Abstract
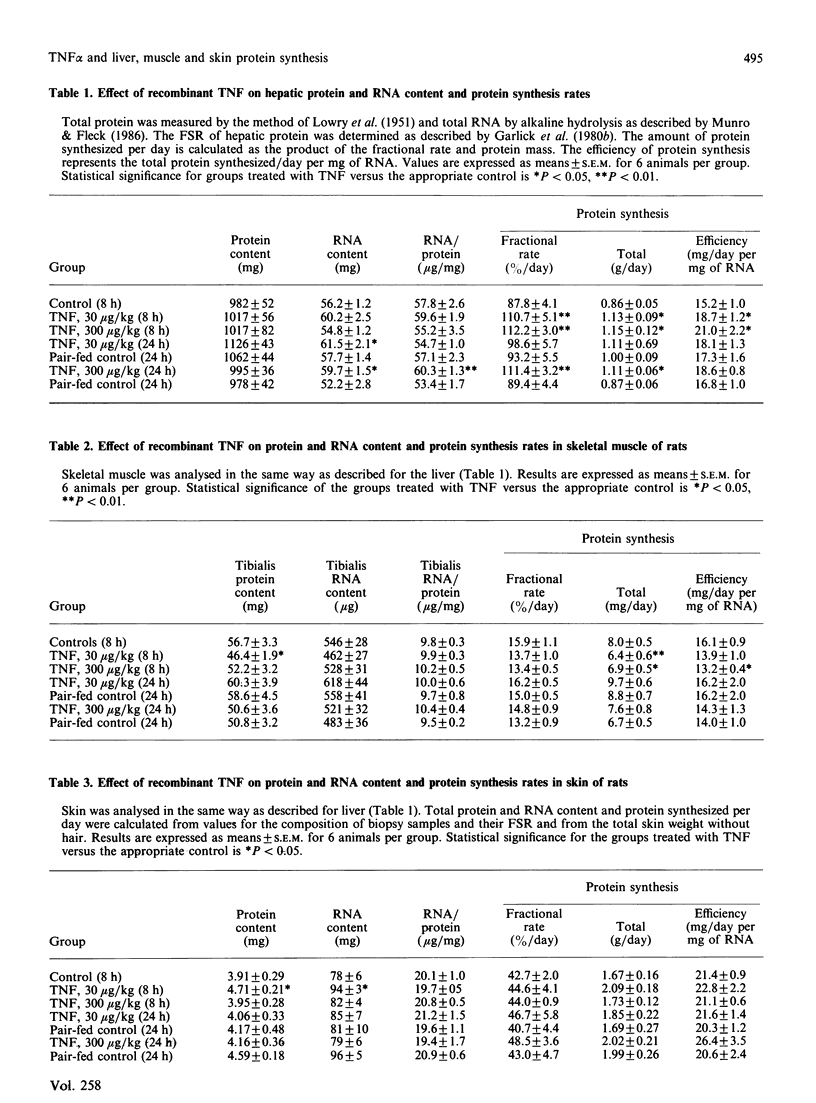
Bacterial endotoxins cause enhanced protein metabolism in liver, and protein catabolism in muscle and skin. These effects may be mediated by cytokines such as interleukin 1 (IL1) and tumour necrosis factor (TNF). The study investigates the timing and magnitude of effects of recombinant human TNF alpha on protein synthesis and protein and RNA content of the liver, tibialis muscle and skin of Wistar rats. Intravenous doses of 30 and 300 micrograms/kg of body weight were used and effects examined 8 h and 24 h after injection. Muscle protein content and synthetic rate were reduced at 8 h post-injection by over 18% and 20% respectively. Protein synthesis returned to normal after the lowest dose but remained depressed 24 h after the highest dose due to the accompanying anorexia. Opposite effects were observed in liver. Protein fractional synthetic rate (FSR) was increased by over 26% at 8 h post-injection and remained elevated 24 h after the higher but not lower dose of TNF. Total protein and RNA contents were significantly higher than controls at this time. Skin protein synthesis was unaffected by TNF; however an increase in protein and RNA content was observed at 8 h post-injection with the lower dose of TNF. Liver and muscle respond in a similar but more rapid way to TNF than to endotoxin. The response of skin is however totally different. While muscle may contribute amino acids for enhanced hepatic protein synthesis following exposure to TNF, skin does not.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Effects of infection on nutritional status and immunity. Fed Proc. 1980 Nov;39(13):3105–3108. [PubMed] [Google Scholar]
- Beisel W. R. Magnitude of the host nutritional responses to infection. Am J Clin Nutr. 1977 Aug;30(8):1236–1247. doi: 10.1093/ajcn/30.8.1236. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin--Tumour necrosis factor: a cytokine that mediates injury initiated by invasive parasites. Parasitol Today. 1987 Nov;3(11):345–346. doi: 10.1016/0169-4758(87)90119-0. [DOI] [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol Lett. 1987 Dec;16(3-4):227–231. doi: 10.1016/0165-2478(87)90151-9. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Fern E. B., Tomkins A. M., Waterlow J. C. Stimulation of protein synthesis and breakdown by vaccination. Br Med J. 1980 Jul 26;281(6235):263–265. doi: 10.1136/bmj.281.6235.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson M. M., Pell J. M., Bates P. C., Millward D. J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986 Apr 15;235(2):329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick-Smith L., Yoder M. C., Polin R. A., Douglas S. D., Erecińska M. Endotoxin-induced changes in nitrogen metabolism. Res Commun Chem Pathol Pharmacol. 1986 Sep;53(3):381–398. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Locksley R. M., Heinzel F. P., Shepard H. M., Agosti J., Eessalu T. E., Aggarwal B. B., Harlan J. M. Tumor necrosis factors alpha and beta differ in their capacities to generate interleukin 1 release from human endothelial cells. J Immunol. 1987 Sep 15;139(6):1891–1895. [PubMed] [Google Scholar]
- McCarthy D. O., Kluger M. J., Vander A. J. The role of fever in appetite suppression after endotoxin administration. Am J Clin Nutr. 1984 Aug;40(2):310–316. doi: 10.1093/ajcn/40.2.310. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Pain V. M., Garlick P. J. Conditions that alter rates of tissue protein synthesis in vivo. Biochem Soc Trans. 1980 Jun;8(3):283–285. doi: 10.1042/bst0080283. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldawer L. L., Andersson C., Gelin J., Lundholm K. G. Regulation of food intake and hepatic protein synthesis by recombinant-derived cytokines. Am J Physiol. 1988 Mar;254(3 Pt 1):G450–G456. doi: 10.1152/ajpgi.1988.254.3.G450. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Svaninger G., Gelin J., Lundholm K. G. Interleukin 1 and tumor necrosis factor do not regulate protein balance in skeletal muscle. Am J Physiol. 1987 Dec;253(6 Pt 1):C766–C773. doi: 10.1152/ajpcell.1987.253.6.C766. [DOI] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985 Oct;70(4):473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S. Effects of tumour necrosis factor and related cytokines on vascular endothelial cells. Ciba Found Symp. 1987;131:170–184. doi: 10.1002/9780470513521.ch12. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Conyers R. A., Bais R., Gamble J. R., Vadas M. A. The effects of recombinant tumour necrosis factor (cachectin) on metabolism in isolated rat adipocyte, hepatocyte and muscle preparations. Biochem J. 1987 Nov 1;247(3):789–792. doi: 10.1042/bj2470789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrado J., Moldawer L. L., Bistrian B. R., Dinarello C. A., Blackburn G. L. Effect of ibuprofen on fever and metabolic changes induced by continuous infusion of leukocytic pyrogen (interleukin 1) or endotoxin. Infect Immun. 1983 Dec;42(3):997–1005. doi: 10.1128/iai.42.3.997-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Goad D., Bosma T., Levine L. Tumor necrosis factor-alpha (cachectin) stimulates bone resorption in mouse calvaria via a prostaglandin-mediated mechanism. Endocrinology. 1987 May;120(5):2029–2036. doi: 10.1210/endo-120-5-2029. [DOI] [PubMed] [Google Scholar]
- Tomkins A. M., Garlick P. J., Schofield W. N., Waterlow J. C. The combined effects of infection and malnutrition on protein metabolism in children. Clin Sci (Lond) 1983 Sep;65(3):313–324. doi: 10.1042/cs0650313. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Cerami A. Physiological responses to cachectin. Ciba Found Symp. 1987;131:88–108. doi: 10.1002/9780470513521.ch7. [DOI] [PubMed] [Google Scholar]
- Waage A. Production and clearance of tumor necrosis factor in rats exposed to endotoxin and dexamethasone. Clin Immunol Immunopathol. 1987 Dec;45(3):348–355. doi: 10.1016/0090-1229(87)90087-0. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Gabrilove J. L., Oettgen H. F., Brennan M. F. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg. 1987 Dec;122(12):1396–1400. doi: 10.1001/archsurg.1987.01400240042007. [DOI] [PubMed] [Google Scholar]


