Abstract
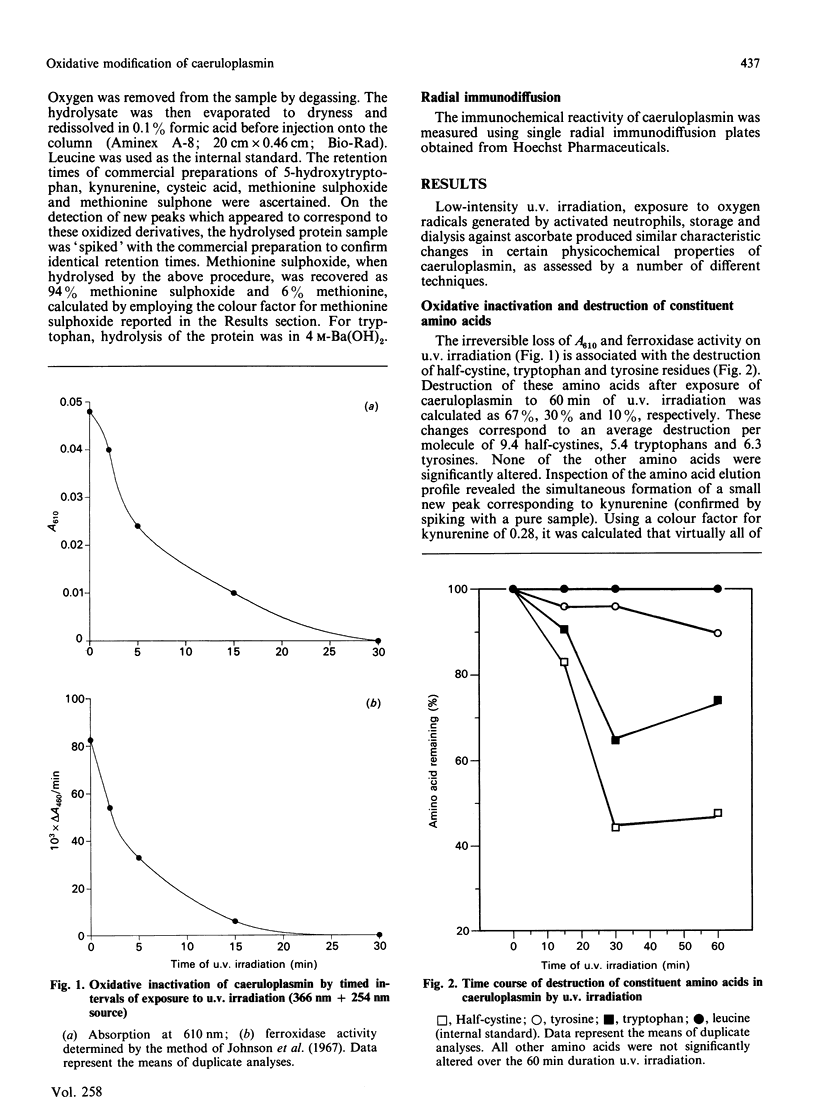
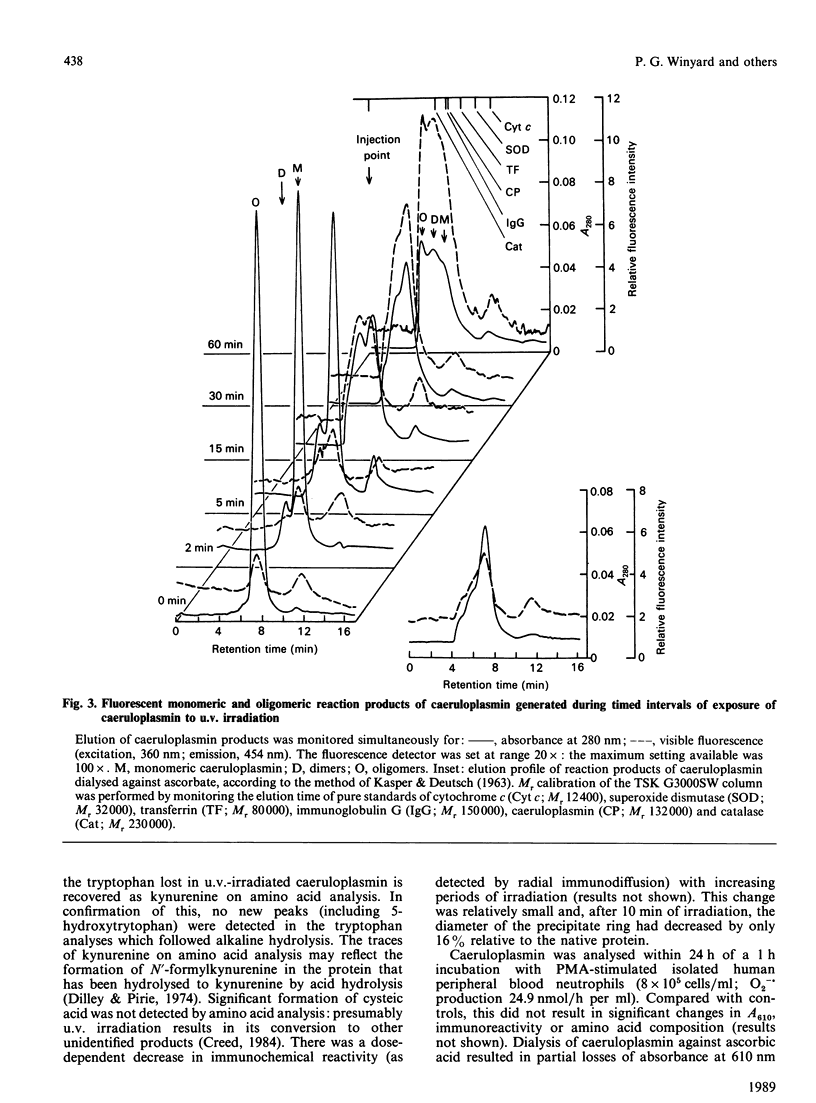
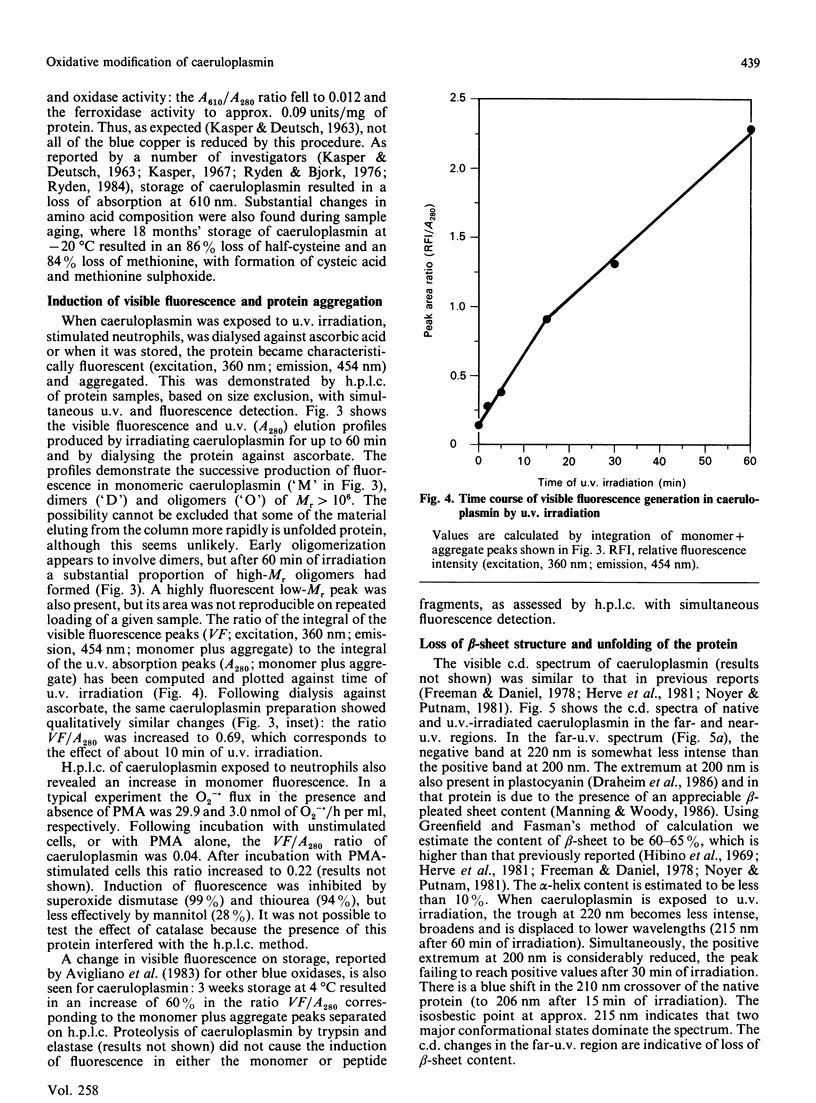
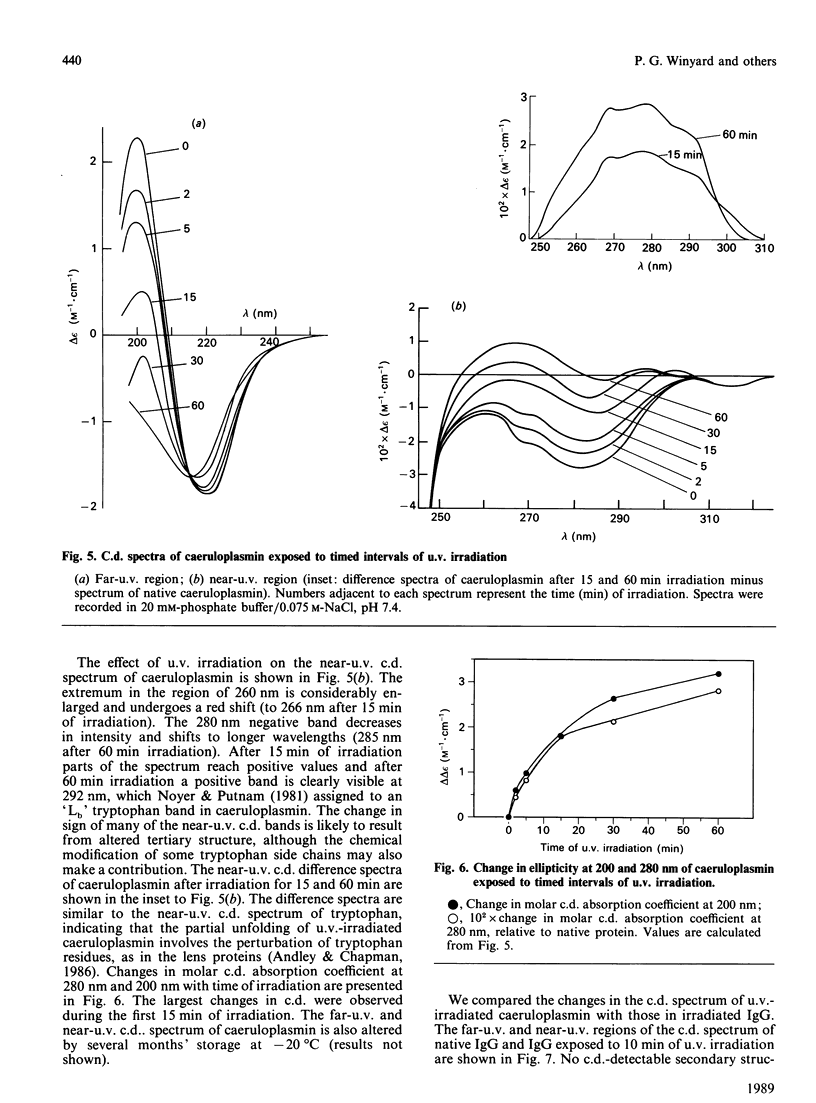
We report the effects of oxidative stress generated by low-intensity u.v. irradiation (366 and 254 nm), dialysis against ascorbate and isolated stimulated neutrophils on some physicochemical properties of caeruloplasmin. Low-intensity u.v. irradiation resulted in a loss of ferroxidase activity and 610 nm absorption, changes previously reported to occur during storage and manipulation of caeruloplasmin. These alterations were found to correspond to aggregation of the protein, induction of visible fluorescence (excitation, 360 nm; emission, 454 nm), changes in c.d. spectra which were indicative of alterations in protein conformation, loss of half-cystine, tryptophan and tyrosine content and loss immunoreactivity. The changes in the far-u.v. c.d. spectrum of caeruloplasmin were more pronounced than those observed for u.v.-irradiated IgG. Similar c.d. changes and induction of fluorescence were observed following dialysis of caeruloplasmin against ascorbate or exposure to stimulated neutrophils. It is concluded that the lability of caeruloplasmin may arise from oxidative modification, in addition to the previously described susceptibility of this protein to proteolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APRISON M. H., HANSON K. M. Mechanism of inactivation of serum oxidase (ceruloplasmin) activity by ultraviolet radiation. Proc Soc Exp Biol Med. 1959 Mar;100(3):643–647. doi: 10.3181/00379727-100-24729. [DOI] [PubMed] [Google Scholar]
- Andley U. P., Chapman S. F. Conformational changes of bovine lens crystallins in a photodynamic system. Photochem Photobiol. 1986 Jul;44(1):67–74. doi: 10.1111/j.1751-1097.1986.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Mahood J. F., Willson R. L., Wolfenden B. S. Does caeruloplasmin dismute superoxide? No. FEBS Lett. 1980 Aug 25;118(1):127–129. doi: 10.1016/0014-5793(80)81233-6. [DOI] [PubMed] [Google Scholar]
- Brailsford S., Lunec J., Winyard P., Blake D. R. A possible role for ferritin during inflammation. Free Radic Res Commun. 1985;1(2):101–109. doi: 10.3109/10715768509056542. [DOI] [PubMed] [Google Scholar]
- Calabrese L., Carbonaro M. An e.p.r. study of the non-equivalence of the copper sites of caeruloplasmin. Biochem J. 1986 Aug 15;238(1):291–295. doi: 10.1042/bj2380291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinum J., Vänngård T. The stoichiometry of the paramagnetic copper and the oxidation-reduction potentials of type I copper in human ceruloplasmin. Biochim Biophys Acta. 1973 Jun 15;310(2):321–330. doi: 10.1016/0005-2795(73)90112-8. [DOI] [PubMed] [Google Scholar]
- Dilley K. J., Pirie A. Changes to the proteins of the human lens nucleus in cataract. Exp Eye Res. 1974 Jul;19(1):59–72. doi: 10.1016/0014-4835(74)90073-6. [DOI] [PubMed] [Google Scholar]
- Draheim J. E., Anderson G. P., Duane J. W., Gross E. L. Plastocyanin conformation. An analysis of its near ultraviolet absorption and circular dichroic spectra. Biophys J. 1986 Apr;49(4):891–900. doi: 10.1016/S0006-3495(86)83717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S., Daniel E. A spectral study of human ceruloplasmin. Biochim Biophys Acta. 1978 May 24;534(1):132–140. doi: 10.1016/0005-2795(78)90483-x. [DOI] [PubMed] [Google Scholar]
- Freeman S., Daniel E. Dissociation and reconstitution of human ceruloplasmin. Biochemistry. 1973 Nov 6;12(23):4806–4810. doi: 10.1021/bi00747a038. [DOI] [PubMed] [Google Scholar]
- Galdston M., Levytska V., Schwartz M. S., Magnússon B. Ceruloplasmin. Increased serum concentration and impaired antioxidant activity in cigarette smokers, and ability to prevent suppression of elastase inhibitory capacity of alpha 1-proteinase inhibitor. Am Rev Respir Dis. 1984 Feb;129(2):258–263. [PubMed] [Google Scholar]
- Garner M. H., Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Grossweiner L. I. Photochemistry of proteins: a review. Curr Eye Res. 1984 Jan;3(1):137–144. doi: 10.3109/02713688408997195. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Hill C., Blake D. R. Copper stimulated phospholipid membrane peroxidation: antioxidant activity of serum and synovial fluid from patients with rheumatoid arthritis. Clin Chim Acta. 1984 May 16;139(1):85–90. doi: 10.1016/0009-8981(84)90195-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Herve M., Garnier A., Tosi L., Steinbuch M. The effects of neutral salts on the conformational transition of ceruloplasmin. Biochim Biophys Acta. 1975 Oct 20;405(2):318–323. doi: 10.1016/0005-2795(75)90097-5. [DOI] [PubMed] [Google Scholar]
- Hervé M., Garnier A., Tosi L., Steinbuch M. Spectroscopic and photoreduction studies of copper chromophores in ceruloplasmin. Eur J Biochem. 1981 May;116(1):177–183. doi: 10.1111/j.1432-1033.1981.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Hibino Y., Samejima T., Kajiyama S., Nosoh Y. On the conformation of porcine ceruloplasmin. Arch Biochem Biophys. 1969 Mar;130(1):617–623. doi: 10.1016/0003-9861(69)90078-2. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Osaki S., Frieden E. A micromethod for the determination of ferroxidase (ceruloplasmin) in human serums. Clin Chem. 1967 Feb;13(2):142–150. [PubMed] [Google Scholar]
- KASPER C. B., DEUTSCH H. F. Physicochemical studies of human ceruloplasmin. J Biol Chem. 1963 Jul;238:2325–2337. [PubMed] [Google Scholar]
- Kasper C. B. Limited trypsin-catalyzed hydrolysis of crystalline human ceruloplasmin. Partial characterization of the digest. Biochemistry. 1967 Oct;6(10):3185–3197. doi: 10.1021/bi00862a029. [DOI] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal K., Bose S. K., Chakrabarti B. Sensitizer-induced conformational changes in lens crystallin--I. Photodynamic action of methylene blue and N-formylkynurenine on bovine alpha-crystallin. Photochem Photobiol. 1986 May;43(5):515–523. doi: 10.1111/j.1751-1097.1986.tb09529.x. [DOI] [PubMed] [Google Scholar]
- Marx G., Chevion M. Site-specific modification of albumin by free radicals. Reaction with copper(II) and ascorbate. Biochem J. 1986 Jun 1;236(2):397–400. doi: 10.1042/bj2360397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyer M., Putnam F. W. A circular dichroism study of undegraded human ceruloplasmin. Biochemistry. 1981 Jun 9;20(12):3536–3542. doi: 10.1021/bi00515a036. [DOI] [PubMed] [Google Scholar]
- Orr C. W. Studies on ascorbic acid. II. Physical changes in catalase following incubation with ascorbate or ascorbate and copper (II). Biochemistry. 1967 Oct;6(10):3000–3006. doi: 10.1021/bi00862a005. [DOI] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]
- Patel S., Rosenthal J. T. Hypercalcemia in carcinoma of prostate. Its cure by orchiectomy. Urology. 1985 Jun;25(6):627–629. doi: 10.1016/0090-4295(85)90297-3. [DOI] [PubMed] [Google Scholar]
- Płonka A., Metodiewa D., Zgirski A., Hilewicz M., Leyko W. ESR evidence of superoxide radical dismutation by human ceruloplasmin. Biochem Biophys Res Commun. 1980 Aug 14;95(3):978–984. doi: 10.1016/0006-291x(80)91569-7. [DOI] [PubMed] [Google Scholar]
- Rydén L., Björk I. Reinvestigation of some physicochemical and chemical properties of human ceruloplasmin (ferroxidase). Biochemistry. 1976 Aug 10;15(16):3411–3417. doi: 10.1021/bi00661a003. [DOI] [PubMed] [Google Scholar]
- Rydén L. Evidence for proteolytic fragments in commercial samples of human ceruloplasmin. FEBS Lett. 1971 Nov 1;18(2):321–325. doi: 10.1016/0014-5793(71)80477-5. [DOI] [PubMed] [Google Scholar]
- Rydén L. Model of the active site in the blue oxidases based on the ceruloplasmin-plastocyanin homology. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6767–6771. doi: 10.1073/pnas.79.22.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Shinar E., Navok T., Chevion M. The analogous mechanisms of enzymatic inactivation induced by ascorbate and superoxide in the presence of copper. J Biol Chem. 1983 Dec 25;258(24):14778–14783. [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Syed M. A., Coombs T. L., Goodman B. A., McPhail D. B. The nature of the copper (II) components of caeruloplasmin. Biochem J. 1982 Oct 1;207(1):183–184. doi: 10.1042/bj2070183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K. C., Linder M. C. Copper transport in rats involving a new plasma protein. Am J Physiol. 1985 Jul;249(1 Pt 1):E77–E88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- Winyard P., Lunec J., Brailsford S., Blake D. Action of free radical generating systems upon the biological and immunological properties of caeruloplasmin. Int J Biochem. 1984;16(12):1273–1278. doi: 10.1016/0020-711x(84)90227-1. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. S., Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1449–1454. doi: 10.1016/0006-291x(80)90113-8. [DOI] [PubMed] [Google Scholar]


