Abstract
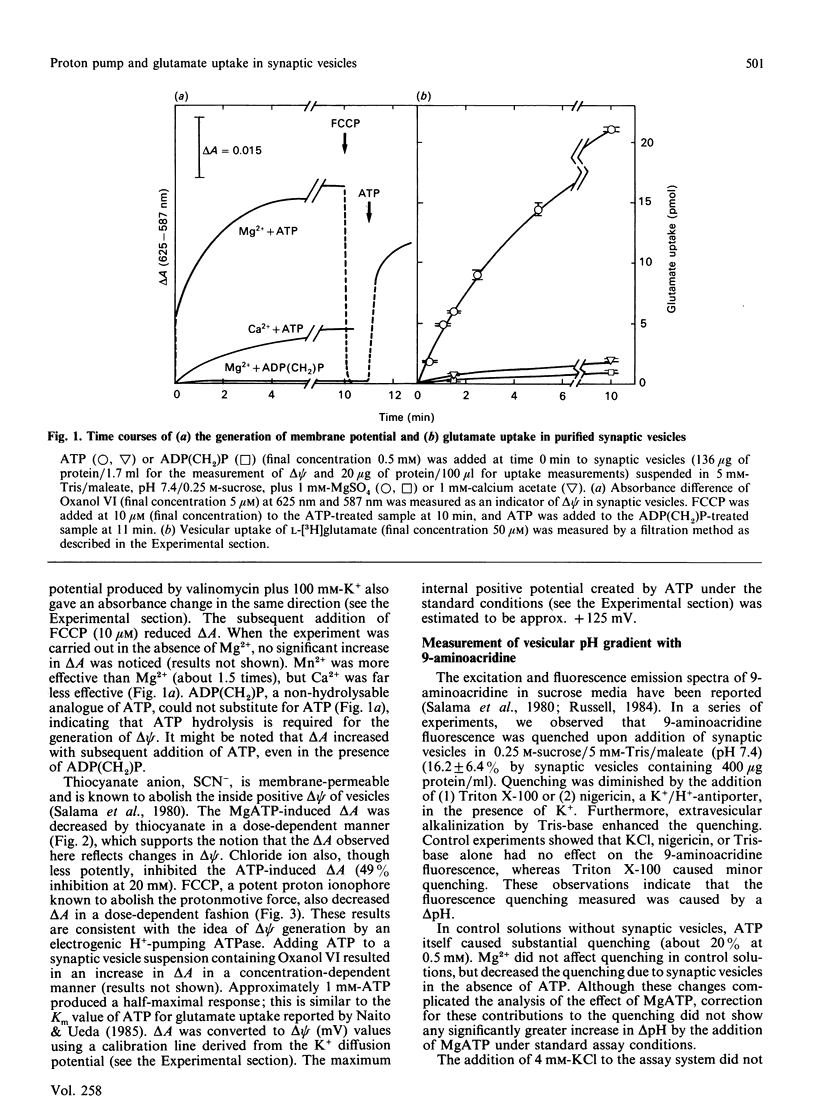
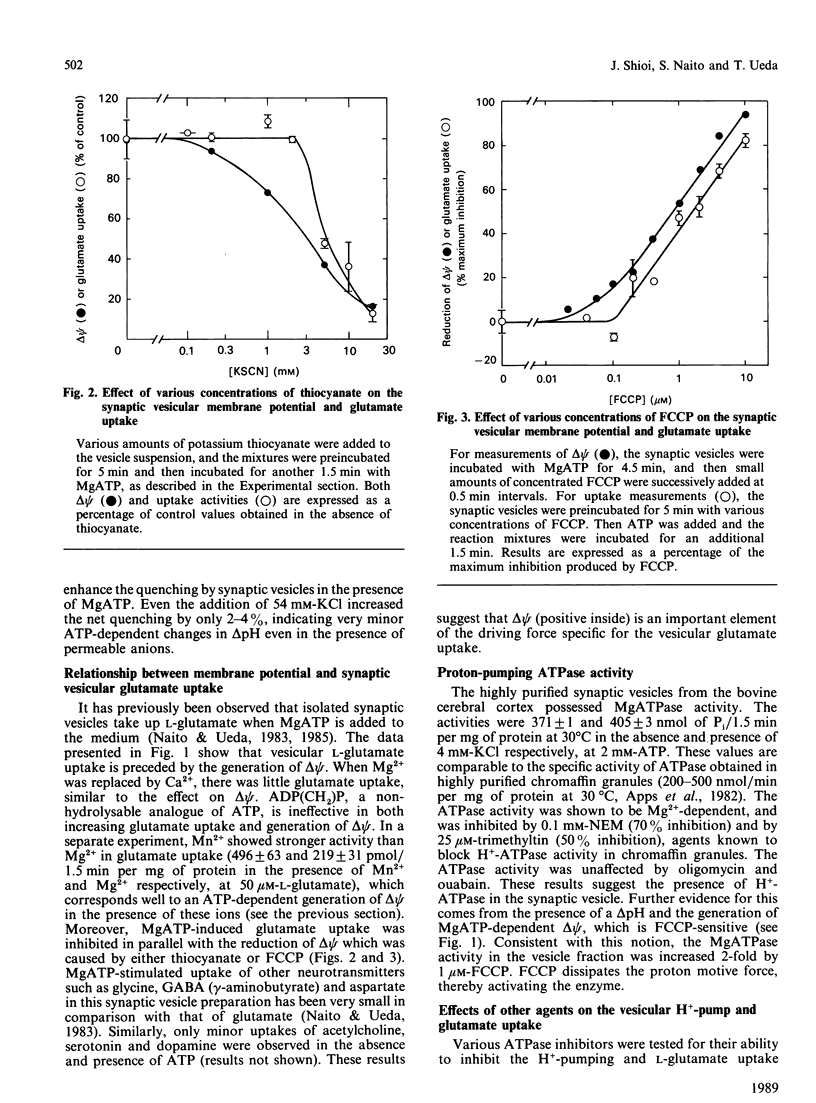
Measurements have been made of the ATP-dependent membrane potential (delta psi) and pH gradient (delta pH) across the membranes of the synaptic vesicles purified from bovine cerebral cortex, using the voltage-sensitive dye bis[3-propyl-5-oxoisoxazol-4-yl]pentamethine oxanol and the delta pH-sensitive fluorescent dye 9-aminoacridine respectively. A pre-existing small delta pH (inside acidic) was detected in the synaptic vesicles, but no additional significant contribution by MgATP to delta pH was observed. In contrast, delta psi (inside positive) increased substantially upon addition of MgATP. This ATP-dependent delta psi was reduced by thiocyanate anion (SCN-), a delta psi dissipator, or carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), a protonmotive-force dissipator. Correspondingly, a substantially larger glutamate uptake occurred in the presence of MgATP, which was inhibited by SCN- and FCCP. A nonhydrolysable analogue of ATP, adenosine 5'-[beta gamma-methylene]triphosphate, did not substitute for ATP in either delta psi generation or glutamate uptake. The results support the hypothesis that a H+-pumping ATPase generates a protonmotive force in the synaptic vesicles at the expense of ATP hydrolysis, and the protonmotive force thus formed provides a driving force for the vesicular glutamate uptake. The delta psi generation by ATP hydrolysis was not affected by orthovanadate, ouabain or oligomycin, but was inhibited by N-ethylmaleimide, quercetin, trimethyltin, 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole and 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid. These results indicate that the H+-pumping ATPase in the synaptic vesicle is similar to that in the chromaffin granule, platelet granule and lysosome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Pedersen P. L. Proton atpases: structure and mechanism. Annu Rev Biochem. 1983;52:801–824. doi: 10.1146/annurev.bi.52.070183.004101. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Pryde J. G., Sutton R. The H+-translocating adenosine triphosphatase of chromaffin granule membranes. Ann N Y Acad Sci. 1982;402:134–145. doi: 10.1111/j.1749-6632.1982.tb25737.x. [DOI] [PubMed] [Google Scholar]
- Carty S. E., Johnson R. G., Scarpa A. Serotonin transport in isolated platelet granules. Coupling to the electrochemical proton gradient. J Biol Chem. 1981 Nov 10;256(21):11244–11250. [PubMed] [Google Scholar]
- Cidon S., Nelson N. Purification of N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J Biol Chem. 1986 Jul 15;261(20):9222–9227. [PubMed] [Google Scholar]
- Fishkes H., Rudnick G. Bioenergetics of serotonin transport by membrane vesicles derived from platelet dense granules. J Biol Chem. 1982 May 25;257(10):5671–5677. [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Hajós F. An improved method for the preparation of synaptosomal fractions in high purity. Brain Res. 1975 Aug 15;93(3):485–489. doi: 10.1016/0006-8993(75)90186-9. [DOI] [PubMed] [Google Scholar]
- Holz R. W. Evidence that catecholamine transport into chromaffin vesicles is coupled to vesicle membrane potential. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5190–5194. doi: 10.1073/pnas.75.10.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C. The internal pH and membrane potential of the insulin-secretory granule. Biochem J. 1982 Apr 15;204(1):171–178. doi: 10.1042/bj2040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Protonmotive force and catecholamine transport in isolated chromaffin granules. J Biol Chem. 1979 May 25;254(10):3750–3760. [PubMed] [Google Scholar]
- Moriyama Y., Takano T., Ohkuma S. Proton translocating ATPase in lysosomal membrane ghosts. Evidence that alkaline Mg2+-ATPase acts as a proton pump. J Biochem. 1984 Apr;95(4):995–1007. doi: 10.1093/oxfordjournals.jbchem.a134726. [DOI] [PubMed] [Google Scholar]
- Naito S., Ueda T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J Biol Chem. 1983 Jan 25;258(2):696–699. [PubMed] [Google Scholar]
- Naito S., Ueda T. Affinity-purified anti-protein I antibody. Specific inhibitor of phosphorylation of protein I, a synaptic protein. J Biol Chem. 1981 Oct 25;256(20):10657–10663. [PubMed] [Google Scholar]
- Naito S., Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985 Jan;44(1):99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Njus D., Kelley P. M., Harnadek G. J. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853(3-4):237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- Njus D., Radda G. K. A potassium ion diffusion potential causes adrenaline uptake in chromaffin-granule 'ghosts'. Biochem J. 1979 Jun 15;180(3):579–585. doi: 10.1042/bj1800579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. T. Delta pH, H+ diffusion potentials, and Mg2+ ATPase in neurosecretory vesicles isolated from bovine neurohypophyses. J Biol Chem. 1984 Aug 10;259(15):9496–9507. [PubMed] [Google Scholar]
- Salama G., Johnson R. G., Scarpa A. Spectrophotometric measurements of transmembrane potential and pH gradients in chromaffin granules. J Gen Physiol. 1980 Feb;75(2):109–140. doi: 10.1085/jgp.75.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]


