Abstract
Background: Anatomical evidence reveals heterogeneous fat distribution in both atrial and ventricular myocardium that are considered normal, but at the same time arrhythmogenic, and numerous cardiac pathophysiological conditions are associated with myocardial fat deposits. The relationship between fatty infiltration, especially in the epicardial layer and its pathophysiological implication is not completely understood. Aim: The aim of this study was to establish a positive or negative relationship between the ventricular burden and several parameters related to right ventricle (RV) adipose tissue – the RV thickness, RV indexed mass, body mass index (BMI), age, gender. Patients, Materials and Methods: Twenty-three patients with documented premature ventricular contractions (PVCs) originating from right ventricular outflow tract based on electrocardiography (ECG) evaluation were hospitalized between January 2018–November 2022 for electrophysiological study and PVCs ablation. Data obtained after collecting the clinical characteristics, ECG, RV measurements from transthoracic echocardiography (TTE), cardiac computed tomography (CT) and magnetic resonance imaging (MRI) were analyzed. Results: A weak positive relationship between the ventricular burden and BMI (r=0.14, p=0.49), tricuspid annular plane systolic excursion (TAPSE) (r=0.07, p=0.7), the RV thickness (r=0.03, p=0.8), epicardial adipose tissue (r=0.13, p=0.55), RV mass indexed (r=0.05, p=0.82) was observed. No clear cut-off of the PVCs burden could be established in terms related to the increase in BMI, RV thickness, epicardial adipose tissue, RV mass indexed. Conclusions: No significant positive or negative relationship between the ventricular burden and the RV thickness, RV indexed mass were found in individuals with a high PVCs originating from right ventricular outflow tract (RVOT) burden.
Keywords: fatty infiltration , premature ventricular contraction , ventricular burden , RV thickness , epicardial adipose tissue
Introduction
Based on anatomical human evidence, heterogeneous fat distribution in both the atrial and ventricular myocardium are considered normal, but at the same time arrhythmogenic. Numerous cardiac pathophysiological conditions are associated with myocardial fat deposits, but the relationship between the fatty infiltration, especially the one from the epicardial layer, and its pathophysiological implication is not completely understood [1]. Data revealed that up to 80% of the myocardial surface can be covered by fat, and almost 20% of the total human heart weight might be represented by fatty tissue [2]. The fat deposits (Figure 1) are frequently found on the free wall of the right ventricle (RV), but also at the apex of the left ventricle (LV). Some studies have shown that despite the fact LV mass exceeds the RV mass, the total amount of fatty tissue is similar in both RV and LV [3]. The myocardial infiltration by abnormal proliferating cells might lead to electrophysiological challenges responsible for micro-fibrosis and, next, to abnormalities of electrical propagation.
Figure 1.
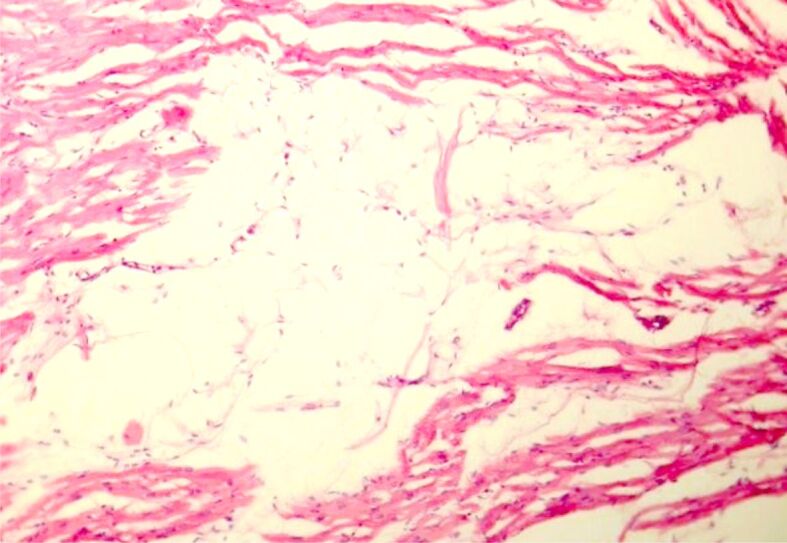
Fatty infiltration at the level of RV myocardium (HE staining, ×100). HE: Hematoxylin–Eosin; RV: Right ventricle
Fibrofatty replacement of the RV is associated with inflammation and represents the hallmark of arrhythmogenic right ventricular cardiomyopathy (ARVC), an established entity responsible for sudden cardiac death in young adults. The fatty infiltration without fibrosis of the RV is another scenario, but its relation to sudden death needs to be further evaluated.
Transthoracic echocardiography (TTE), cardiac computed tomography (CT), and cardiac magnetic resonance imaging (MRI) have been used to investigate cardiac fatty tissue deposits, each modality presenting advantages and limitations [4] (Table 1).
Table 1.
Imaging modality frequently used for fat tissue evaluation (advantages and limits)
|
Imaging modality |
Advantages |
Limitations |
|
TTE |
Widely available</p> <p>Not expensive |
Limited measurement of thickness |
|
CT |
Precise volume, area measurement</p> <p>Precise thickness measurement |
Ionization radiation |
|
MRI |
Precise volume, area measurement</p> <p>Precise thickness measurement |
Expensive</p> <p>Not feasible for extreme obese patients |
CT: Computed tomography; MRI: Magnetic resonance imaging; TTE: Transthoracic echocardiography
Myocardial fatty tissue develops with aging and can be observed at CT examinations, especially in the RV-free wall and right ventricular outflow tract (RVOT). Data showed that the myocardium thickness is either normal or increased and the RV exhibits normal values as diameter and volume in elderly patients [5]. These values seem to be completely different when compared to pathological conditions, such as ARVC, myocardial infarction, chronic ischemia, lipomatous hypertrophy of the inter-atrial septum, cardiac lipoma, dilated cardiomyopathy, or cardiomyopathies caused by muscular dystrophy.
The most common imaging findings observed in patients with ARVC are represented by a thin RV-free wall and RVOT caused by subepicardial fatty infiltration, the presence of fatty tissue in the RV moderator band, RV trabeculae, septum, and abnormal wall motion and dilated RV. Although epicardial fat is considered a normal variant, fatty infiltration of the myocardium is frequently arrhythmogenic (adipositas cordis) and seems to be associated with pathophysiological conditions [6].
The layers of fatty tissue surrounding the heart are divided into intra- and extra-pericardial. The extra-pericardial fat represents thoracic adipose tissue located external to the parietal pericardium and receives blood supply from non-coronary sources [7]. The intrapericardial fat is divided into epicardial and pericardial fat. Epicardial fat lies between the outer wall of the myocardium and the visceral layer of the pericardium, while pericardial fat is displayed anteriorly to the epicardial fat, between the visceral and parietal pericardium [2]. The epicardial fat is located close to the myocardium, shares the same microcirculation with the myocardium from the coronary arteries, and is metabolically active being a source of several adipokines and inflammatory cytokines [8].
Aim
While the relationship between the fatty infiltration, especially in the epicardial layer and its pathophysiological implication is not completely understood, the purpose of this study was to establish a positive or negative relationship between the ventricular burden and several parameters related to RV adipose tissue – the RV thickness, RV indexed mass, body mass index (BMI), age, gender.
Patients, Materials and Methods
Forty-two patients with documented premature ventricular contractions (PVCs) originating from the RVOT based on electrocardiography (ECG) evaluation were hospitalized in the Department of Cardiology, MedLife European Hospital Polisano, Sibiu, Romania, between January 2018–November 2022, for electrophysiological study and PVCs ablation (Figure 2).
Figure 2.
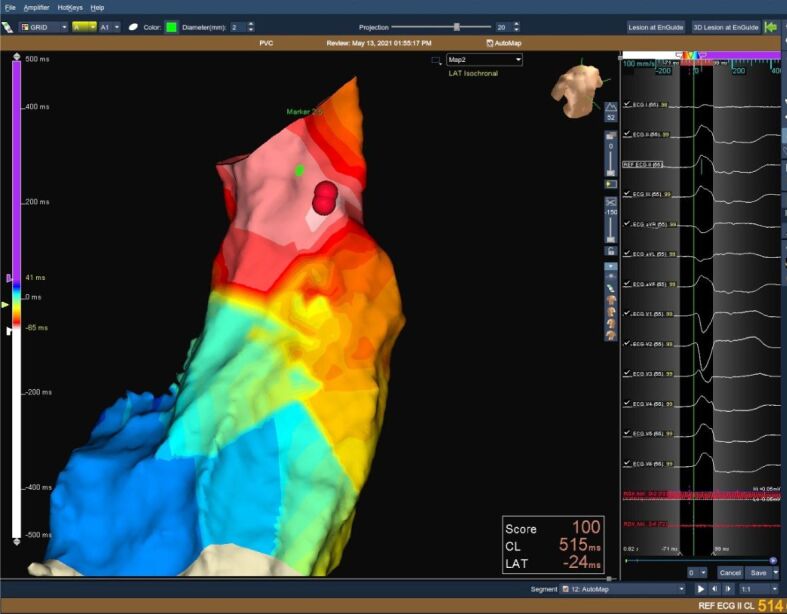
3D reconstructed image of the RVOT (left panel) and 12-lead ECG (right panel) showing LBBB morphology PVCs with late transition in V4 R/S >1 in leads II, III, AVF. The presence of QRS notching and the lower R amplitude in the inferior leads is highly suggestive of a free wall origin. 3D: Three-dimensional; AVF: Augmented voltage foot; ECG: Electrocardiography; LBBB: Left bundle branch block; PVC: Premature ventricular contraction; RVOT: Right ventricular outflow tract
We identified 23 patients in whom both cardiac CT and MRI were performed before or after the procedure, and they were enrolled in our study. Data were retrospectively retrieved from the imaging archiving and communication system.
For all 23 patients, PVCs originating from the RVOT were documented on 12-lead ECG based on the presence of a left bundle branch block (LBBB) morphology, an inferior axis, and late precordial transition. Each patient underwent complete TTE with the evaluation of left ventricular ejection fraction (LVEF), RV diameter, and thickness, RVOT diameter measured above the aortic valve, tricuspid annular plane systolic excursion (TAPSE), RV four chamber longitudinal strain, and absence or presence of epicardial RV fatty tissue and its thickness. The RV’s thickness and the epicardial fatty tissue were measured along the wall of the zone with clearly defined endocardial and epicardial margins on short and long-axis view images. Maximum and minimum thickness of the free wall were noted for each patient (Figure 3).
Figure 3.
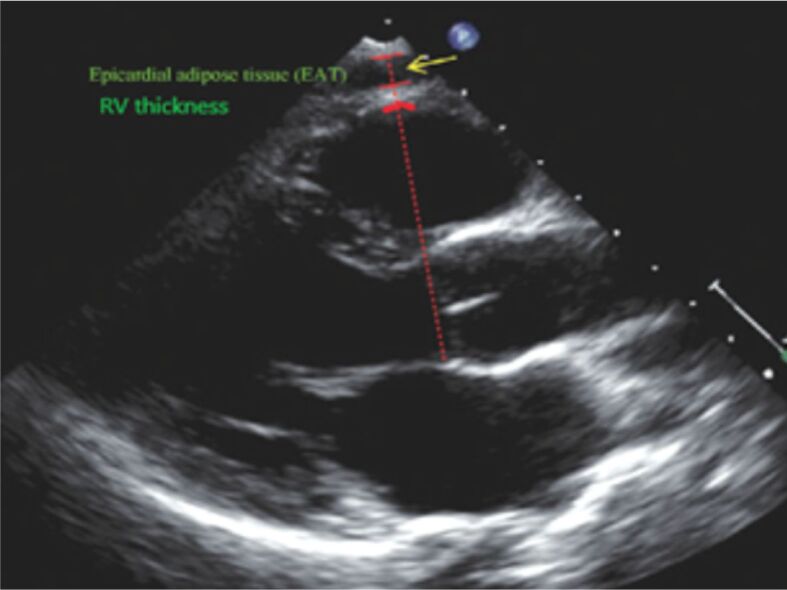
The epicardial fat thickness and the thickness of the RV free wall are measured by TTE from the PLA view, using the aortic annulus as an anatomic reference. The epicardial adipose tissue is represented by an echo-free space between the pericardial layers (yellow arrow). The RV thickness is measured between the inferior red lines. PLA: Parasternal long axis; RV: Right ventricle; TTE: Transthoracic echocardiography
Cardiac CT angiography with ECG gating was performed for each patient (Figure 4). Both endocardial and epicardial margins were manually traced on the end-diastolic short-axis images. Next, RV end-diastolic and end-systolic volumes, as well as right ventricular ejection fraction (RVEF) using Simpson’s rule. The software calculated the epicardial fat volume (EFV), as well as the mean density of the epicardial adipose volume (EAV) (expressed in HU), its standard deviation (SD), and cardiac height. Next, the parameters were indexed for body surface area (BSA).
Figure 4.
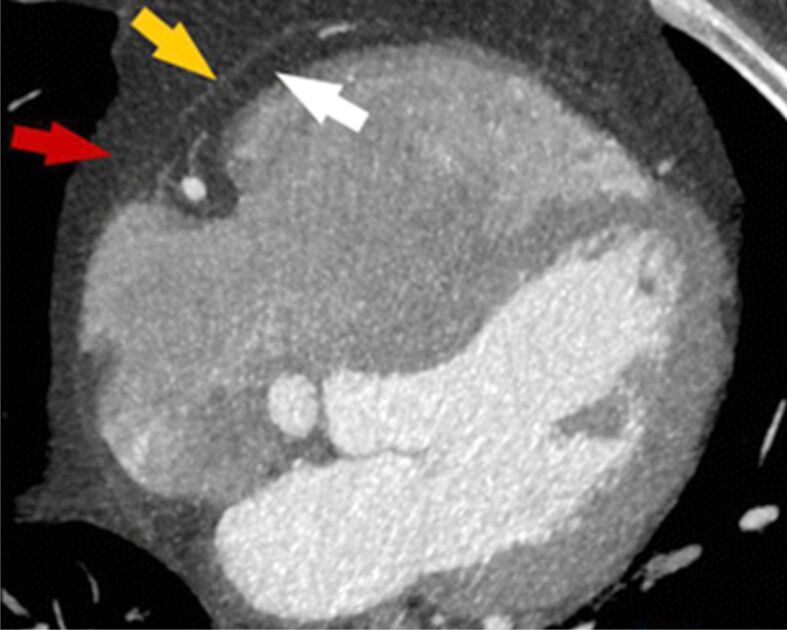
Cardiac CT view: the white arrow reveals the epicardial fat, the yellow arrow depicts the pericardium and the red one – the pericardial fat. CT: Computed tomography
ECG-gated MRIs were also reviewed. Endocardial borders were manually traced at end-diastole, and next at end-systole in the short-axis view. Only volumes below the pulmonary valve were included. Also, RV ejection fraction and wall motion were analyzed. Three lines were drawn to mark the myo-epicardial margin, the parietal and the visceral borders of the pericardium and the lateral border of the paracardial fatty tissue. Based on these three lines, the areas of epicardial, para and pericardial fatty tissue were calculated. Using the modified Simpson’s rule, the corresponding volumes were also estimated.
Statistical analysis
For analysis, IBM Statistical Package for Social Sciences (SPSS) Statistics (version 20.0; IBM Corp., Armonk, NY, USA) was used. Continuous variables were presented as median and mean ± SD. Categorical variables are presented as counts and percentages. Comparison tests were used for both parametric and nonparametric variables. Single sample t-test, one-way analysis of variance (ANOVA) test, and Pearson’s univariate correlation coefficient were used to compare categorical variables.
Results
Clinical and ECG characteristics are presented in Table 2. Data obtained after analyzing the RV measurements from TTE, cardiac CT, and MRI are presented in Table 3. The values of TTE, CT, and MRI parameters from our patients were like the data obtained from the general healthy population – LVEF (p=0.20), RV thickness (p=0.73), EAV (p=0.73), RV mass/BSA (p=0.58).
Table 2.
Clinical characteristics of the patients
|
Clinical characteristics |
Values |
|
Females [ n (%)] |
14 (60.86%) |
|
Males [ n (%)] |
9 (39.14%) |
|
Age (mean ± SD) [years] |
42.25±11.27 |
|
Body weight (mean ± SD) [kg] |
71.20±11.11 |
|
BMI (mean ± SD) [kg/m 2 ] |
24.33±3.47 |
|
BSA (mean ± SD) [m 2 ] |
1.81±0.18 |
|
PVCs burden (mean ± SD) |
19.54±6.02 |
|
History of sudden cardiac death |
0 |
|
ECG |
|
|
▪ Tall R waves in V1 and V2 suggesting RVH |
1 patient |
|
▪ Nonspecific ST-segment depression or T-wave inversions in precordial leads |
2 patients |
BMI: Body mass index; BSA: Body surface area; ECG: Electrocardiography; n: No. of cases; PVC: Premature ventricular contraction; RVH: Right ventricular hypertrophy; SD: Standard deviation
Table 3.
Ventricular parameters of the RV calculated with TTE, CT and MRI
|
Ventricular parameters of the RV |
Values |
|
TTE – parameters measures (mean ± SD) |
|
|
▪ LVEF [%] |
57.33±4.54 |
|
▪ RV diameter (PLA) [mm] |
25.33±3.17 |
|
▪ RV diameter (PSA) [mm] |
28.21±5.28 |
|
▪ RVOT diameter (PSA) [mm] |
25.04±5.81 |
|
▪ RV thickness [mm] |
3.29±1.09 |
|
▪ TAPSE [mm] |
19.5±3.31 |
|
▪ RV global strain |
-24.5±3.1 |
|
▪ RV free wall strain |
-27.5±3.8 |
|
▪ Epicardial fat thickness [mm] |
3.2±1.1 |
|
CT – parameters measures (mean ± SD) |
|
|
▪ RV end-diastolic volume/BSA [mL/m 2 ] |
74.12±21.3 |
|
▪ RV end-systolic volume/BSA [mL/m 2 ] |
41.31±17.56 |
|
▪ RVEF [%] |
45.71±9.1 |
|
▪ EFV [mL] |
69.83±39.71 |
|
▪ EAV density [HU] |
-71.19±8.27 |
|
MRI – parameters measures (mean ± SD) |
|
|
▪ RV end-diastolic volume/BSA [mL/m 2 ] |
72.2±17.31 |
|
▪ RV end-systolic volume/BSA [mL/m 2 ] |
26.41±14.63 |
|
▪ RVEF [%] |
41.71±6.3 |
|
▪ RV mass/BSA [g/m 2 ] |
36.12±7.84 |
|
▪ Late gadolinium enhancement |
0 patients |
BSA: Body surface area; CT: Computed tomography; EAV: Epicardial adipose volume; EFV: Epicardial fat volume; HU: Hounsfield units; LVEF: Left ventricular ejection fraction; MRI: Magnetic resonance imaging; PLA: Parasternal long axis; PSA: Parasternal short axis; RV: Right ventricle; RVEF: Right ventricular ejection fraction; RVOT: Right ventricular outflow tract; SD: Standard deviation; TAPSE: Tricuspid annular plane systolic excursion; TTE: Transthoracic echocardiography
Six out of 23 (26.08%) patients presented right ventricular intramyocardial fat, detected on both cardiac CT and MRI. The morphology of adipose tissue was described as linear in two patients and patchy infiltration for four patients. Fatty deposits were detected in the RV-free wall (five patients), and both the RV-free wall and RVOT (one patient). The fatty replacement was observed in elderly patients and more in men (three out of nine patients) than in women (three out of 14 patients). There was no significant fibrosis or inflammation detected in any of the cases.
In our study, ECG signs of RV hypertrophy were observed in one patient, but not confirmed on TTE, CT, or MRI. Another two patients presented T-wave inversion in the precordial leads on ECG. TTE detected an increase in RV wall thickness, an increase in the EFV, and RV mass indexed. There was no significant fibrosis or inflammation detected in any of the cases or signs of the RV systolic dysfunction.
RV hypertrophy at the level of the free wall was detected in 14.28% (three out of 23 patients) (p=0.001). Global RV hypertrophy was detected in six out of 14 (42.85%) female patients (p=0.73) when a cut-off limit of 35 g/m2 was established, and in four out of nine (44.4%) male patients (p=0.57) using a cut-off limit of 41 g/m2. The upper normal limit of EFV was set at 100 mL, as previously proposed by Sarin et al. [9]. EFV>100 mL was recorded in 26.08% (6/23) of patients (p=0.01).
Epicardial fat thickness ranged from 1 mm to 11.5 mm, without significant differences between genders (6.78±2.17 mm in females versus 6.35±2.98 mm in men) (p=0.34), probably because of similar BMI in women and men. These results are like those previously reported [10, 11].
The ventricular burden obtained on 24-hour Holter ECG monitoring varied between 9% to 30%. A weak positive relationship between the ventricular burden and BMI (r=0.14, p=0.49), TAPSE (r=0.07, p=0.70), the RV thickness (r=0.03, p=0.8), epicardial adipose tissue (r=0.13, p=0.55), RV mass indexed (r=0.05, p=0.82) was observed. No clear cut-off of PVCs burden as 25% could be established in terms related to the increase in the BMI, RV thickness, epicardial adipose tissue, and RV mass indexed. A negative non-significant relationship was observed for the ventricular burden and the age (r=-0.13, p=0.55) and, for the ventricular burden and the epicardial thickness (r=-0.05, p=0.82).
Discussions
No significant positive or negative relationship between the ventricular burden and the parameters investigated in this study was found. The strongest positive correlation was observed with BMI (r=0.14, p=0.49), and the epicardial adipose tissue (r=0.13, p=0.55) while aging presents a negative relationship with the burden of RVOT ectopics.
Physiological cardiac fatty tissue is normally present in adults not affected by any cardiac disease, without any consequences. Foci of intramyocardial adipose tissue extending from the epicardium is a common finding during cardiac CT, observed more frequently in the RV than in the LV. The prevalence of intramyocardial fat detection from our study is like the one observed in other studies of 16–43% [13], but with differences between genders, probably caused by the reduced number of patients included in our study and by the fact that our population is slightly younger from previous publications. In the series of results obtained from the autopsies, focal RV fatty tissue was observed in 85% of patients free of cardiac diseases [13].
Both cardiac CT and MRI can detect physiological RV myocardial adipose tissue either with linear or patchy morphology located in the free wall of RV, in the RVOT, and the anterolateral or apical segments, usually with a preserved myocardial thickness [14]. Other frequent locations are represented by the trabeculae, the RV moderator band, the LV apex, and the interventricular septum. In our study, adipose deposits were observed only in the RV-free wall and RVOT. The absence of other locations as mentioned in previous studies is caused by the limited number of patients included in our study. Compared to the other studies, in two patients was observed an increase in the myocardial thickness and EAV, confirmed by CT and MRI. This fact could be explained by the fact that RV fatty infiltration can extend from the epicardium through the myocardium until the subendocardial layer, causing an increase in the myocardial wall thickness [15].
It is known that the increase in the prevalence and the degree of RV ectopic intramyocardial adipose deposits is a part of the aging process [12]. Setting a cut-off age limit of 54 years, our study confirmed this data, and we observed a significant increase in the RV thickness (p=0.01), and in the EAV (p=0.001), but not for the RV mass indexed BSA (p=0.11) (Table 4). Some of the parameters evaluated, as the RV mass and the RV volumes are body size dependent and are usually indexed to BSA, with values being greater in men compared to women, and decreasing with aging.
Table 4.
Relationship between age and RV thickness, EAV and RV mass indexed BSA in women and men
|
Gender (age) |
Parameters |
||
|
RV thickness (mean ± SD) [mm] |
EAV (mean ± SD) [mL] |
RV mass indexed BSA (mean ± SD) [g/m 2 ] |
|
|
Women |
|||
|
▪ <54 years |
2.4±0.66 |
33.54±7.57 |
42.8±17.5 |
|
▪ ≥54 years |
4±1.41 |
38±10.24 |
99±51.19 |
|
Men |
|||
|
▪ <54 years |
3.42±0.49 |
79±44.07 |
36.57±5.15 |
|
▪ ≥54 years |
4.66±0.47 |
135.33±4.18 |
47±2.94 |
BSA: Body surface area; EAV: Epicardial adipose volume; RV: Right ventricle; SD: Standard deviation
The relationship between RV adipose tissue with obesity remains still unclear [12]. Sometimes, when the adipose fatty foci have a circumscribed morphology resembling cardiac tumors or are detected intracavitary as in the case of adipose degeneration of the RV moderator band, it is paramount to recognize the most common locations of physiological fatty tissue and to correlate these findings with the clinical data toward an adequate interpretation. Obesity is associated with an increase in BSA-indexed RV mass.
In this study, both men and women exhibited an increase in the epicardial adipose tissue and the RV index mass with increasing BMI (Table 5). These results are like those previously published [16]. There was a positive relationship between BMI and RV thickness only in women (p=0.02), and not in men, suggesting a disproportionate RV thickness increase in women. The sex difference in BMI on RV thickness can be explained by a differential effect of adipose tissue mass on RV modeling. Because some of the patients in our study presented both arterial hypertension and hypercholesterolemia, risk factors involved in RV remodeling, it is difficult to interpret these results. These details raise the question of whether the RV in women is more prone to the effects of increased body fat mass [17].
Table 5.
Relationship between BMI and RV thickness, EAV and RV mass indexed BSA in women and men
|
Gender (BMI) |
Parameters |
||
|
RV thickness (mean ± SD) [mm] |
EAV (mean ± SD) [mL] |
RV mass indexed BSA (mean ± SD) [g/m 2 ] |
|
|
Women |
|||
|
▪ <24.9 kg/m 2 |
2.86±01.14 |
41.66±18.09 |
31.66±6.28 |
|
▪ ≥25 kg/m 2 |
4.2±0.97 |
90.6±49.49 |
38.8±9.3 |
|
p =0.02 |
p =0.014 |
p =0.007 |
|
|
Men |
|||
|
▪ <24.9 kg/m 2 |
3.2±0.74 |
74.83±33.63 |
35.33±4.26 |
|
▪ ≥25 kg/m 2 |
4±0.89 |
120.02±41.4 |
46.25±3.34 |
|
p =0.1 |
p =0.03 |
p =0.02 |
BMI: Body mass index; BSA: Body surface area; EAV: Epicardial adipose volume; RV: Right ventricle; SD: Standard deviation
Study limitations
Our study presents several limitations. First, we could only evaluate the associations and not the causality. Secondly, we could not obtain all clinical information for a minority of patients because of the retrospective study model. No power calculation was done before the study. Although the echocardiographic, CT, and MRI investigations were performed by experienced examiners, sometimes, for some patients, the image quality was challenging and thus, the measurements were limited by the quality of those images or missing. This issue prevented us from creating an adequate and complete multivariate analysis.
Conclusions
No significant positive or negative relationship between the ventricular burden and the RV thickness, and RV-indexed mass were found in individuals with a high PVCs burden originating from RVOT. The strongest positive correlation was observed between BMI and the epicardial adipose tissue, while aging presents a negative relationship with the burden of RVOT ectopics.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Anumonwo JMB, Herron T. Fatty infiltration of the myocardium and arrhythmogenesis: potential cellular and molecular mechanisms. Front Physiol. 2018;9:2–2. doi: 10.3389/fphys.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular, and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 3.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, Bordi C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic, and hypertrophic hearts. Cardiovasc Pathol. 2004;13(6):313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2016;38(17):1294–1302. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 5.Kimura F, Matsuo Y, Nakajima T, Nishikawa T, Kawamura S, Sannohe S, Hagiwara N, Sakai F. Myocardial fat at cardiac imaging: how can we differentiate pathologic from physiologic fatty infiltration. Radiographics. 2010;30(6):1587–1602. doi: 10.1148/rg.306105519. [DOI] [PubMed] [Google Scholar]
- 6.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements, and systematic review of main outcomes. Arq Bras Cardiol. 2013;101(1):e18–e28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153(6):907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1–1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarin S, Wenger C, Marwaha A, Qureshi A, Go BDM, Woomert CA, Clark K, Nassef LA, Shirani J. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008;102(6):767–771. doi: 10.1016/j.amjcard.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Aydın AM, Kayalı A, Poyraz AK, Aydın K. The relationship between coronary artery disease, pericoronary epicardial adipose tissue thickness. J Int Med Res. 2015;43(1):17–25. doi: 10.1177/0300060514558323. [DOI] [PubMed] [Google Scholar]
- 11.Tanami Y, Jinzaki M, Kishi S, Matheson M, Vavere AL, Rochitte CE, Dewey M, Chen MY, Clouse ME, Cox C, Kuribayashi S, Lima JA, Arbab-Zadeh A. Lack of association between epicardial fat volume and extent of coronary artery calcification, severity of coronary artery disease, or presence of myocardial perfusion abnormalities in a diverse, symptomatic patient population: results from the CORE320 multicenter study. Circ Cardiovasc Imaging. 2015;8(3):e002676–e002676. doi: 10.1161/CIRCIMAGING.114.002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobi AH, Gohari A, Zalta B, Stein MW, Haramati LB. Ventricular myocardial fat: CT findings and clinical correlates. J Thorac Imaging. 2007;22(2):130–135. doi: 10.1097/01.rti.0000213576.39774.68. [DOI] [PubMed] [Google Scholar]
- 13.Tansey DK, Aly Z, Sheppard MN. Fat in the right ventricle of the normal heart. Histopathology. 2005;46(1):98–104. doi: 10.1111/j.1365-2559.2005.02054.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim E, Choe YH, Han BK, Kim SM, Kim JS, Park SW, Sung J. Right ventricular fat infiltration in asymptomatic subjects: observations from ECG-gated 16-slice multidetector CT. J Comput Assist Tomogr. 2007;31(1):22–28. doi: 10.1097/01.rct.0000236416.05267.6c. [DOI] [PubMed] [Google Scholar]
- 15.Imada M, Funabashi N, Asano M, Uehara M, Hori Y, Ueda M, Komuro I. Epidemiology of fat replacement of the right ventricular myocardium determined by multislice computed tomography using a logistic regression model. Int J Cardiol. 2007;119(3):410–413. doi: 10.1016/j.ijcard.2006.07.174. [DOI] [PubMed] [Google Scholar]
- 16.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JAC, Bluemke DA, Kawut SM. Obesity and right ventricular structure and function: the MESA-Right Ventricle study. Chest. 2012;141(2):388–395. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rider OJ, Lewis AJM, Lewandowski AJ, Ntusi N, Nethononda R, Petersen SE, Francis JM, Pitcher A, Banerjee R, Leeson P, Neubauer S. Obese subjects show sex-specific differences in right ventricular hypertrophy. Circ Cardiovasc Imaging. 2015;8(1):e002454–e002454. doi: 10.1161/CIRCIMAGING.114.002454. [DOI] [PubMed] [Google Scholar]


