Abstract
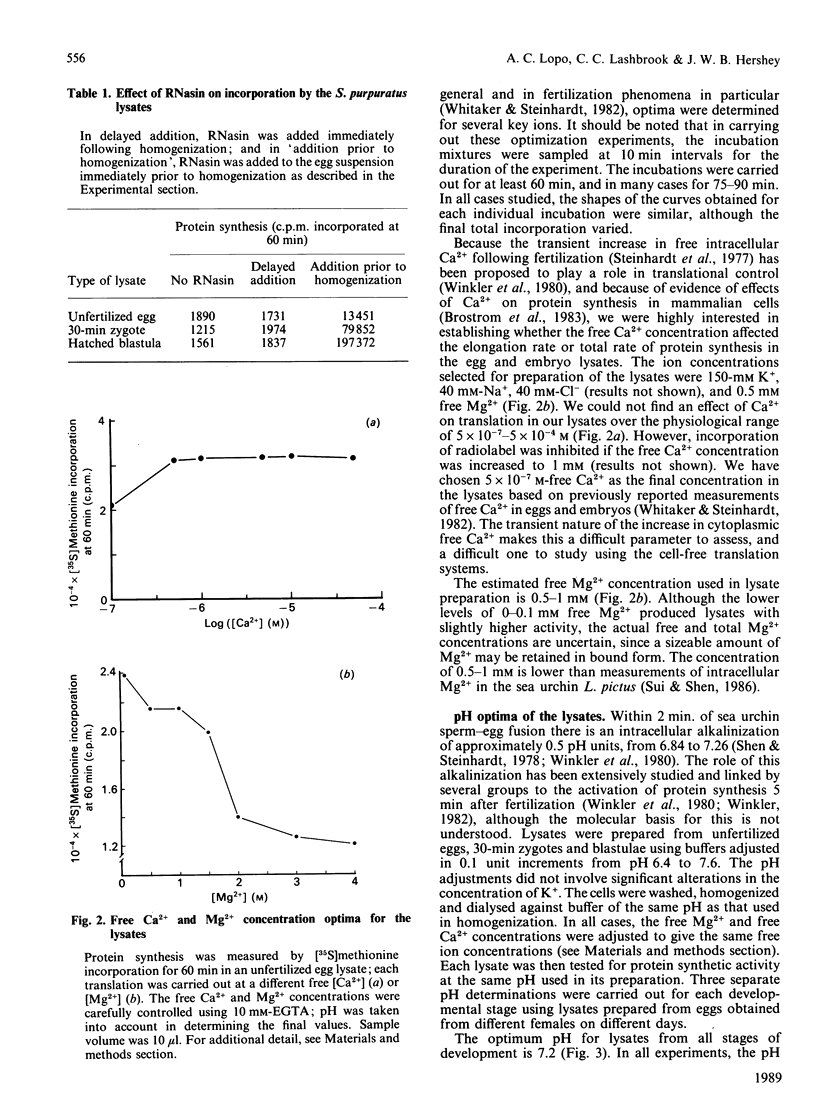
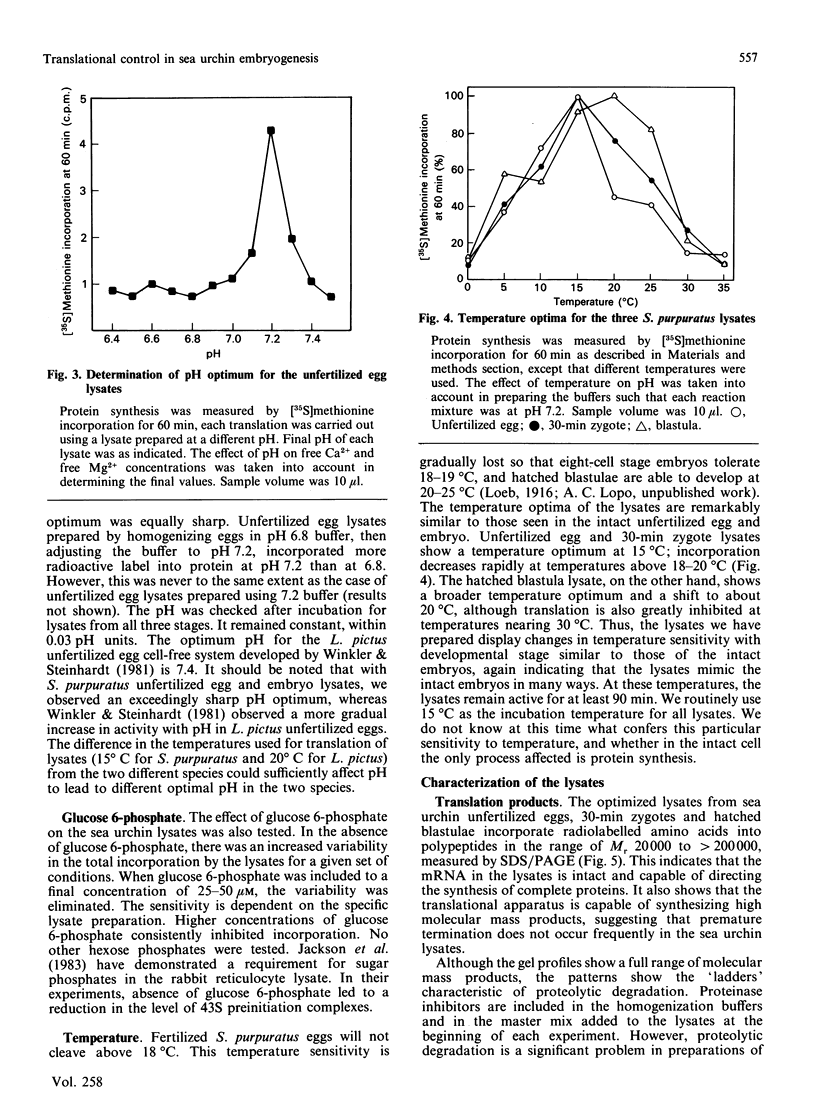
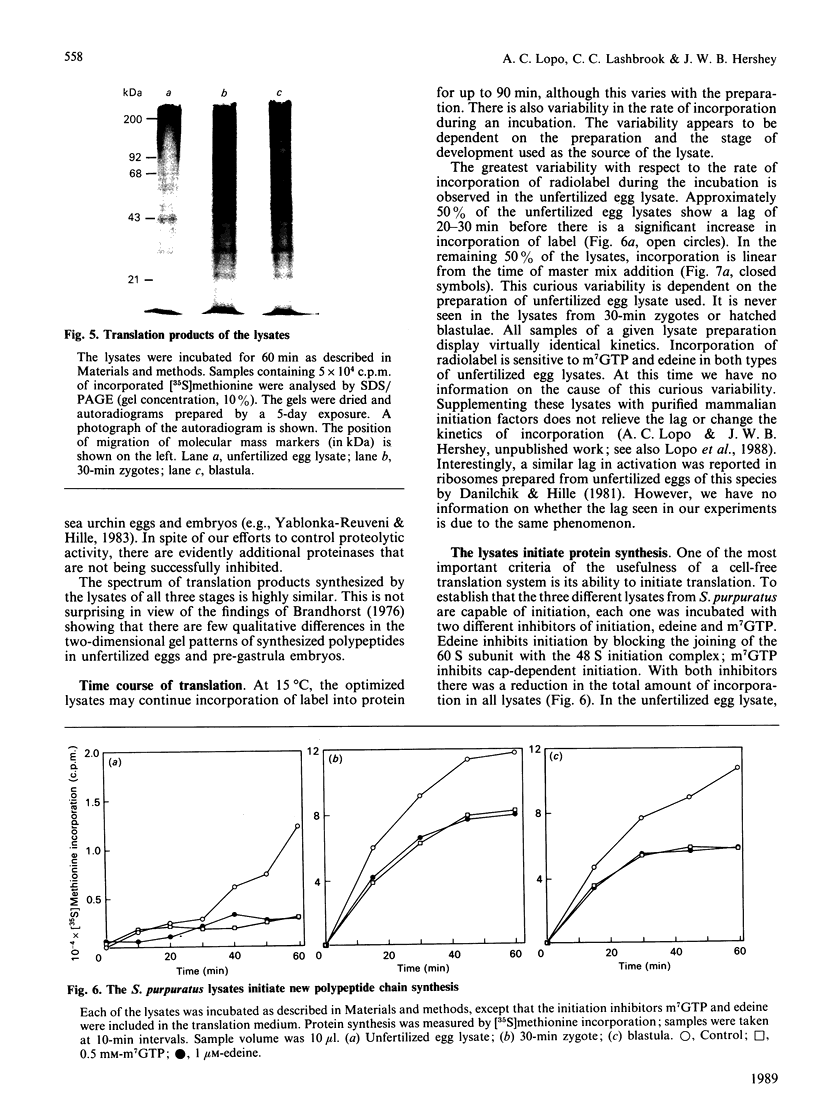
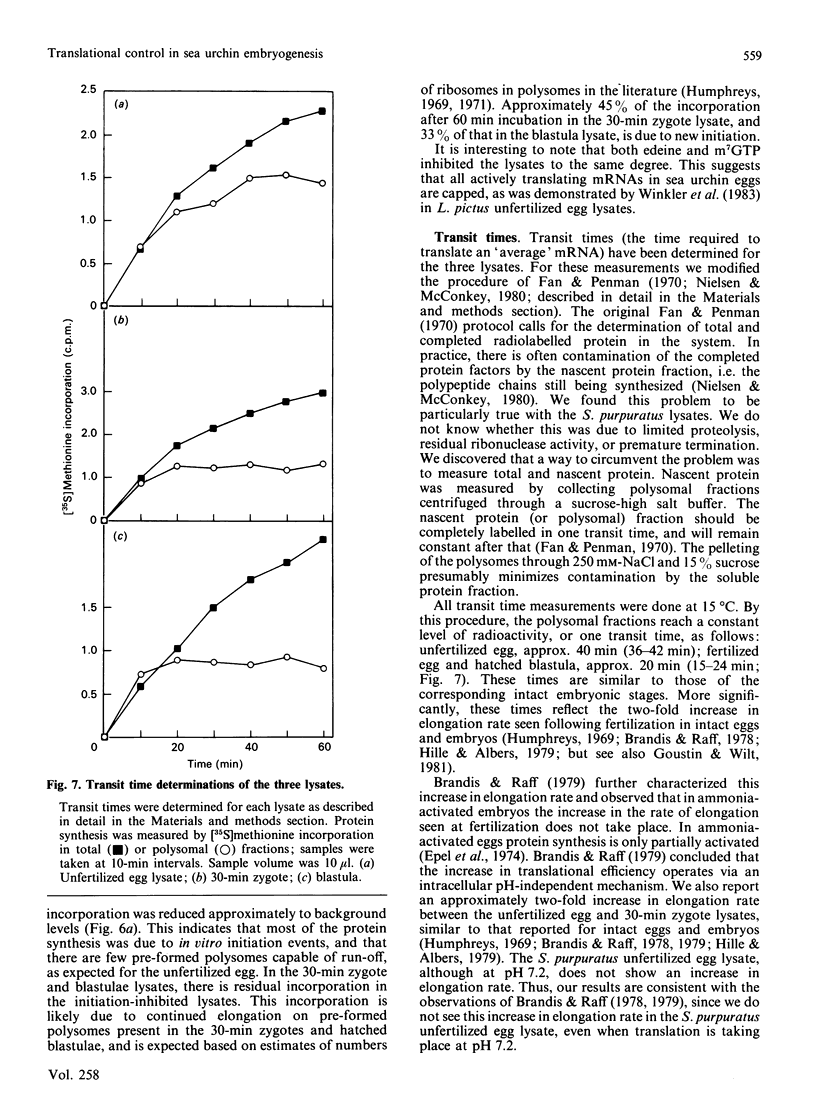
We have developed and characterized cell-free systems active in translation from unfertilized eggs, 30-min zygotes and hatched blastulae of the sea urchin Strongylocentrotus purpuratus. The ion concentrations selected for preparation of the lysates were 150 mM-K+, 40 mM-Na+, 40 mM-Cl-, 5 x 10(-7) M free Ca2+ and 1 mM free Mg2+. It was necessary to include the ribonuclease inhibitor RNas in the preparations to obtain full activity consistently. The pH optimum was 7.2 and was extremely sharp for the three S. purpuratus lysates. The temperature optima of the three lysates were remarkably similar to those of the intact unfertilized egg and embryos. Lysates from unfertilized egg and 30-min zygotes showed a temperature optimum at 15 degrees C. The hatched blastula lysate showed a broader temperature optimum with a shift to about 20 degrees C. The optimized lysates incorporated radiolabelled amino acids into polypeptides for up to 90 min. The polypeptides synthesized ranged in Mr from 200,000 to 20,000, suggesting that the mRNA in the lysates was intact and capable of directing the synthesis of complete polypeptides. Furthermore, the three lysates were capable of initiation, as demonstrated by inhibition of initiation using the inhibitors edeine and 7-methylguanosine 5'-triphosphate (m7GTP). At 15 degrees C, the transit times for the three lysates were: unfertilized egg, 40 min; 30-min zygotes and hatched blastula lysates, 20 min. These transit times are similar to those of intact eggs and embryos, and significantly, reflect the two-fold increase in elongation rate seen following fertilization in intact embryos. Thus, these lysates display many features and characteristic responses typical of intact eggs and embryos, indicating that the lysates should be useful tools for the analysis of translation control in early embryogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Brandis J. W., Raff R. A. Elevation of protein synthesis is a complex response to fertilisation. Nature. 1979 Mar 29;278(5703):467–469. doi: 10.1038/278467a0. [DOI] [PubMed] [Google Scholar]
- Brandis J. W., Raff R. A. Translation of oogenetic mRNA in sea urchin eggs and early embryos. Demonstration of a change in translational efficiency following fertilization. Dev Biol. 1978 Nov;67(1):99–113. doi: 10.1016/0012-1606(78)90303-2. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Bocckino S. B., Brostrom M. A. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983 Dec 10;258(23):14390–14399. [PubMed] [Google Scholar]
- Davidson E. H., Hough-Evans B. R., Britten R. J. Molecular biology of the sea urchin embryo. Science. 1982 Jul 2;217(4554):17–26. doi: 10.1126/science.6178156. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. S., Harper A. E. Characterization of a protein synthesis system from rat liver. Translation of endogenous and exogenous messenger RNA. J Biol Chem. 1984 Aug 10;259(15):9922–9928. [PubMed] [Google Scholar]
- Epel D. Protein synthesis in sea urchin eggs: a "late" response to fertilization. Proc Natl Acad Sci U S A. 1967 Apr;57(4):899–906. doi: 10.1073/pnas.57.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D., Steinhardt R., Humphreys T., Mazia D. An analysis of the partial metabolic derepression of sea urchin eggs by ammonia: the existence of independent pathways. Dev Biol. 1974 Oct;40(2):245–255. doi: 10.1016/0012-1606(74)90127-4. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Foerder C. A., Shapiro B. M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino R., Metafora S., Felicetti L., Raisman J. Properties of the ribosomal salt wash from unfertilized and fertilized sea urchin eggs and its effect on natural mRNA translation. Biochim Biophys Acta. 1973 Jun 23;312(2):377–391. doi: 10.1016/0005-2787(73)90382-1. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Wilt F. H. Protein synthesis, polyribosomes, and peptide elongation in early development of Strongylocentrotus purpuratus. Dev Biol. 1981 Feb;82(1):32–40. doi: 10.1016/0012-1606(81)90426-7. [DOI] [PubMed] [Google Scholar]
- Hansen L. J., Huang W. I., Jagus R. Inhibitor of translational initiation in sea urchin eggs prevents mRNA utilization. J Biol Chem. 1987 May 5;262(13):6114–6120. [PubMed] [Google Scholar]
- Hille M. B., Albers A. A. Efficiency of protein synthesis after fertilisation of sea urchin eggs. Nature. 1979 Mar 29;278(5703):469–471. doi: 10.1038/278469a0. [DOI] [PubMed] [Google Scholar]
- Humphreys T. Efficiency of translation of messenger-RNA before and after fertilization in sea urchins. Dev Biol. 1969 Nov;20(5):435–458. doi: 10.1016/0012-1606(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Humphreys T. Measurements of messenger RNA entering polysomes upon fertilization of sea urchin eggs. Dev Biol. 1971 Oct;26(2):201–208. doi: 10.1016/0012-1606(71)90122-9. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Translation of maternal messenger ribonucleoprotein particles from sea urchin in a cell-free system from unfertilized eggs and product analysis. Dev Biol. 1978 Oct;66(2):375–385. doi: 10.1016/0012-1606(78)90246-4. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Herbert P., Campbell E. A., Hunt T. The roles of sugar phosphates and thiol-reducing systems in the control of reticulocyte protein synthesis. Eur J Biochem. 1983 Mar 15;131(2):313–324. doi: 10.1111/j.1432-1033.1983.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopo A. C., Lashbrook C. C., Infante D., Infante A. A., Hershey J. W. Translational initiation factors from sea urchin eggs and embryos: functional properties are highly conserved. Arch Biochem Biophys. 1986 Oct;250(1):162–170. doi: 10.1016/0003-9861(86)90713-7. [DOI] [PubMed] [Google Scholar]
- Lopo A. C., MacMillan S., Hershey J. W. Translational control in early sea urchin embryogenesis: initiation factor eIF4F stimulates protein synthesis in lysates from unfertilized eggs of Strongylocentrotus purpuratus. Biochemistry. 1988 Jan 12;27(1):351–357. doi: 10.1021/bi00401a053. [DOI] [PubMed] [Google Scholar]
- Mandley E. N., Lopo A. C. Putative nuclease-sensitive control element in unfertilized eggs of the sea urchin Lytechinus pictus. Biochem Biophys Res Commun. 1987 Jun 15;145(2):921–926. doi: 10.1016/0006-291x(87)91053-9. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., McConkey E. H. Evidence for control of protein synthesis in HeLa cells via the elongation rate. J Cell Physiol. 1980 Sep;104(3):269–281. doi: 10.1002/jcp.1041040302. [DOI] [PubMed] [Google Scholar]
- Regier J. C., Kafatos F. C. Absolute rates of protein synthesis in sea urchins with specific activity measurements of radioactive leucine and leucyl-tRNA. Dev Biol. 1977 Jun;57(2):270–283. doi: 10.1016/0012-1606(77)90214-7. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D., Payne J. E. Methods for quantitating sea urchin sperm-egg binding. Exp Cell Res. 1973 Nov;82(1):227–235. doi: 10.1016/0014-4827(73)90265-6. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Winkler M. M., Lashbrook C., Hershey J. W., Mukherjee A. K., Sarkar S. The cytoplasmic 4 S translation inhibitory RNA species of chick embryonic muscle. Effect on mRNA binding to 43 S initiation complex. J Biol Chem. 1983 Dec 25;258(24):15141–15145. [PubMed] [Google Scholar]
- Winkler M. M., Nelson E. M., Lashbrook C., Hershey J. W. Multiple levels of regulation of protein synthesis at fertilization in sea urchin eggs. Dev Biol. 1985 Feb;107(2):290–300. doi: 10.1016/0012-1606(85)90312-4. [DOI] [PubMed] [Google Scholar]
- Winkler M. M., Steinhardt R. A., Grainger J. L., Minning L. Dual ionic controls for the activation of protein synthesis at fertilization. Nature. 1980 Oct 9;287(5782):558–560. doi: 10.1038/287558a0. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z., Hille M. B. Isolation and distribution of elongation factor 2 in eggs and embryos of sea urchins. Biochemistry. 1983 Oct 25;22(22):5205–5212. doi: 10.1021/bi00291a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]



