Abstract
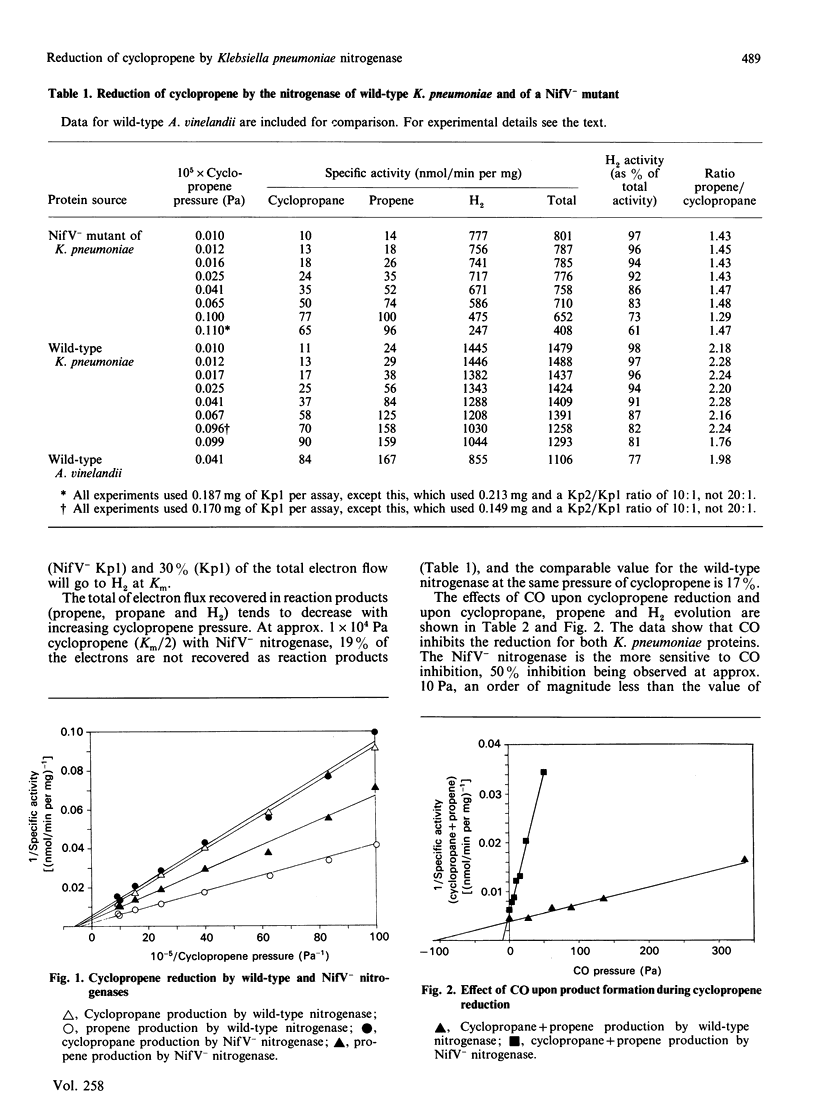
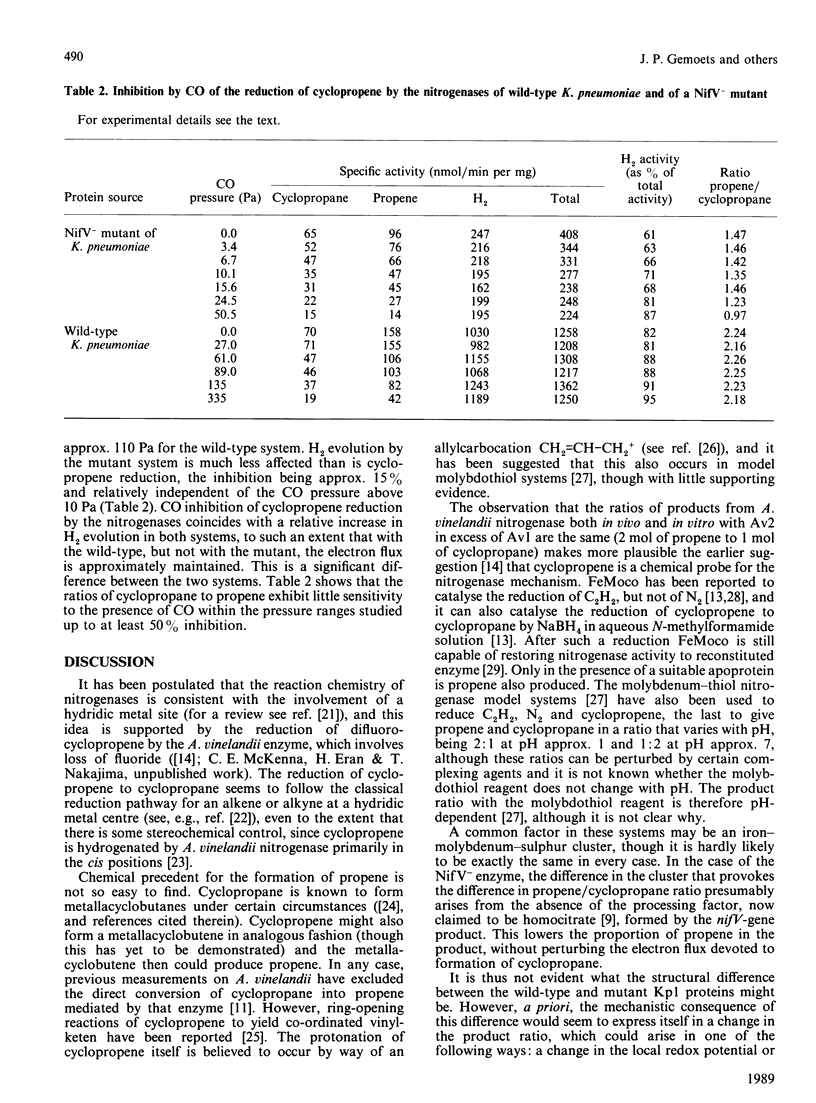
The nitrogenase from wild-type Klebsiella pneumoniae reduces cyclopropene to cyclopropane and propene in the ratio 1:2 at pH 7.5. We show in this paper that the nitrogenase from a nifV mutant of K. pneumoniae also reduces cyclopropene to cyclopropane and propene, but the ratio of products is now 1:1.4. However, both nitrogenases exhibit the same Km for cyclopropene (2.1 x 10(4) +/- 0.2 x 10(4) Pa), considerably more than the Km for the analogous reaction with Azotobacter vinelandii nitrogenase under the same conditions (5.1 x 10(3) Pa). Analysis of the data shows that the different product ratio arises from the slower production of propene compared with cyclopropane by the mutant nitrogenase. During turnover, both nitrogenases use a large proportion of the electron flux for H2 production. CO inhibits the reduction of cyclopropene by both K. pneumoniae proteins, but the mutant nitrogenase exhibits 50% inhibition at approx. 10 Pa, whereas the corresponding value for the wild-type nitrogenase is approx. 110 Pa. However, H2 evolution by the mutant enzyme is much less affected than is cyclopropene reduction. CO inhibition of cyclopropene reduction by the nitrogenases coincides with a relative increase in H2 evolution, so that in the wild-type (but not the mutant) the electron flux is approximately maintained. The cyclopropane/propene production ratios are little affected by the presence of CO within the pressure ranges studied at least up to 50% inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Robertson A. D., Cerny R. L., Hayes R. N., Imperial J., Shah V. K., Ludden P. W. Identification of the V factor needed for synthesis of the iron-molybdenum cofactor of nitrogenase as homocitrate. 1987 Oct 29-Nov 4Nature. 329(6142):855–857. doi: 10.1038/329855a0. [DOI] [PubMed] [Google Scholar]
- McKenna C. E., McKenna M. C., Higa M. T. Letter: Chemical probes of nitrogenase. 1. Cyclopropene. Nitrogenase-catalyzed reduction to propene and cyclopropane. J Am Chem Soc. 1976 Jul 21;98(15):4657–4659. doi: 10.1021/ja00431a059. [DOI] [PubMed] [Google Scholar]
- McKenna C. E., McKenna M. C., Huang C. W. Low stereoselectivity in methylacetylene and cyclopropene reductions by nitrogenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4773–4777. doi: 10.1073/pnas.76.10.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Dixon R. A. Requirement of nifV gene for production of wild-type nitrogenase enzyme in Klebsiella pneumoniae. Nature. 1981 Aug 13;292(5824):655–656. doi: 10.1038/292655a0. [DOI] [PubMed] [Google Scholar]
- McLean P. A., Smith B. E., Dixon R. A. Nitrogenase of Klebsiella pneumoniae nifV mutants. Biochem J. 1983 Jun 1;211(3):589–597. doi: 10.1042/bj2110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Chisnell J. R., Brill W. J. Acetylene reduction by the iron-molybdenum cofactor from nitrogenase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):232–236. doi: 10.1016/0006-291x(78)91654-6. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


