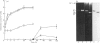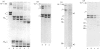Abstract
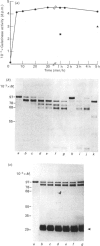
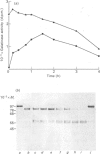
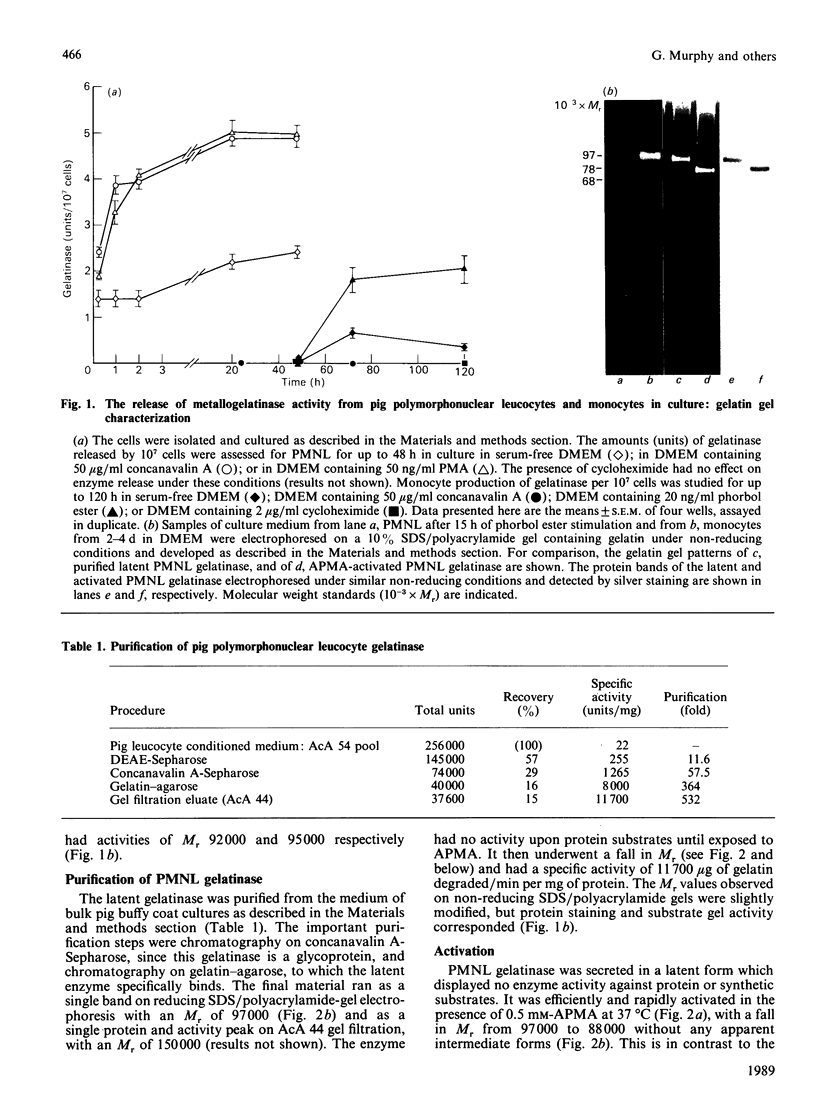
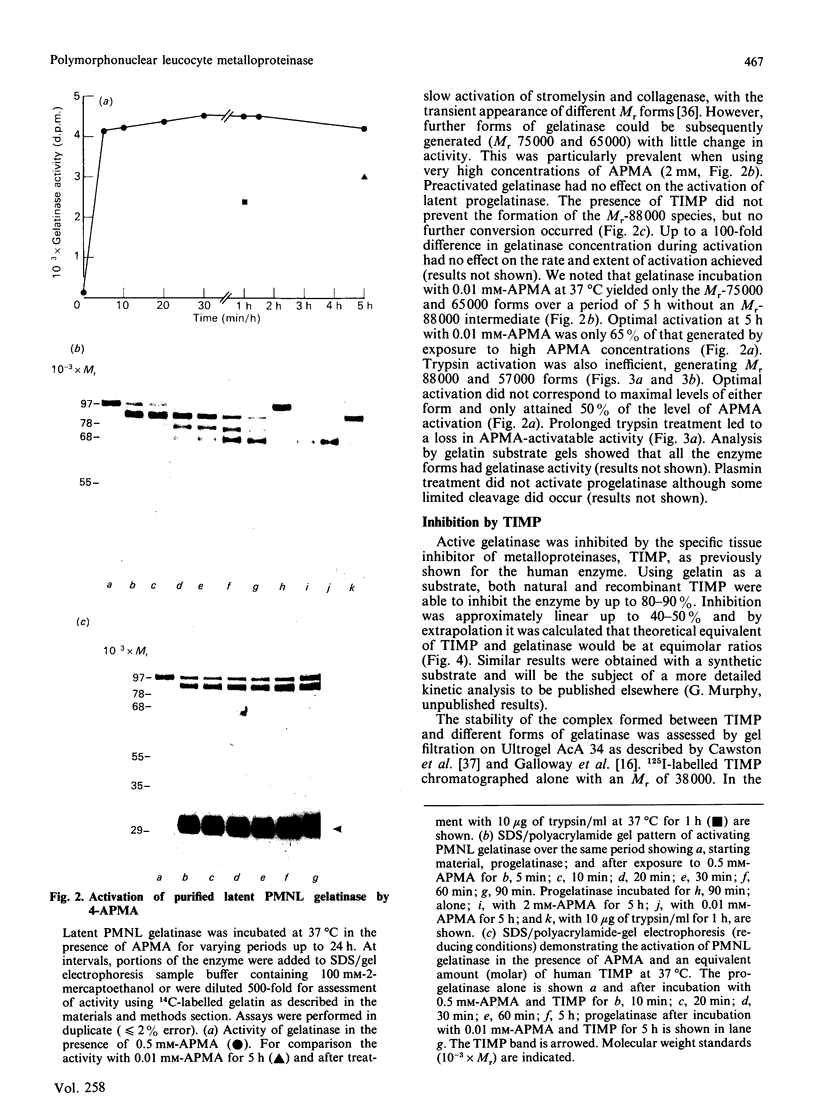
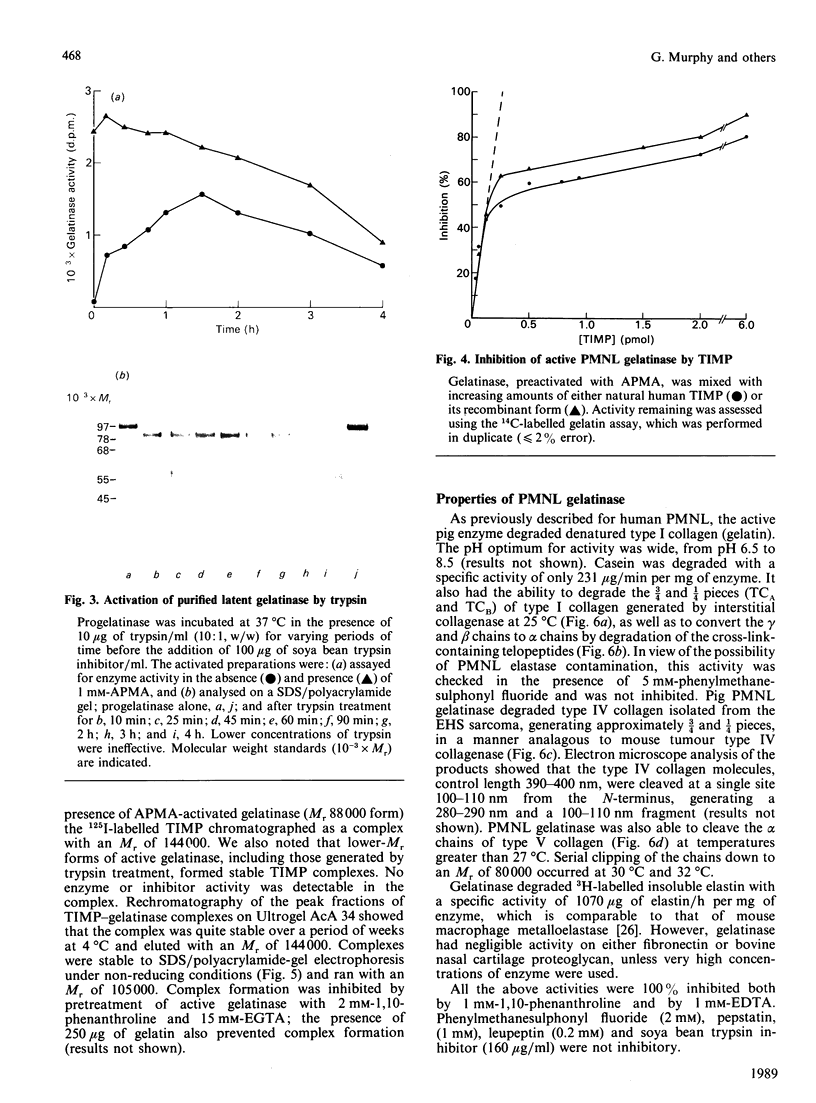
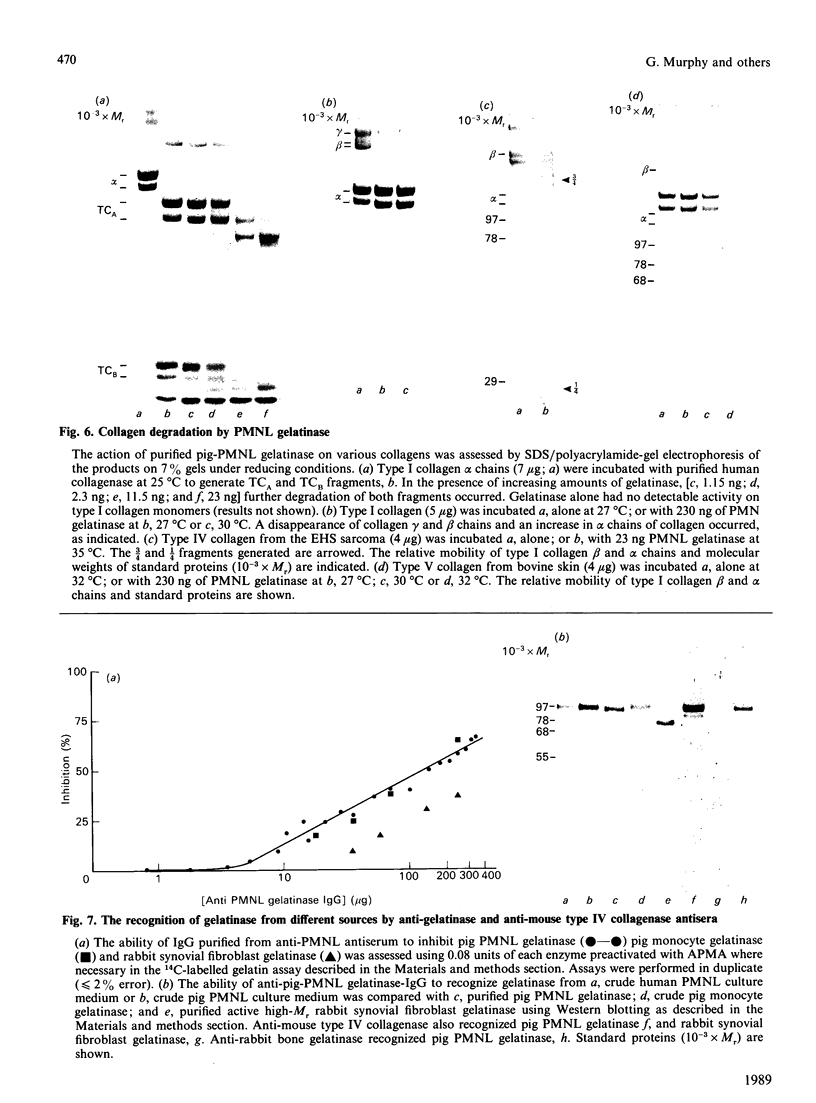
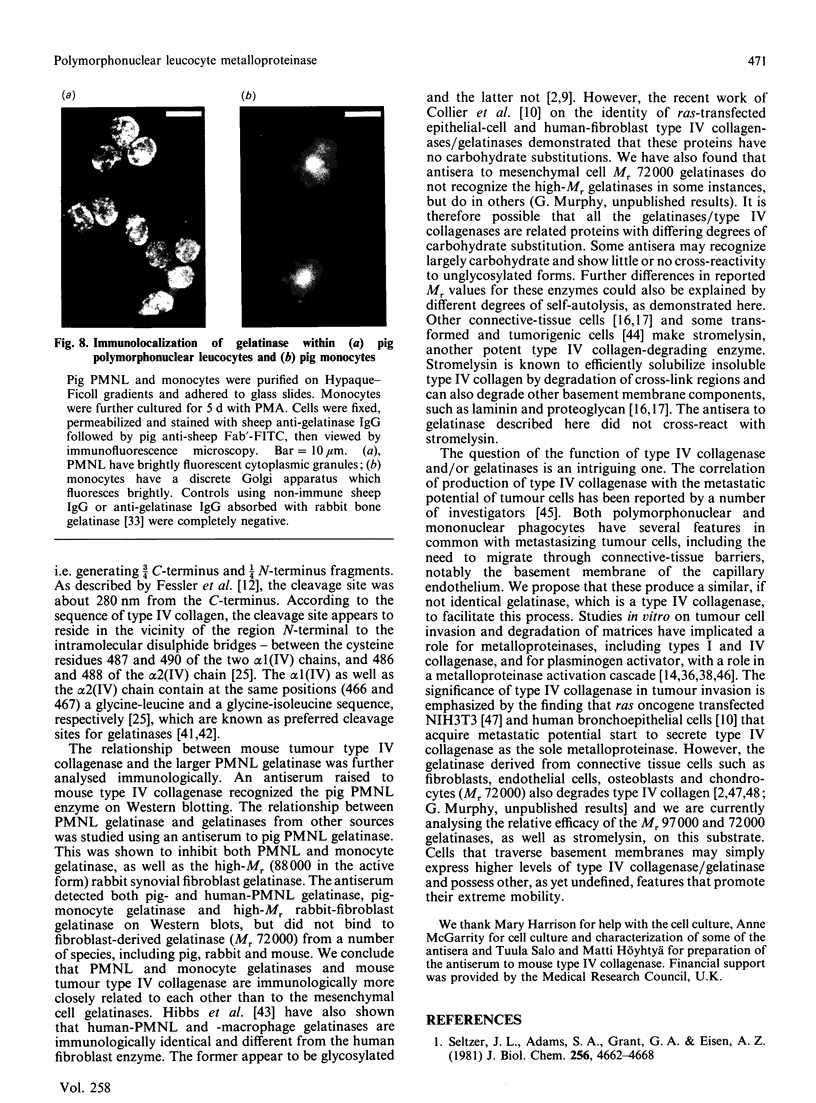
The metalloproteinase 'gelatinase' stored in the granules of pig polymorphonuclear leucocytes has been purified in the latent form. The enzyme is secreted as an Mr 97,000 proenzyme that can be activated in the presence of 4-aminophenylmercuric acetate (APMA) by self-cleavage to generate lower-Mr species, of which an Mr 88,000 form was the most active. Trypsin-initiated activation generated different Mr gelatinases of much lower specific activity. Activation was slowed but not prevented by the presence of the tissue inhibitor of metalloproteinases, TIMP. The activated gelatinase formed a stable complex (Mr 144,000) with TIMP, in a Zn2+- and Ca2+-dependent manner, and complex formation was inhibited by the presence of the substrate gelatin. Similar to the human granulocyte gelatinase, the organomercurial-activated pig enzyme degraded gelatin and TCA and TCB fragments of type I collagen, as well as elastin and types IV and V collagen. The degradation of type IV collagen was shown, both by polyacrylamide-gel electrophoresis and by electron microscopic analysis, to generate 3/4 and 1/4 fragments as described for mouse tumour type IV collagenase. Furthermore, an antiserum raised to mouse type IV collagenase recognized the pig granulocyte gelatinase. An antiserum to the pig polymorphonuclear leucocyte gelatinase recognized other high-Mr gelatinases, including those from human granulocytes, pig monocytes and rabbit connective tissue cells, but not the Mr 72,000 enzyme from connective tissue cells. These data suggest that there are two distinct major forms of gelatinolytic activity that also cause specific cleavage of type IV collagen. These enzymes are associated with a wide variety of normal connective tissue and haemopoietic cells, as well as many tumour cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazel D., Pollner R., Oberbäumer I., Kühn K. Human basement membrane collagen (type IV). The amino acid sequence of the alpha 2(IV) chain and its comparison with the alpha 1(IV) chain reveals deletions in the alpha 1(IV) chain. Eur J Biochem. 1988 Feb 15;172(1):35–42. doi: 10.1111/j.1432-1033.1988.tb13852.x. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Murphy G., Mercer E., Galloway W. A., Hazleman B. L., Reynolds J. J. The interaction of purified rabbit bone collagenase with purified rabbit bone metalloproteinase inhibitor. Biochem J. 1983 May 1;211(2):313–318. doi: 10.1042/bj2110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Davies M., Thomas G. J., Martin J., Lovett D. H. The purification and characterization of a glomerular-basement-membrane-degrading neutral proteinase from rat mesangial cells. Biochem J. 1988 Apr 15;251(2):419–425. doi: 10.1042/bj2510419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Duncan K. G., Fessler J. H., Salo T., Tryggvason K. Characterization of the procollagen IV cleavage products produced by a specific tumor collagenase. J Biol Chem. 1984 Aug 10;259(15):9783–9789. [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbisa S., Ballin M., Daga-Gordini D., Fastelli G., Naturale M., Negro A., Semenzato G., Liotta L. A. Transient expression of type IV collagenolytic metalloproteinase by human mononuclear phagocytes. J Biol Chem. 1986 Feb 15;261(5):2369–2375. [PubMed] [Google Scholar]
- Gavrilovic J., Hembry R. M., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases (TIMP) regulates extracellular type I collagen degradation by chondrocytes and endothelial cells. J Cell Sci. 1987 Mar;87(Pt 2):357–362. doi: 10.1242/jcs.87.2.357. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Reynolds J. J. Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci. 1985 Feb;73:105–119. doi: 10.1242/jcs.73.1.105. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Robey P. G., Abe S. Partial purification and characterization of a neutral protease which cleaves type IV collagen. Biochemistry. 1981 Jan 6;20(1):100–104. doi: 10.1021/bi00504a017. [DOI] [PubMed] [Google Scholar]
- Mainardi C. L., Hibbs M. S., Hasty K. A., Seyer J. M. Purification of a type V collagen degrading metalloproteinase from rabbit alveolar macrophages. Coll Relat Res. 1984 Dec;4(6):479–492. doi: 10.1016/s0174-173x(84)80014-x. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Murphy G., Bretz U., Baggiolini M., Reynolds J. J. The latent collagenase and gelatinase of human polymorphonuclear neutrophil leucocytes. Biochem J. 1980 Nov 15;192(2):517–525. doi: 10.1042/bj1920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Galloway W. A., Barnes M. J., Bunning R. A., Mercer E., Reynolds J. J., Burgeson R. E. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem J. 1981 Dec 1;199(3):807–811. doi: 10.1042/bj1990807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem J. 1982 Apr 1;203(1):209–221. doi: 10.1042/bj2030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Woessner J. F., Jr An improved assay for proteases and polysaccharidases employing a cartilage proteoglycan substrate entrapped in polyacrylamide particles. Anal Biochem. 1980 Sep 15;107(2):385–392. doi: 10.1016/0003-2697(80)90400-5. [DOI] [PubMed] [Google Scholar]
- Nakano T., Scott P. G. Purification and characterization of a gelatinase produced by fibroblasts from human gingiva. Biochem Cell Biol. 1986 May;64(5):387–393. doi: 10.1139/o86-054. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Rhodes R. K., Miller E. J. Physicochemical characterization and molecular organization of the collagen A and B chains. Biochemistry. 1978 Aug 22;17(17):3442–3448. doi: 10.1021/bi00610a003. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Curry V. A., Sarsfield S. J. Purification to homogeneity of pig leucocyte catabolin, a protein that causes cartilage resorption in vitro. Biochem J. 1983 Nov 1;215(2):385–392. doi: 10.1042/bj2150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Keski-Oja J., Turpeenniemi-Hujanen T., Tryggvason K. Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells--role in metastasis. Int J Cancer. 1982 Nov 15;30(5):669–673. doi: 10.1002/ijc.2910300520. [DOI] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Tryggvason K. Purification and characterization of a murine basement membrane collagen-degrading enzyme secreted by metastatic tumor cells. J Biol Chem. 1983 Mar 10;258(5):3058–3063. [PubMed] [Google Scholar]
- Sapolsky A. I., Sheff M. F., Matsuta K., Howell D. S., Moskowitz R. W., Goldberg V. M., Norby D. P., Malemud C. J. 'Gelatinase-like' activity from articular chondrocytes in monolayer culture. Biochim Biophys Acta. 1983 Apr 5;762(2):227–231. doi: 10.1016/0167-4889(83)90075-7. [DOI] [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Vartio T., Hovi T. Polypeptide composition of human macrophage gelatinase. Acta Chem Scand B. 1987 Nov;41(10):754–756. doi: 10.3891/acta.chem.scand.41b-0754. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., Winterbourn C. C. Gelatinase contributes to the degradation of glomerular basement membrane collagen by human neutrophils. Coll Relat Res. 1988 Mar;8(2):113–122. doi: 10.1016/s0174-173x(88)80023-2. [DOI] [PubMed] [Google Scholar]
- Weingarten H., Feder J. Cleavage site specificity of vertebrate collagenases. Biochem Biophys Res Commun. 1986 Sep 30;139(3):1184–1187. doi: 10.1016/s0006-291x(86)80302-3. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. R., Lin T. Y. Human polymorphonuclear leukocyte collagenase and gelatinase. Comparison of certain enzymatic properties. Int J Biochem. 1984;16(12):1321–1329. doi: 10.1016/0020-711x(84)90235-0. [DOI] [PubMed] [Google Scholar]