. E. Digestive capacity predicts diet diversity in Neotropical frugivorous bats. J. Anim. Ecol.84, 1396–1404 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Burant, C. F., Takeda, J., Brot-Laroche, E., Bell, G. I. & Davidson, N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem.267, 14523–14526 (1992). [PubMed] [Google Scholar]
- 50.Mizuno, T. M., Lew, P. S. & Jhanji, G. Regulation of the fructose transporter gene Slc2a5 expression by glucose in cultured microglial cells. Int. J. Mol. Sci.22, 12668 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navale, A. M. & Paranjape, A. N. Glucose transporters: physiological and pathological roles. Biophys. Rev.8, 5–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veys, K. et al. Role of the GLUT1 glucose transporter in postnatal CNS angiogenesis and blood-brain barrier integrity. Circ. Res.127, 466–482 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia58, 221–232 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Gerhart, D. Z., Broderius, M. A., Borson, N. D. & Drewes, L. R. Neurons and microvessels express the brain glucose transporter protein GLUT3. Proc. Natl Acad. Sci USA.89, 733–737 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter, E. A. & Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev.93, 993–1017 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Smith, M. D. et al. Less is more: an adaptive branch-site random effects model for efficient detection of episodic diversifying selection. Mol. Biol. Evol.32, 1342–1353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pond, S. L. K., Frost, S. D. W. & Muse, S. V. HyPhy: hypothesis testing using phylogenies. Bioinformatics21, 676–679 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Pond, S. L. K. et al. HyPhy 2.5—a customizable platform for evolutionary hypothesis testing using phylogenies. Mol. Biol. Evol.37, 295–299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol.42, 243–246 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann, W. & Hauser, F. The P-domain or trefoil motif: a role in renewal and pathology of mucous epithelia? Trends Biochem. Sci18, 239–243 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Quistgaard, E. M., Löw, C., Moberg, P., Trésaugues, L. & Nordlund, P. Structural basis for substrate transport in the GLUT-homology family of monosaccharide transporters. Nat. Struct. Mol. Biol.20, 766–768 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Yasutake, H., Goda, T. & Takase, S. Dietary regulation of sucrase–isomaltase gene expression in rat jejunum. Biochim. Biophys. Acta BBA1243, 270–276 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Arita, H. T. & Santos-del-Prado, K. Conservation biology of nectar-feeding bats in Mexico. J. Mammal.80, 31–41 (1999). [Google Scholar]
- 65.McNab, B. K. Energetics and the distribution of vampires. J. Mammal.54, 131–144 (1973). [Google Scholar]
- 66.Horner, M. A., Fleming, T. H. & Sahey, C. T. Foraging behaviour and energetics of a nectar-feeding bat, Leptonycteris curasoae (Chiroptera: Phyllostomidae). J. Zool.244, 575–586 (1998). [Google Scholar]
- 67.Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell143, 1174–1189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freitas, M. B. et al. Reduced insulin secretion and glucose intolerance are involved in the fasting susceptibility of common vampire bats. Gen. Comp. Endocrinol.183, 1–6 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Simpson, I. A. et al. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol.-Endocrinol. Metab.295, E242–E253 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hutcheon, J. M., Kirsch, J. A. & Garland, T. Jr A comparative analysis of brain size in relation to foraging ecology and phylogeny in the Chiroptera. Brain. Behav. Evol.60, 165–180 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Hall, R. P. et al. Find the food first: an omnivorous sensory morphotype predates biomechanical specialization for plant based diets in phyllostomid bats. Evolution75, 2791–2801 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Amaral, T. S., Pinheiro, E. C., Freitas, M. B. & Aguiar, L. M. S. Low energy reserves are associated with fasting susceptibility in Neotropical nectar bats Glossophaga soricina. Braz. J. Biol.79, 165–168 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Potter, J. H. T. et al. Dietary diversification and specialization in neotropical bats facilitated by early molecular evolution. Mol. Biol. Evol.38, 3864–3883 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher, R. B. & Parsons, D. S. The gradient of mucosal surface area in the small intestine of the rat. J. Anat.84, 272–282 (1950). [PMC free article] [PubMed] [Google Scholar]
- 75.Riesenfeld, G., Sklan, D., Bar, A., Eisner, U. & Hurwitz, S. Glucose absorption and starch digestion in the intestine of the chicken. J. Nutr.110, 117–121 (1980). [DOI] [PubMed] [Google Scholar]
- 76.Wright, E. M., Sala-Rabanal, M., Ghezzi, C. & Loo, D. D. F. in Physiology of the Gastrointestinal Tract, 6th edn (ed. Said, H. M.) 1051–1062 (Elsevier, 2018).
- 77.Silva, C. et al. Comparative study on the small and large intestines of the bats Artibeus planirostris and Diphylla ecaudata: influence of food habits on morphological parameters. Acta Chiropterologica22, 435–448 (2020). [Google Scholar]
- 78.Park, H. & Hall, R. The gross anatomy of the tongues and stomachs of eight new world bats. Trans. Kans. Acad. Sci.54, 64–72 (1951). [Google Scholar]
- 79.Nicolson, S. W. Sweet solutions: nectar chemistry and quality. Philos. Trans. R. Soc. B377, 20210163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Göttlinger, T., Schwerdtfeger, M., Tiedge, K. & Lohaus, G. What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species. Front. Plant Sci.10, 205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hilton, W. A. The morphology and development of intestinal folds and villi in vertebrates. Am. J. Anat.1, 459–505 (1902). [Google Scholar]
- 82.Walton, K. D., Mishkind, D., Riddle, M. R., Tabin, C. J. & Gumucio, D. L. Blueprint for an intestinal villus: species-specific assembly required. Wiley Interdiscip. Rev. Dev. Biol.7, e317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strobel, S., Encarnação, J. A., Becker, N. I. & Trenczek, T. E. Histological and histochemical analysis of the gastrointestinal tract of the common pipistrelle bat (Pipistrellus pipistrellus). Eur. J. Histochem.59, 2477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scillitani, G., Zizza, S., Liquori, G. E. & Ferri, D. Lectin histochemistry of gastrointestinal glycoconjugates in the greater horseshoe bat, Rhinolophus ferrumequinum (Schreber, 1774). Acta Histochem.109, 347–357 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Gadelha-Alves, R., Rozensztranch, A. M. D. S. & Rocha-Barbosa, O. Comparative intestinal histomorphology of five species of phyllostomid bats (Phyllostomidae, Microchiroptera): ecomorphological relations with alimentary habits. Int. J. Morphol.26, 591–602 (2008). [Google Scholar]
- 86.Novaes, R. L. M. et al. First evidence of frugivory in Myotis (Chiroptera, Vespertilionidae, Myotinae). Biodivers. Data J. 10.3897/BDJ.3.e6841 (2015). [DOI] [PMC free article] [PubMed]
- 87.Makanya, A., John, M., Mayhew, T., Tschanz, S. & Burri, P. A stereological comparison of villous and microvillous surfaces in small intestines of frugivorous and entomophagous bats: species, inter-individual and craniocaudal differences. J. Exp. Biol.200, 2415–2423 (1997). [DOI] [PubMed] [Google Scholar]
- 88.Makanya, A. N., Self, T. J., Warui, C. N. & Mwangi, D. K. Gut morphology and morphometry in the epauletted wahlbergs fruit bat (Epomophorus wahlbergi, Sundevall, 1846). Acta Biol. Hung.52, 75–89 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Caviedes-Vidal, E. et al. Paracellular absorption: a bat breaks the mammal paradigm. PLoS ONE3, e1425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brun, A. et al. High paracellular nutrient absorption in intact bats is associated with high paracellular permeability in perfused intestinal segments. J. Exp. Biol.217, 3311–3317 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Brun, A. et al. Morphological bases for intestinal paracellular absorption in bats and rodents. J. Morphol.280, 1359–1369 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Fasulo, S. V. Absorción intestinal en mamíferos. Evaluación comparativa de la ruta paracelular en mamíferos terrestres y voladores. Mastozool. Neotropical21, 180–181 (2014). [Google Scholar]
- 93.Price, E. R., Brun, A., Caviedes-Vidal, E. & Karasov, W. H. Digestive adaptations of aerial lifestyles. Physiology30, 69–78 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Shirazi-Beechey, S. P., Moran, A. W., Batchelor, D. J., Daly, K. & Al-Rammahi, M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc. Nutr. Soc.70, 185–193 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Ali, R. S. et al. Glucose transporter expression and regulation following a fast in the ruby-throated hummingbird, Archilochus colubris. J. Exp. Biol.223, jeb229989 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meng, F., Zhu, L., Huang, W., Irwin, D. M. & Zhang, S. Bats: body mass index, forearm mass index, blood glucose levels and SLC2A2 genes for diabetes. Sci Rep.6, 29960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinheiro, E. C., Taddei, V. A., Migliorini, R. H. & Kettelhut, I. C. Effect of fasting on carbohydrate metabolism in frugivorous bats (Artibeus lituratus and Artibeus jamaicensis). Comp. Biochem. Physiol. B143, 279–284 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Tobin, V. et al. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes57, 555–562 (2008). [DOI] [PubMed] [Google Scholar]
- 99.Suarez, R. K. & Welch, K. C. Sugar metabolism in hummingbirds and nectar bats. Nutrients9, 743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Voigt, C. C. & Speakman, J. R. Nectar-feeding bats fuel their high metabolism directly with exogenous carbohydrates. Funct. Ecol.21, 913–921 (2007). [Google Scholar]
- 101.Marks, J., Carvou, N. J. C., Debnam, E. S., Srai, S. K. & Unwin, R. J. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J. Physiol.553, 137–145 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ait-Omar, A. et al. GLUT2 accumulation in enterocyte apical and intracellular membranes. Diabetes60, 2598–2607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gromova, L. V., Fetissov, S. O. & Gruzdkov, A. A. Mechanisms of glucose absorption in the small intestine in health and metabolic diseases and their role in appetite regulation. Nutrients13, 2474 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freeman, P. W. in Bat Biology and Conservation (eds Kunz T. H. & Racey, P. A.) 140–156 (Smithsonian Institution Scholarly Press, 1998).
- 105.Rojas, D., Vale, A., Ferrero, V. & Navarro, L. When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Mol. Ecol.20, 2217–2228 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Santana, S. E., Strait, S. & Dumont, E. R. The better to eat you with: functional correlates of tooth structure in bats. Funct. Ecol.25, 839–847 (2011). [Google Scholar]
- 107.Kurta, A., Bell, G. P., Nagy, K. A. & Kunz, T. H. Energetics of pregnancy and lactation in freeranging little brown bats (Myotis lucifugus). Physiol. Zool.62, 804–818 (1989). [Google Scholar]
- 108.Voigt, C. Reproductive energetics of the nectar-feeding bat Glossophaga soricina (Phyllostomidae). J. Comp. Physiol. B173, 79–85 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Bürkner, P. C. Advanced Bayesian multilevel modeling with the R package brms. R J.10, 395–411 (2018). [Google Scholar]
- 110.Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics35, 526–528 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol.3, 217–223 (2012). [Google Scholar]
- 112.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
- 113.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ishida, M. et al. The comparative anatomy of the folds, fossae, and adhesions around the duodenojejunal flexure in mammals. Folia Morphol.77, 286–292 (2018). [DOI] [PubMed] [Google Scholar]
- 115.consortium, U. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res.49, D480–D489 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buels, R. et al. JBrowse: a dynamic web platform for genome visualization and analysis. Genome Biol.17, 66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spoor, S. et al. Tripal v3: an ontology-based toolkit for construction of FAIR biological community databases. Database2019, baz077 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics25, 1972–1973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dylus, D. et al. How to build phylogenetic species trees with OMA. F1000Research9, 511 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller-Butterworth, C. M. et al. A family matter: conclusive resolution of the taxonomic position of the long-fingered bats, miniopterus. Mol. Biol. Evol.24, 1553–1561 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Lim, B. Review of the origins and biogeography of bats in South America. Chiropt. Neotrop.15, 391–410 (2009). [Google Scholar]
- 122.Rojas, D., Warsi, O. & Davalos, L. Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant neotropical diversity. Syst. Biol.65, 432–448 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Original analysis generated by Hyphy in .json format per gene.
Html links to predicted proteins.
Unprocessed qPCR data.
Multiple raw image files within folder.
Raw data for intestinal measurements
Average glucose tolerance test (GTT) values from 0, 10, 30 and 60 used for AUC.
Values for average glucose tolerance test (GTT) per species.
Data used for calculations.
HCR source data per enterocyte.
Data Availability Statement
Original data underlying this manuscript can be publically accessed from the Stowers Original Data Repository at http://www.stowers.org/research/publications/LIBPB-2406. Original genome data can be found in Extended Data Table 3. Our Genome browsers are publically available here: https://simrbase.stowers.org/bats_sugar_assimilation. Source data are provided with this paper.