Abstract
Introduction: Gonadal pediatric tumors are rare, ranking fourth (6%) among pediatric tumors, by Surveillance, Epidemiology, and End Results Program (https://seer.cancer.gov/). They have vague symptoms, leading to late discovery, but early detection and identifying its risk factors result in favorable prognosis and reduction of its incidence respectively. Patients, Material and Methods: A 10-year retrospective study identified peculiarities and risk factors in 210 children till age 17 with (para)gonadal tumors. Results: Stress, pollution (agricultural chemicals, insecticides and metal mine), obesity, breastfeeding ≤5 months, malformations [mainly non-genetic related 67/87 (77%), especially eye malformation – 64%], hormone, smoking, positive heredo-genetic history, rural residence area, abnormal birth weight, and menstruation disorders showed an increased gonadal malignancy risk; relative risk ratio (RR): 1.33, 1.30, 1.34, 1.11, 1.65, 1.16, 1.36, 1.10, 1.00, 1.08 and 1.15 folds, respectively. RR for histopathological subtypes: immature teratoma (IT) (pollution – 1.75, Rhesus positive – 3.41), dysgerminoma (menstruation disorders – 2.80), granulosa cell tumor (stress – 2.10, menstruation disorders – 2.80), mucinous cystadenomas (obesity – 2.84, no postnatal vaccine – 3.71), mature teratomas (stress – 2.35, malformations – 2.18) and serous cystadenomas (breastfeeding ≤5 months – 2.53), dependent variables being mixed germ cell tumors (GCTs) and cysts. Children presenting with bleeding (73%), abdominal distention (62%), elevated tumor markers (91%), (multilocular) solid tumor (88% and 100%), tumor size >10 cm (65%), GCTs (74%), death (100%), metastases (100%), viruses (77%), loss of appetite (68%), and weight (85%), had gonadal malignant tumors, especially mixed GCTs and IT. Conclusions: Avoiding these risk factors will prevent and reduce gonadal pediatric tumors. Investigating children presenting with the listed peculiarities, especially if exposed to the mentioned risk factors, will enable early gonadal tumor identification, successful patient management, and favorable prognosis.
Keywords: testicular tumor , ovarian tumor , risk factors , gonadal tumor peculiarities , children
Introduction
Pediatric testicular and ovarian tumors are rare, with an incidence rate of 0.3–12:100 000 and 2.6–10.7:100 000, peaking below age one (18:1000 000) and at 15–19 years (28:1000 000) [1, 2, 3, 4, 5]. Although curable with favorable prognosis, this is possible only when discovered early [1, 2, 3, 4, 5]. However, there are obstacles to achieving this, making it a challenge, as gonadal tumors have an unclear multi-etiology and vague manifestation, and hence easily misdiagnosed. This was observed in 70% of women with ovarian tumors who presented late, leading to outcomes like death or infertility [1, 2, 3, 6]. A group of studies showed that the daily cost of hospitalization of pediatric cancer patients is 70% higher than for other diseases, and they stay an average of eight days more in the hospital; also, delayed diagnoses increase patients’ hospital costs [7, 8].
The Romanian Society of Pediatric Onco-Hematology and the National Registry of Childhood Cancers published a 2010–2021 study showing that annually, the prevalence of gonadal and germ cell tumors (GCTs) among other pediatric tumors was 3.4% for 0–14 years, 12.2% for 15–19 years and 5% for 0–19 years [5, 9, 10]. The pediatric cancer patients’ survival in Romania was 72% (2010–2017) for 0–19 years, which is below the mean of European countries (81%); also, the survival rate in children with gonadal and GCTs dropped from 85% (2010–2013) to 81% (2014–2017), and 6% increase was observed in ages 0–14 [9, 10, 11]. Cancer remains the leading cause of disease related death in children, and an increase in the incidence rate and mortality rate from 1975 is recognized, especially in Western countries; also, a 25% increase in the incidence rate is predicted by 2025 in Europe [2, 12, 13]. The incidence rate of testicular and ovarian tumors in Europe is 12% and 10.7%, unlike in North America 8.3% and 8.1%, in Africa 0.3% and 4.9%, and in Asian populations 2.9% and and 8.1%, respectively [1, 2, 4]. World Health Organization (WHO) recently reported that cancer awareness, early detection of pediatric tumors, administering appropriate treatment, and availability of cancer facilities and medications varied significantly between Western and Eastern European countries, leading to a 9–57% mortality rate, varying health of survivors; hence, actions for equality across Europe is required [14].
Aim
Our study, therefore, aims to identify children with (para)gonadal tumors, analyze the peculiar clinical features presented, and evaluate the risk factors to which they were exposed. This will allow us to easily identify malignancy and its histopathological (HP) subtype quickly, possibly before surgery. This will also enable better patient management, favorable prognosis, minimal side effects, and prevention or reduction of childhood tumors by avoiding identified high-risk factors.
Patients, Materials and Methods
We conducted a retrospective study from 2010–2020 of the peculiarities and risk factors of gonadal tumors at Emergency Children Hospitals in Iaşi, Bacău, and Timişoara (Louis Ţurcanu) that attends to pediatric patients from western Romania. The study participants included 210 pediatric patients, with 164 being female, 98 patients had malignant (para)gonadal tumors and 112 had benign tumors. Patients were at prepubertal (one month–nine years) to pubertal ages (10–17 years).
Data
We collected data from patients’ files, questionnaires, and telephone interviews. The data included demographic details, diagnosis, presentations (localization, size, tumor stage, and grade), loculation type, pain location and radiation, prenatal diagnosis, presence of signs and symptoms such as appendicitis, vomiting, constipation, loss of appetite, weight loss, leukocytosis, fever, bleeding, abdominal distention, viral infection, ascites, and urinary tract infection (UTI). We also collected data on the presence of elevated tumor markers, metastasis, recurrence, compressed organs, asymptomatic cases, mortality and gonadectomy. For risks factors, patients’ data was collected included obesity, mother’s age, length of breastfeeding, disorders of sexual development, stress, hormones, smoking, heredo-genetic history, abnormal birthweight, residential area, pollution, Rhesus (Rh) factor, vitamin D, vaccination, menstrual disorders, and malformations.
Informed consent
Informed verbal and written consent was obtained from the participants’ parents.
Ethical approval
The study was performed in compliance with all institutional policies, according to the Helsinki Declaration’s tenets, the World Medical Association (WMA), Romania’s regulations, Medical Research Council Guidelines for Good Clinical Practice in Clinical Trials, WHO Guidelines, and approved by the Timişoara, Iaşi, Arad and Bacău Emergency Children Hospital Ethical Committee (Approval No. 124/2020–16922 on 11.12.2020) and the Scientific Research Ethics Commission (Approval No. 59/12.12.2018).
Tumor markers analysis
We obtained blood (5 mL) from fasting patients before administering any treatment and afterward to verify the patients’ condition. We stored the blood at 25°C for one hour, then centrifuged at 4000 rpm to isolate the blood serum. As per the manufacturer’s directives (Mannheim, Germany), the markers were measured by an electric chemiluminescence analyzer with alpha-fetoprotein (AFP, 9.92 IU/mL), beta-human chorionic gonadotropin (β-HCG, 5.3 IU/l), lactate dehydrogenase (LDH, 0–300 U/L), cancer antigen-125 (CA-125, 0–35 kU/L), carcinoembryonic antigen (CEA, 0–5.3 ng/mL), neuron-specific enolase (NSE, 0–16 ng/mL) and human epididymis protein 4 (HE4, 0–70 pmol/L) kits and cutoff marks were accordingly. Inhibin B hormone, anti-Müllerian hormone (AMH), and estradiol were measured based on age.
Statistical analysis
We used Stata ver. 17 (2021) (StataCorp, Texas, TX, USA) multinomial logistic regression for relative risk ratio (RR), estimation coefficient (β), 95% confidence interval (CI), p-values <0.05, and the results in percentages.
Histopathology study
From the Pathology Service of the Emergency Children Hospitals of Bacău, Iaşi, Arad, and Timişoara, expert pathologists analyzed gonadal tissue specimens macro- and microscopically to identify HP components and determined the diagnoses. An average of two or more sample fragments per patient of testicular or ovarian specimens were harvested by tissue excision, and ≥6 slides per patient underwent Hematoxylin–Eosin (HE) and immunohistochemical (IHC) staining. Fixation of the tissue was performed by placing the sample in a 10% neutral buffered formalin solution for 48 hours. The sample was processed, paraffin-embedded, sectioned into 3 μm sections, deparaffinized, and rehydrated. The sections were then ready for staining using the usual HE technique. Leica Autostainer XL was used for morphological staining (Leica Biosystems Newcastle Ltd., Newcastle upon Tyne, UK). The HE-stained sections were examined on multiple slides with a Leica DM 750 optical microscope and Leica AirLab Microsystems GmbH to identify the HP components. If a rare or suspected malignant tumor was observed, the section was taken for further IHC analysis. Heat-induced epitope retrieval was conducted to retrieve antigen reactivity in the paraffin-embedded sample tissue (formalin-fixed) using the Novocastra mouse monoclonal antibody by Leica Biosystems (Newcastle Ltd., Newcastle upon Tyne, UK). The antibodies, their clones, and dilutions performed at 25°C for 30 minutes, include: anti-cytokeratin 8/18 (CK8/18) (5D3, 1:200), anti-Ki67 (MM1, 1:200), anti-vimentin (V9, 1:800), anti-alpha smooth muscle actin (α-SMA) (αsm-1, 1:50), anti-S100 (EP32, 1:100), anti-placental alkaline phosphatase (PLAP) (8A9, 1:50), anti-cluster of differentiation (CD)10 (56C6, 1:100), anti-octamer-binding protein 3/4 (OCT3/4) (N1NK, 1:100), anti-myogenin (Myf4) (LO26, 1:40), anti-epithelial membrane antigen (EMA) (GP1.4, 1:200), anti-desmin (DE-R-11, 1:200), anti-CD30 (JCM182, 1:100), anti-AFP (C3, 1:100), anti-p53 (DO-7, 1:800) per the manufacturer’s guidelines.
Besides markers, physical and radiological examination, we performed surgical interventions to verify tumor presence for staging, treatment, and postoperative pathology diagnostics. Histopathology using HE staining and IHC played an extremely vital role in investigating and concretely identifying patients with malignancy, from specimen macroscopic and microscopic examination for postoperative staging and grading; to identify lymph node metastasis, lymphovascular invasion, and retroperitoneal nodal, used in deciding the treatment and estimating patients survival prognoses. Histopathology diagnosis, classification, and staging was done using the WHO Classification of Tumors of the Genital Organs and the Urinary System criteria.
Results
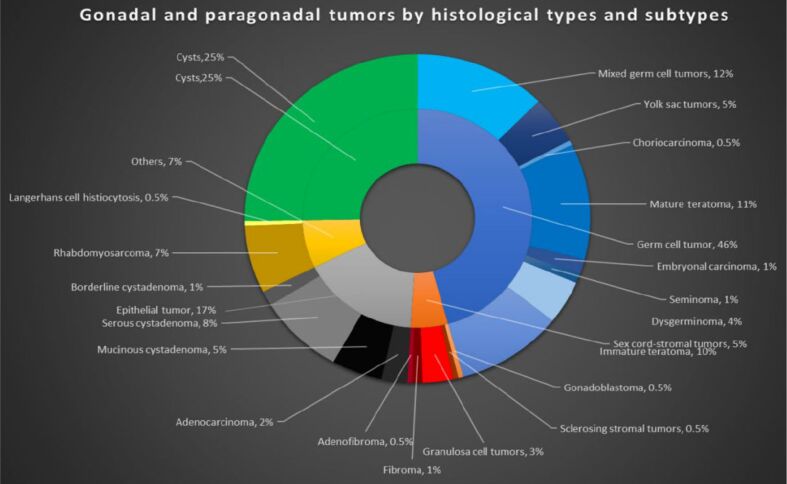
Our study population consisted of 210 patients: 96 had GCTs, nine had sex-cord stromal tumors (SCSTs), 35 had epithelial tumors, and 53 had cysts; also, a patient had a combination of SCSTs and GCTs (gonadoblastoma), another had SCST and epithelial tumor (adenofibroma), while 15 patients had other types of tumors like rhabdomyosarcoma (RMS) and Langerhans cell histiocytosis (LCH) (Figure 1).
Figure 1.
Histological types and subtype of (para)gonadal tumors in our study
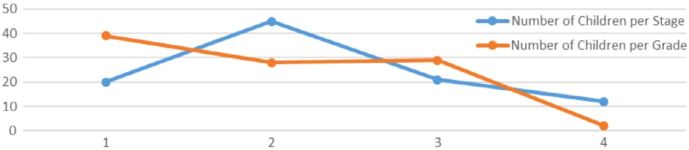
The male-to-female ratio in this study is 1:1.45; also, the majority of patients presented at stage 2 – 45/98 (46%) and at grade 1 – 39/98 (40%) (Figure 2). Gonadectomy was performed on 27 (24%) patients with benign tumors due to large tumor size, if vital organs are compressed, or in cases of necrotic torsioned gonads. In comparison, nine (9%) patients with malignancy had their gonads spared because of the patient’s and family’s decisions, considering if both gonads were involved, tumor type, stage, grade, and size. Tumor obstruction led to 21% of UTI cases. The patients with viruses in Table 1 had human immunodeficiency virus (HIV), human herpesvirus 1–6, hepatitis, tuberculosis, and rubella. The asymptomatic presentation reported was observed in two boys whose malignant tumors were found coincidentally on admission after involvement in accidents. Of the 35 patients with appendicitis, 54% had their tumor identified during appendicectomy, and for the other 45%, the reverse was the case.
Figure 2.
The number of children per tumor stage and grade in our study
Table 1.
The peculiarities observed in our study population, for the total number of patients with benign and malignant tumors, accordingly, using percentages [n, (%)] and p-values
|
Peculiarities |
Total |
Malignant tumors |
Bening tumors |
p -value |
|
Tumor types |
210 |
Total : 98 IT: 21 (10%) RMS: 14 (6.7%) DYS: 9 (4.3%) Mixed GCT: 26 (12.4%) SEM: 2 (1%) AC: 5 (2.4%) LCH: 1 (0.5%) EC: 3 (1.4%) GrCT: 6 (2.9%) CHOR: 1 (0.5%) YST: 10 (4.8%) |
Total : 112 Gonadoblastoma: 1 (0.5%) SST: 1 (0.5%) MT: 24 (11.4) Fibroma: 2 (1%) AF: 1 (5%) MCA: 10 (4.8%) SCA: 17 (8.1%) BCA: 3 (1.4%) Cysts: 53 (25%) |
|
|
Age [years] |
||||
|
Prepuberty (0–9) |
56 |
41 (73%) |
15 (27%) |
0.001 |
|
Puberty (10–17) |
154 |
57 (37%) |
97 (63%) |
|
|
Sex |
||||
|
Male |
47 |
40 (85%) |
7 (15%) |
0.001 |
|
Female |
163 |
58 (36%) |
105 (64%) |
|
|
Size [cm] |
||||
|
<5 |
37 |
10 (27%) |
27 (73%) |
0.001 |
|
5–10 |
110 |
47 (43%) |
63 (57%) |
|
|
>10 |
63 |
41 (65%) |
22 (35%) |
|
|
Tumor location |
||||
|
Right |
79 |
32 (41%) |
47 (49%) |
0.373 |
|
Left |
110 |
55 (50%) |
55 (50%) |
|
|
Both sides |
21 |
11 (52%) |
10 (48%) |
|
|
Pain location |
||||
|
Right |
61 |
30 (49%) |
31 (51%) |
0.932 |
|
Left |
104 |
48 (49%) |
56 (51%) |
|
|
Both sides |
38 |
18 (47%) |
20 (53%) |
|
|
Pain radiates to leg |
53 |
26 (49%) |
27 (51%) |
0.687 |
|
Repetition |
20 |
13 (65%) |
7 (35%) |
0.084 |
|
Death |
4 |
4 (100%) |
0 (0%) |
0.031 |
|
Prenatal diagnosis |
8 |
2 (25%) |
6 (75%) |
0.210 |
|
Gonadectomy |
116 |
89 (77%) |
27 (23%) |
0.001 |
|
Asymptomatic |
7 |
2 (29%) |
5 (71%) |
0.329 |
|
Appendicitis |
35 |
16 (46%) |
19 (54%) |
0.901 |
|
Loss of appetite |
59 |
40 (68%) |
19 (32%) |
0.001 |
|
Loss of weight |
33 |
28 (85%) |
5 (15%) |
0.001 |
|
Leukocytosis |
54 |
19 (35%) |
35 (65%) |
0.050 |
|
Fever |
16 |
5 (31%) |
11 (69%) |
0.191 |
|
Constipation |
21 |
13 (62%) |
8 (38%) |
0.140 |
|
Vomit |
9 |
2 (22%) |
7 (78%) |
0.329 |
|
Bleeding |
26 |
19 (73%) |
7 (27%) |
0.001 |
|
Abdominal distention |
63 |
39 (62%) |
24 (38%) |
0.004 |
|
Ascites |
14 |
10 (71%) |
4 (29%) |
0.055 |
|
GCT |
97 |
72 (74%) |
25 (26%) |
0.001 |
|
Not GCT |
113 |
26 (23%) |
87 (77%) |
|
|
Loculation |
||||
|
Unilocular cyst |
39 |
0 (0%) |
39 (100%) |
0.001 |
|
Unilocular cyst + solid |
22 |
2 (9%) |
20 (91%) |
|
|
Multilocular cyst |
33 |
5 (15%) |
28 (85%) |
|
|
Multilocular cyst + solid |
50 |
29 (58%) |
21 (42%) |
|
|
Multilocular solid |
33 |
29 (88%) |
4 (12%) |
|
|
Solid |
33 |
33 (100%) |
0 (0%) |
|
|
Compressed organs |
36 |
20 (56%) |
16 (44%) |
0.240 |
|
Viruses |
26 |
20 (77%) |
6 (23%) |
0.001 |
|
Elevated tumor markers |
88 |
80 (91%) |
8 (9%) |
0.001 |
AC: Adenocarcinoma; AF: Adenofibroma; BCA: Borderline cystadenoma; CHOR: Choriocarcinoma; DYS: Dysgerminoma; EC: Embryonal carcinoma; GCT: Germ cell tumor; GrCT: Granulosa cell tumor; IT: Immature teratoma; LCH: Langerhans cell histiocytosis; MCA: Mucinous cystadenoma; MT: Mature teratoma; n: No. of cases; RMS: Rhabdomyosarcoma; SCA: Serous cystadenoma; SEM: Seminoma; SST: Sclerosing stromal tumor; YST: Yolk sac tumor.
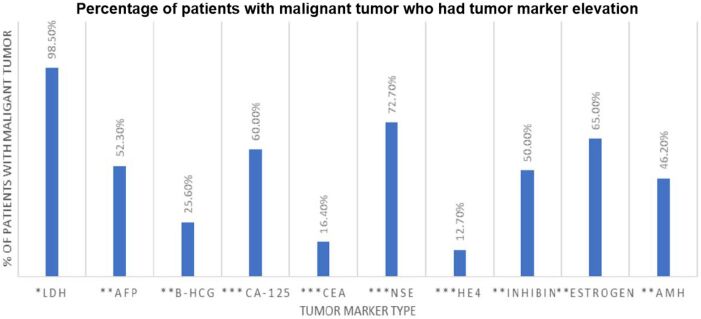
The surgical approaches used were open surgery (70%), laparoscopy (24%), and robotic surgery (6%). Additionally, in our study, 34 (16.2%) patients had metastasis, the <5-year overall survival was 32.3% and for >5 years was 66.7%. The elevation of tumor markers among malignant tumor patients is shown in Figure 3. Furthermore, LDH was elevated in two patients with large cysts, three with mature teratoma (MT), a patient with serous cystadenoma (SCA), and another with borderline cystadenoma (BCA); and CA-125 was slightly elevated in three patients with MTs, a patient with SCAs, one with cyst and another with adenofibroma.
Figure 3.
Tumor marker elevation in our malignant tumor study population. AFP: Alpha-fetoprotein; AMH: Anti-Müllerian hormone; β-HCG: Beta-human chorionic gonadotropin; LDH: Lactate dehydrogenase; CA-125: Cancer antigen-125; CEA: Carcinoembryonic antigen; NSE: Neuron-specific enolase; HE4: Human epididymis protein 4
Further studies done to identify significant peculiarities in each tumor type are shown in Table 2 and Table 3. Excluding cases where no patient had that peculiar presentation, we observed that the percentage of patients with the mentioned morphology in Table 4, recognized in literature was >60%, except for 25 cases from the 48 peculiarities for each of the 10 malignant tumor types reported (25/480, 5.2%), observed in yolk sac tumor (YST), immature teratoma (IT), dysgerminoma (DYS), RMS, granulosa cell tumor (GrCT). For benign tumors, in Table 5, the exception was eight cases from the 39 peculiarities for each of the nine benign tumor types reported (8/351, 2.3%), observed in patients with SCA, mucinous cystadenoma (MCA) and BCA. The relative risk of getting gonadal malignant tumors with an increase in each unit of the risk factors analyzed is reported in Table 6. Eighty-five percent of the patients who were exposed to pollution reported constant exposure to agricultural chemicals, metal mining, and insecticides. The estimation coefficient (β) showing the chances of getting the different malignant and benign tumor types with an increase in each unit of the risk factors is presented in Table 7 and Table 8; also, patients who had mixed GCT and cysts were used as the dependent variable. Patients with eye malformations 28/87 (32%) was the highest among non-genetic malformations, and disorder of sex development (DSD) 17/28 (60.7%) was the highest among genetic-related malformations (Table 9).
Table 2.
The peculiarities observed in the different malignant tumor types found in our study population, using percentages [n, (%)]
|
Peculiarities of malignant tumors |
Total |
YST |
Mixed GCT |
IT |
DYS |
SEM |
AC |
CHOR |
RMS |
EC |
LCH |
GrCT |
|
Age [years] |
||||||||||||
|
Prepuberty (0–9) |
42 |
6 (14.3%) |
9 (22%) |
9 (22%) |
2 (5%) |
0 (0%) |
2 (5%) |
0 (0%) |
7 (17%) |
2 (5%) |
1 (2%) |
3 (7%) |
|
Puberty (10–17) |
56 |
4 (7%) |
17 (30%) |
12 (21%) |
7 (12%) |
2 (4%) |
3 (5%) |
1 (2%) |
7 (12%) |
1 (2%) |
0 (0%) |
3 (5%) |
|
Sex |
||||||||||||
|
Male |
40 |
4 (10%) |
17 (43%) |
7 (18%) |
1 (3%) |
2 (5%) |
0 (0%) |
1 (3%) |
8 (20%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Female |
58 |
6 (10%) |
9 (16%) |
14 (24%) |
8 (14%) |
0 (0%) |
5 (9%) |
0 (0%) |
6 (10%) |
3 (5%) |
1 (2%) |
6 (10%) |
|
Size [cm] |
||||||||||||
|
<5 |
10 |
1 (10%) |
1 (10%) |
2 (20%) |
2 (20%) |
0 (0%) |
1 (10%) |
0 (0%) |
2 (20%) |
1 (10%) |
0 (0%) |
0 (0%) |
|
5–10 |
47 |
4 (9%) |
15 (32%) |
10 (21%) |
4 (9%) |
1 (2%) |
1 (2%) |
1 (2%) |
8 (17%) |
1 (2%) |
1 (2%) |
1 (2%) |
|
>10 |
41 |
5 (12%) |
10 (24%) |
9 (22%) |
3 (7%) |
1 (2%) |
3 (7%) |
0 (0%) |
4 (10%) |
1 (2%) |
0 (0%) |
5 (12%) |
|
Tumor location |
||||||||||||
|
Right |
32 |
3 (9%) |
9 (28%) |
4 (13%) |
5 (16%) |
1 (3%) |
2 (6%) |
1 (3%) |
6 (19%) |
0 (0%) |
0 (0%) |
1 (3%) |
|
Left |
55 |
7 (13%) |
16 (29%) |
15 (27%) |
4 (7%) |
1 (2%) |
1 (2%) |
0 (0%) |
6 (11%) |
2 (4%) |
0 (0%) |
3 (5%) |
|
Both sides |
11 |
0 (0%) |
1 (9%) |
2 (18%) |
0 (0%) |
0 (0%) |
2 (18%) |
0 (0%) |
2 (18%) |
1 (9%) |
1 (9%) |
2 (18%) |
|
Pain location |
||||||||||||
|
Right |
30 |
3 (10%) |
8 (27%) |
4 (13%) |
5 (17%) |
1 (3%) |
2 (7%) |
1 (3%) |
5 (17%) |
0 (0%) |
0 (0%) |
1 (3%) |
|
Left |
48 |
6 (12%) |
17 (34%) |
13 (26%) |
3 (6%) |
0 (0%) |
1 (2%) |
0 (0%) |
5 (10%) |
2 (4%) |
0 (0%) |
3 (6%) |
|
Both sides |
18 |
1 (6%) |
1 (6%) |
4 (22%) |
1 (6%) |
1 (6%) |
2 (11%) |
0 (0%) |
4 (22%) |
1 (6%) |
1 (6%) |
2 (11%) |
|
Pain radiates to leg |
26 |
3 (6%) |
25 (49%) |
12 (24%) |
2 (4%) |
0 (0%) |
2 (4%) |
0 (0%) |
4 (8%) |
1 (2%) |
0 (0%) |
2 (4%) |
|
Repetition |
13 |
1 (8%) |
3 (23%) |
1 (8%) |
0 (0%) |
0 (0%) |
2 (15%) |
0 (0%) |
4 (31%) |
1 (8%) |
0 (0%) |
1 (8%) |
|
Death |
4 |
0 (0%) |
2 (50%) |
0 (0%) |
1 (25%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (25%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Prenatal diagnosis |
2 |
0 (0%) |
0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (50%) |
|
Gonadectomy |
89 |
9 (10%) |
26 (29%) |
18 (20%) |
6 (7%) |
2 (2%) |
5 (6%) |
1 (1%) |
12 (13%) |
3 (3%) |
1 (1%) |
6 (7%) |
|
Asymptomatic |
2 |
0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Appendicitis |
16 |
0 (0%) |
4 (25%) |
2 (13%) |
3 (19%) |
0 (0%) |
2 (13%) |
0 (0%) |
3 (19%) |
2 (13%) |
0 (0%) |
0 (0%) |
|
Loss of appetite |
40 |
3 (8%) |
12 (30%) |
7 (18%) |
2 (5%) |
1 (3%) |
2 (5%) |
0 (0%) |
8 (20%) |
1 (3%) |
1 (3%) |
3 (8%) |
|
Loss of weight |
28 |
1 (4%) |
10 (36%) |
7 (25%) |
1 (4%) |
0 (0%) |
1 (4%) |
0 (0%) |
4 (14%) |
1 (4%) |
1 (4%) |
2 (7%) |
|
Leukocytosis |
19 |
3 (16%) |
4 (21%) |
5 (26%) |
1 (5%) |
0 (0%) |
2 (11%) |
0 (0%) |
1 (5%) |
3 (16%) |
0 (0%) |
0 (0%) |
|
Fever |
5 |
0 (0%) |
1 (20%) |
4 (80%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Constipation |
13 |
1 (8%) |
1 (8%) |
3 (23%) |
2 (15%) |
0 (0%) |
1 (8%) |
0 (0%) |
1 (8%) |
1 (8%) |
0 (0%) |
3 (23%) |
|
Vomit |
2 |
0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Bleeding |
19 |
1 (5%) |
6 (32%) |
3 (16%) |
3 (16%) |
0 (0%) |
2 (11%) |
0 (0%) |
2 (11%) |
0 (0%) |
0 (0%) |
2 (11%) |
|
Abdominal distention |
39 |
4 (10%) |
7 (18%) |
9 (23%) |
3 (8%) |
1 (3%) |
4 (10%) |
0 (0%) |
6 (15%) |
1 (3%) |
0 (0%) |
4 (10%) |
|
Ascites |
10 |
1 (10%) |
4 (40%) |
2 (20%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
3 (30%) |
|
GCT |
72 |
10 (14%) |
26 (36%) |
21 (29%) |
9 (13%) |
2 (3%) |
0 (0%) |
1 (1%) |
0 (0%) |
3 (4%) |
0 (0%) |
0 (0%) |
|
Not GCT |
26 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
5 (19%) |
0 (0%) |
14 (54%) |
0 (0%) |
1 (4%) |
6 (23%) |
|
Loculation |
||||||||||||
|
Unilocular cyst |
0 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Unilocular cyst + solid |
2 |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Multilocular cyst |
5 |
0 (0%) |
0 (0%) |
2 (40%) |
1 (20%) |
0 (0%) |
1 (20%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (20%) |
0 (0%) |
|
Multilocular cyst + solid |
29 |
3 (10%) |
9 (31%) |
6 (21%) |
2 (7%) |
1 (3%) |
0 (0%) |
1 (3%) |
2 (7%) |
2 (7%) |
0 (0%) |
3 (10%) |
|
Multilocular solid |
29 |
2 (7%) |
4 (14%) |
13 (45%) |
2 (7%) |
0 (0%) |
2 (7%) |
0 (0%) |
4 (14%) |
0 (0%) |
0 (0%) |
2 (7%) |
|
Solid |
33 |
5 (15%) |
12 (36%) |
0 (0%) |
4 (12%) |
1 (3%) |
1 (3%) |
0 (0%) |
8 (24%) |
1 (3%) |
0 (0%) |
1 (3%) |
|
Compressed organs |
20 |
5 (25%) |
6 (30%) |
3 (15%) |
1 (5%) |
0 (0%) |
1 (5%) |
0 (0%) |
2 (10%) |
1 (5%) |
0 (0%) |
1 (5%) |
|
Grade |
||||||||||||
|
1 |
4 (10%) |
4 (10%) |
16 (41%) |
5 (13%) |
0 (0%) |
0 (0%) |
0 (0%) |
5 (13%) |
0 (0%) |
0 (0%) |
5 (13%) |
|
|
2 |
1 (4%) |
9 (32%) |
5 (18%) |
3 (11%) |
1 (4%) |
3 (11%) |
1 (4%) |
4 (14%) |
0 (0%) |
1 (4%) |
0 (0%) |
|
|
3 |
5 (17%) |
12 (41%) |
0 (0%) |
0 (0%) |
1 (3%) |
2 (7%) |
0 (0%) |
5 (17%) |
3 (10%) |
0 (0%) |
1 (3%) |
|
|
4 |
0 (0%) |
1 (50%) |
0 (0%) |
1 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Stage |
||||||||||||
|
1 |
1 (5%) |
2 (10%) |
6 (30%) |
4 (20%) |
1 (5%) |
2 (10%) |
0 (0%) |
2 (10%) |
0 (0%) |
1 (5%) |
1 (5%) |
|
|
2 |
3 (7%) |
12 (27%) |
14 (31%) |
2 (4%) |
1 (2%) |
1 (2%) |
1 (2%) |
6 (13%) |
1 (2%) |
0 (0%) |
4 (9%) |
|
|
3 |
3 (14%) |
6 (29%) |
1 (5%) |
2 (10%) |
0 (0%) |
2 (10%) |
0 (0%) |
4 (19%) |
2 (10%) |
0 (0%) |
1 (5%) |
|
|
4 |
3 (25%) |
6 (50%) |
0 (0%) |
1 (8%) |
0 (0%) |
0 (0%) |
0 (0%) |
2 (17%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Viruses |
20 |
0 (0%) |
6 (30%) |
4 (20%) |
1 (5%) |
1 (5%) |
2 (10%) |
0 (0%) |
1 (5%) |
1 (5%) |
0 (0%) |
4 (20%) |
|
Elevated tumor markers |
80 |
10 (13%) |
26 (33%) |
11 (14%) |
7 (9%) |
2 (3%) |
4 (5%) |
1 (1%) |
10 (13%) |
3 (4%) |
1 (1%) |
5 (6%) |
AC: Adenocarcinoma; CHOR: Choriocarcinoma; DYS: Dysgerminoma; EC: Embryonal carcinoma; GCT: Germ cell tumor; GrCT: Granulosa cell tumor; IT: Immature teratoma; LCH: Langerhans cell histiocytosis; n: No. of cases; RMS: Rhabdomyosarcoma; SEM: Seminoma; YST: Yolk sac tumor.
Table 3.
The peculiarities observed in the different benign tumor types found in our study population, using percentages [n, (%)]
|
Peculiarities of benign tumors |
Total |
SCA |
MCA |
BCA |
AF |
Fibroma |
MT |
Gonadoblastoma |
SST |
Cysts |
|
Age [years] |
||||||||||
|
Prepuberty (0–9) |
15 |
2 (13%) |
1 (7%) |
0 (0%) |
0 (0%) |
0 (0%) |
6 (40%) |
0 (0%) |
0 (1%) |
6 (40%) |
|
Puberty (10–17) |
97 |
15 (15%) |
9 (9%) |
3 (3%) |
1 (1%) |
2 (2%) |
18 (19%) |
1 (1%) |
1 (1%) |
47 (49%) |
|
Sex |
||||||||||
|
Male |
7 |
2 (29%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
2 (29%) |
0 (0%) |
0 (0%) |
3 (43%) |
|
Female |
105 |
15 (14%) |
10 (10%) |
3 (3%) |
1 (1%) |
2 (2%) |
22 (21%) |
1 (1%) |
1 (1%) |
50 (48%) |
|
Size [cm] |
||||||||||
|
<5 |
27 |
8 (30%) |
3 (11%) |
1 (4%) |
0 (0%) |
0 (0%) |
3 (11%) |
0 (0%) |
0 (0%) |
12 (44%) |
|
5–10 |
63 |
6 (10%) |
5 (8%) |
1 (2%) |
1 (2%) |
0 (0%) |
19 (30%) |
0 (0%) |
0 (0%) |
31 (49%) |
|
>10 |
22 |
3 (14%) |
2 (9%) |
1 (5%) |
0 (0%) |
2 (9%) |
2 (9%) |
1 (5%) |
1 (5%) |
10 (45%) |
|
Tumor location |
||||||||||
|
Right |
47 |
10 (21%) |
4 (9%) |
2 (4%) |
0 (0%) |
2 (4%) |
7 (15%) |
0 (0%) |
0 (0%) |
22 (47%) |
|
Left |
55 |
6 (11%) |
5 (9%) |
1 (2%) |
1 (2%) |
0 (0%) |
16 (29%) |
0 (0%) |
1 (2%) |
25 (45%) |
|
Both sides |
10 |
1 (10%) |
1 (10%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (10%) |
1 (10%) |
0 (0%) |
6 (60%) |
|
Pain location |
||||||||||
|
Right |
31 |
7 (22.6%) |
3 (9.7%) |
0 (0%) |
0 (0%) |
1 (3.2%) |
6 (19.4%) |
0 (0%) |
0 (0%) |
12 (38.7%) |
|
Left |
56 |
7 (12.5%) |
7 (12.5%) |
1 (1.8%) |
0 (0%) |
0 (0%) |
15 (26.8%) |
0 (0%) |
1 (1.8%) |
28 (50%) |
|
Both sides |
20 |
2 (10%) |
0 (0%) |
2 (10%) |
1 (5%) |
1 (5%) |
2 (10%) |
0 (0%) |
0 (0%) |
11 (55%) |
|
Pain radiates to leg |
27 |
6 (22%) |
4 (15%) |
1 (4%) |
1 (4%) |
0 (0%) |
10 (37%) |
1 (4%) |
0 (0%) |
4 (15%) |
|
Repetition |
7 |
1 (14%) |
0 (0%) |
2 (29%) |
0 (0%) |
1 (14%) |
1 (14%) |
0 (0%) |
0 (0%) |
2 (29%) |
|
Prenatal diagnosis |
6 |
1 (17%) |
1 (17%) |
1 (17%) |
0 (0%) |
0 (0%) |
1 (17%) |
0 (0%) |
0 (0%) |
2 (33%) |
|
Gonadectomy |
27 |
5 (19%) |
2 (7%) |
2 (7%) |
1 (4%) |
0 (0%) |
9 (33%) |
0 (0%) |
0 (0%) |
8 (30%) |
|
Asymptomatic |
5 |
1 (20%) |
1 (20%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (20%) |
0 (0%) |
0 (0%) |
2 (40%) |
|
Appendicitis |
19 |
2 (11%) |
4 (21%) |
1 (5%) |
0 (0%) |
1 (5%) |
2 (11%) |
0 (0%) |
0 (0%) |
9 (47%) |
|
Loss of appetite |
19 |
9 (47%) |
5 (26%) |
2 (11%) |
1 (5%) |
0 (0%) |
1 (5%) |
0 (0%) |
0 (0%) |
1 (5%) |
|
Loss of weight |
5 |
2 (40%) |
1 (20%) |
2 (40%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Leukocytosis |
35 |
9 (26%) |
3 (9%) |
1 (3%) |
1 (3%) |
1 (3%) |
5 (14%) |
0 (0%) |
0 (0%) |
15 (43%) |
|
Fever |
11 |
1 (9%) |
2 (18%) |
0 (0%) |
0 (0%) |
1 (9%) |
2 (18%) |
0 (0%) |
0 (0%) |
5 (45%) |
|
Constipation |
8 |
2 (25%) |
1 (13%) |
0 (0%) |
0 (0%) |
0 (0%) |
2 (25%) |
0 (0%) |
0 (0%) |
3 (38%) |
|
Vomit |
7 |
0 (0%) |
2 (29%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
5 (71%) |
|
Bleeding |
7 |
0 (0%) |
2 (29%) |
0 (0%) |
1 (14%) |
1 (14%) |
1 (14%) |
0 (0%) |
0 (0%) |
2 (29%) |
|
Abdominal distention |
24 |
2 (8%) |
1 (4%) |
1 (4%) |
0 (0%) |
2 (8%) |
9 (38%) |
1 (4%) |
1 (4%) |
7 (29%) |
|
Ascites |
4 |
1 (25%) |
1 (25%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (25%) |
0 (0%) |
0 (0%) |
1 (25%) |
|
GCT |
25 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
24 (96%) |
1 (4%) |
0 (0%) |
0 (0%) |
|
Not GCT |
87 |
17 (20%) |
10 (11%) |
3 (3%) |
1 (1%) |
2 (2%) |
0 (0%) |
0 (0%) |
1 (1%) |
53 (61%) |
|
Loculation |
||||||||||
|
Unilocular cyst |
39 |
5 (13%) |
1 (3%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
33 (85%) |
|
Unilocular cyst + solid |
20 |
7 (35%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
4 (20%) |
1 (5%) |
0 (0%) |
8 (40%) |
|
Multilocular cyst |
28 |
2 (7%) |
9 (32%) |
0 (0%) |
1 (4%) |
1 (4%) |
5 (18%) |
0 (0%) |
0 (0%) |
10 (36%) |
|
Multilocular cyst + solid |
21 |
3 (14%) |
0 (0%) |
1 (5%) |
0 (0%) |
1 (5%) |
13 (62%) |
0 (0%) |
1 (5%) |
2 (10%) |
|
Multilocular solid |
4 |
0 (0%) |
0 (0%) |
2 (50%) |
0 (0%) |
0 (0%) |
2 (50%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Solid |
0 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
Compressed organs |
16 |
6 (38%) |
2 (13%) |
1 (6%) |
0 (0%) |
0 (0%) |
1 (6%) |
0 (0%) |
0 (0%) |
6 (38%) |
|
Viruses |
6 |
2 (33%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
2 (33%) |
0 (0%) |
1 (17%) |
1 (17%) |
|
Elevated tumor markers |
8 |
1 (13%) |
1 (13%) |
2 (25%) |
0 (0%) |
0 (0%) |
3 (38%) |
0 (0%) |
0 (0%) |
1 (13%) |
AF: Adenofibroma; BCA: Borderline cystadenoma; GCT: Germ cell tumor; MCA: Mucinous cystadenoma; MT: Mature teratoma; n: No. of cases; SCA: Serous cystadenoma; SST: Sclerosing stromal tumor.
Table 4.
Clinico-morphological presentation in children with malignant tumors in our study
|
Children with malignant tumors – clinico-morphological presentation |
||||||||||
|
Peculiarities |
YST endodermal sinus pattern* |
IT # |
DYS $ |
SEM % |
AC^ |
CHOR & |
RMS @ |
EC ¥ |
LCH § |
GrCT ‡ |
|
Percent |
70% |
86% |
67% |
100% |
100% |
100% |
71% |
100% |
100% |
83% |
|
Age [years] |
||||||||||
|
Prepuberty (0–9) |
67% |
88.9% |
50% |
0% |
100% |
0% |
57% |
100% |
100% |
100% |
|
Puberty (10–17) |
75% |
83.3% |
63% |
100% |
100% |
100% |
86% |
100% |
0% |
67% |
|
Sex |
||||||||||
|
Male |
50% |
100% |
100% |
100% |
0% |
100% |
0% |
0% |
0% |
|
|
Female |
83% |
79% |
63% |
0% |
100% |
0% |
50% |
100% |
100% |
83% |
|
Size [cm] |
||||||||||
|
<5 |
100% |
100% |
50% |
0% |
100% |
0% |
50% |
100% |
0% |
0% |
|
5–10 |
75% |
80% |
100% |
100% |
100% |
100% |
100% |
100% |
100% |
100% |
|
>10 |
60% |
89% |
33% |
100% |
100% |
0% |
25% |
100% |
0% |
80% |
|
Tumor location |
||||||||||
|
Right |
67% |
25% |
60% |
100% |
100% |
100% |
83% |
0% |
0% |
100% |
|
Left |
63% |
93% |
75% |
100% |
100% |
0% |
67% |
100% |
0% |
67% |
|
Both sides |
0% |
50% |
0% |
0% |
100% |
0% |
50% |
100% |
100% |
100% |
|
Pain location |
||||||||||
|
Right |
67% |
100% |
80% |
100% |
100% |
100% |
80% |
0% |
0% |
100% |
|
Left |
67% |
85% |
33% |
0% |
100% |
0% |
80% |
100% |
0% |
100% |
|
Both sides |
100% |
75% |
100% |
100% |
100% |
0% |
50% |
100% |
100% |
50% |
|
Pain radiates to leg |
100% |
83% |
100% |
0% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Repetition |
100% |
100% |
0% |
0% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Death |
0% |
0% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
0% |
|
Prenatal diagnosis |
0% |
0% |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
100% |
|
Gonadectomy |
78% |
83% |
67% |
100% |
100% |
100% |
83% |
100% |
100% |
83% |
|
Asymptomatic |
0% |
100% |
0% |
0% |
0% |
0% |
100% |
0% |
0% |
0% |
|
Appendicitis |
0% |
100% |
100% |
0% |
100% |
0% |
100% |
100% |
0% |
0% |
|
Loss of appetite |
100% |
100% |
100% |
100% |
100% |
0% |
100% |
100% |
100% |
100% |
|
Loss of weight |
100% |
100% |
100% |
0% |
100% |
0% |
100% |
100% |
100% |
100% |
|
Leukocytosis |
100% |
100% |
100% |
0% |
100% |
0% |
100% |
100% |
0% |
0% |
|
Fever |
0% |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
Constipation |
100% |
100% |
100% |
0% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Vomit |
0% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
0% |
0% |
|
Bleeding |
100% |
100% |
100% |
0% |
100% |
0% |
100% |
0% |
0% |
100% |
|
Abdominal distention |
100% |
67% |
100% |
100% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Ascites |
100% |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
100% |
|
GCT |
70% |
86% |
67% |
100% |
0% |
100% |
0% |
100% |
0% |
0% |
|
Not GCT |
0% |
0% |
0% |
0% |
100% |
0% |
71% |
0% |
100% |
83% |
|
Loculation |
||||||||||
|
Unilocular cyst |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
Unilocular cyst + solid |
0% |
0% |
0% |
0% |
100% |
0% |
0% |
0% |
0% |
0% |
|
Multilocular cyst |
0% |
100% |
100% |
0% |
100% |
0% |
0% |
0% |
100% |
0% |
|
Multilocular cyst + solid |
33% |
100% |
100% |
100% |
0% |
100% |
50% |
100% |
0% |
67% |
|
Multilocular solid |
100% |
77% |
50% |
0% |
100% |
0% |
75% |
0% |
0% |
100% |
|
Solid |
80% |
0% |
50% |
100% |
100% |
0% |
75% |
100% |
0% |
100% |
|
Compressed organs |
100% |
100% |
100% |
0% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Grade |
||||||||||
|
1 |
50% |
88% |
80% |
0% |
0% |
0% |
60% |
0% |
0% |
80% |
|
2 |
100% |
80% |
33% |
100% |
100% |
100% |
75% |
0% |
100% |
0% |
|
3 |
80% |
0% |
0% |
100% |
100% |
0% |
80% |
100% |
0% |
100% |
|
4 |
0% |
0% |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
Stage |
||||||||||
|
1 |
100% |
67% |
50% |
100% |
100% |
0% |
50% |
0% |
100% |
100% |
|
2 |
33% |
93% |
100% |
100% |
100% |
100% |
50% |
100% |
0% |
75% |
|
3 |
67% |
100% |
50% |
0% |
100% |
0% |
100% |
100% |
0% |
100% |
|
4 |
100% |
0% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
0% |
|
Viruses |
0% |
100% |
100% |
100% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Elevated tumor markers |
70% |
82% |
71% |
100% |
100% |
100% |
80% |
100% |
100% |
80% |
AC: Adenocarcinoma; CHOR: Choriocarcinoma; DYS: Dysgerminoma; EC: Embryonal carcinoma; GCT: Germ cell tumor; GrCT: Granulosa cell tumor; IT: Immature teratoma; LCH: Langerhans cell histiocytosis; RMS: Rhabdomyosarcoma; SEM: Seminoma; YST: Yolk sac tumor. Peculiarities: *: Anastomosing network of labyrinthine-like spaces lined by primitive tumor cells; formation of vaguely glomeruloid perivascular structures, Schiller–Duval bodies. #: Mature elements from all three germ layers, mixed with immature elements, mostly neuroectodermal (variable amounts). $: Nests of large, uniform polygonal cells with clear cytoplasm and distinct cell membranes alveolar pattern; fibrous septa containing T-cells (cytotoxic), epithelioid histiocytes separating the tumors. %: Uniform cells with distinct borders and pale clear cytoplasm; fibrous septa with lymphocytes. ^: Invasion by malignant glands. &: Biphasic with large syncytiotrophoblasts and mononucleated cytotrophoblasts; distinct cell membrane and pale cytoplasm. @: Primitive mesenchymal and spindle cells with variable degrees of skeletal muscle differentiation. ¥: Solid, glandular growth pattern; cells are polygonal, crowded with cell (indistinct) borders, large and primitive. §: Langerhans cells with abundant, pale eosinophilic cytoplasm, irregular and elongated nuclei with prominent nuclear grooves and folds. ‡: Sheet of small, bland, cuboidal to polygonal cells with scanty cytoplasm; uniform angulated and often grooved nuclei.
Table 5.
Clinico-morphological presentation in children with benign tumors in our study
|
Children with benign tumors – clinico-morphological presentation | |||||||||
|
Peculiarities |
SCA* |
MCA # |
BCA $ |
AF % |
Fibroma^ |
MT & |
Gonadoblastoma @ |
SCT ¥ |
Cysts § |
|
Percent |
65% |
70% |
67% |
100% |
100% |
100% |
100% |
100% |
85% |
|
Age [years] |
|||||||||
|
Prepuberty (0–9) |
50% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Puberty (10–17) |
67% |
67% |
67% |
100% |
100% |
100% |
100% |
100% |
83% |
|
Sex |
|||||||||
|
Male |
100% |
0% |
0% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Female |
60% |
70% |
67% |
100% |
100% |
100% |
100% |
100% |
84% |
|
Size [cm] |
|||||||||
|
<5 |
75% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
83% |
|
5–10 |
67% |
60% |
100% |
100% |
0% |
100% |
0% |
0% |
81% |
|
>10 |
33% |
50% |
100% |
0% |
100% |
100% |
100% |
100% |
100% |
|
Tumor location |
|||||||||
|
Right |
80% |
75% |
50% |
0% |
100% |
100% |
0% |
0% |
83% |
|
Left |
33% |
60% |
100% |
100% |
0% |
100% |
0% |
100% |
76% |
|
Both sides |
100% |
100% |
0% |
0% |
0% |
100% |
100% |
0% |
100% |
|
Pain location |
|||||||||
|
Right |
57% |
100% |
0% |
0% |
100% |
100% |
0% |
0% |
75% |
|
Left |
71% |
57% |
100% |
0% |
0% |
100% |
0% |
100% |
93% |
|
Both sides |
100% |
0% |
50% |
100% |
100% |
100% |
0% |
0% |
91% |
|
Pain radiates to leg |
100% |
100% |
100% |
100% |
0% |
100% |
100% |
0% |
100% |
|
Repetition |
100% |
0% |
100% |
0% |
100% |
100% |
0% |
0% |
100% |
|
Elevated tumor markers |
100% |
100% |
100% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Prenatal diagnosis |
100% |
100% |
100% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Gonadectomy |
100% |
100% |
100% |
100% |
0% |
100% |
(0% |
0% |
100% |
|
Asymptomatic |
100% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Appendicitis |
100% |
100% |
100% |
0% |
100% |
100% |
0% |
0% |
100% |
|
Loss of appetite |
78% |
100% |
100% |
100% |
0% |
100% |
0% |
0% |
100% |
|
Loss of weight |
100% |
100% |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
|
Leukocytosis |
89% |
100% |
100% |
100% |
100% |
100% |
0% |
0% |
80% |
|
Fever |
100% |
100% |
0% |
0% |
100% |
100% |
0% |
0% |
100% |
|
Constipation |
100% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Vomit |
0% |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
100% |
|
Bleeding |
0% |
100% |
0% |
100% |
100% |
100% |
0% |
0% |
100% |
|
Abdominal distention |
100% |
100% |
100% |
0% |
100% |
100% |
100% |
100% |
100% |
|
Ascites |
100% |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
100% |
|
GCT |
0% |
0% |
0% |
0% |
0% |
100% |
100% |
0% |
0% |
|
Not GCT |
65% |
70% |
67% |
100% |
100% |
0% |
0% |
100% |
85% |
|
Loculation |
|||||||||
|
Unilocular cyst |
100% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
85% |
|
Unilocular cyst + solid |
86% |
0% |
0% |
0% |
0% |
100% |
100% |
0% |
100% |
|
Multilocular cyst |
0% |
78% |
0% |
100% |
100% |
100% |
0% |
0% |
90% |
|
Multilocular cyst + solid |
0% |
0% |
0% |
0% |
100% |
100% |
0% |
100% |
0% |
|
Multilocular solid |
0% |
0% |
100% |
0% |
0% |
100% |
0% |
0% |
0% |
|
Solid |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
Compressed organs |
100% |
100% |
100% |
0% |
0% |
100% |
0% |
0% |
100% |
|
Viruses |
100% |
0% |
0% |
0% |
0% |
100% |
0% |
100% |
100% |
AF: Adenofibroma; BCA: Borderline cystadenoma; GCT: Germ cell tumor; MCA: Mucinous cystadenoma; MT: Mature teratoma; SCA: Serous cystadenoma; SST: Sclerosing stromal tumor. Peculiarities: *: Small, unilocular cysts lined by a single layer of tall, columnar cuboid epithelium, ciliated cells. #: Multilocular cystic neoplasm composed of multiple cysts and glands lined by a single layer of bland mucinous epithelium. $: Numerous slender to bulbous, irregularly contoured papillae with fibrous, myxoid cores and hierarchical branching pattern; pseudostratified, crowded and lined with epithelial tufting. %: Predominant fibrous stroma, with glands and cysts forming a minor component. ^: Well circumscribed unencapsulated, variably cellular fascicular storiform growth of tumor cells within discreet collagenous stroma. &: Mixture of mature, benign tissues composed of ectodermal, mesodermal and endodermal components in varying degrees. @: Primitive germ cells, sex cord stromal cells surrounded by ovarian type stroma. ¥: Alternating cellular and hypocellular areas impart a pseudo-lobular appearance. §: Conjunctive fibrous layer of cylindrical epithelium; inner layer of granulosa cell; eosinophilic cytoplasm; primordial follicle; primary and secondary follicles present
Table 6.
Multinomial logistic regression of RRs and 95% CIs and p-values of risk factors found in our study among patients with malignant tumors, using patients with benign tumors as the control group
|
Patients’ risks |
Total ( n =210) |
Malignant tumor [ n , (%)] |
Univariate RR |
p -value |
95%CI |
Multivariate RR |
p -value |
95%CI |
|
Obesity |
109 |
72/98 (73.5%) |
2.78 |
0.001 |
1.93–4.00 |
1.34 |
0.001 |
1.29–2.55 |
|
Mother’s age [years] |
||||||||
|
≤20 |
24 |
18/98 (18.4%) |
Constant |
Constant |
Constant |
Constant |
Constant |
Constant |
|
>20–25 |
50 |
17/98 (17.3%) |
0.40 |
0.001 |
0.24–0.63 |
0.72 |
0.100 |
-3.26– -0.94 |
|
>25–30 |
60 |
15/98 (15.3%) |
0.33 |
0.001 |
0.20–0.54 |
0.69 |
0.393 |
-3.486– -1.182 |
|
>30–35 |
32 |
18/98 (18.4%) |
0.73 |
0.105 |
0.50–1.06 |
1.14 |
0.660 |
-2.239–0.213 |
|
>35–40 |
40 |
30/98 (30.6%) |
0.95 |
0.73 |
0.71–1.27 |
1.10 |
0.680 |
-1.468–1.027 |
|
Breastfeeding ≤5 months |
100 |
72/98 (73.5%) |
3.20 |
0.001 |
2.20–4.63 |
1.11 |
0.001 |
1.578–2.883 |
|
DSD |
17 |
10/98 (10.2%) |
1.42 |
0.104 |
0.93–2.16 |
0.79 |
0.179 |
-0.356–1.908 |
|
Stress |
97 |
70/98 (71.4%) |
3.04 |
0.001 |
2.13–4.34 |
1.33 |
0.001 |
1.526–2.824 |
|
Hormone |
21 |
15/98 (15.3%) |
1.68 |
0.001 |
1.23–2.31 |
1.16 |
0.018 |
1.217–2.345 |
|
Smoking |
96 |
69/98 (70.4%) |
2.74 |
0.001 |
1.94–3.86 |
1.36 |
0.001 |
1.329–2.594 |
|
Positive heredo-genetic history |
63 |
47/98 (48.0%) |
2.24 |
0.001 |
1.71–2.92 |
1.10 |
0.001 |
1.154–2.612 |
|
Abnormal birth weight |
34 |
25/98 (25.5%) |
1.72 |
0.001 |
1.30–2.29 |
1.08 |
0.003 |
1.442–2.104 |
|
Residence area |
||||||||
|
Rural |
97 |
59/98 (60.2%) |
1.82 |
0.001 |
1.33–2.49 |
1.00 |
0.001 |
1.559–1.733 |
|
Urban |
113 |
39/98 (39.8%) |
||||||
|
Pollution |
100 |
70/98 (71.4%) |
2.98 |
0.001 |
2.08–4.25 |
1.30 |
0.022 |
1.119–1.505 |
|
Rh positive |
159 |
81 (82.7%) |
1.61 |
0.037 |
1.03–2.51 |
1.11 |
0.860 |
-0.737–0.611 |
|
No postnatal vitamin D |
47 |
20/98 (20.4%) |
0.80 |
0.751 |
0.25–2.68 |
0.83 |
0.855 |
-0.964–0.430 |
|
No postnatal vaccination |
42 |
18/98 (18.4%) |
0.86 |
0.470 |
0.58–1.29 |
1.08 |
0.453 |
0.288–1.907 |
|
Menstrual disorders |
36 |
25/98 (25.5%) |
1.62 |
0.001 |
1.21–2.18 |
1.15 |
0.001 |
1.957–3.339 |
|
Malformations |
102 |
77/98 (78.6%) |
4.07 |
0.001 |
2.67–6.20 |
1.65 |
0.001 |
1.559–1.733 |
|
UTI |
85 |
45/98 (45.9%) |
1.35 |
0.049 |
1.00–1.81 |
1.28 |
0.053 |
0.006–1.146 |
CI: Confidence interval; DSD: Disorder of sex development; n: No. of cases; Rh: Rhesus; RR: Relative risk ratio; UTI: Urinary tract infection
Table 7.
Multinomial logistic regression of estimation coefficient (β), 95% CIs and p-values of risk factors found in our study among patients with malignant tumors using patients with mixed GCT as the dependent variable
|
Risks |
n (%) |
IT total n , β coefficient ( p -value) |
YST total n , β coefficient ( p -value) |
DYS total n , β coefficient ( p -value) |
GrCT total n , β coefficient ( p -value) |
RMS total n , β coefficient ( p -value) |
ADC total n , β coefficient ( p -value) |
|
Univariate |
|||||||
|
Obesity |
72 (73.5%) |
16, 0.04 (0.953) |
6, 0.08 (0.316) |
7, 0.045 (0.958) |
5, 0.40 (0.733) |
9, 0.62 (0.396) |
3, 0.80 (0.436) |
|
Mother’s age [years] |
|||||||
|
≤20 |
18 (18.4%) |
Constant |
|||||
|
>20–25 |
17 (17.3%) |
3, 0.18 (0.872) |
0, -16.12 (0.994) |
2, -0.47 (0.736) |
0, 17.04 (0.995) |
5, 0.10 (0.100) |
0, 16.82 (0.990) |
|
>25–30 |
15 (15.3%) |
5, 0.51 (0.629) |
2, -0.41 (0.733) |
0, -15.58 (0.994) |
0, 17.09 (0.994) |
2, -1.10 (0.309) |
0, 16.86 (0.995) |
|
>30–35 |
18 (18.4%) |
5, 0.92 (0.403) |
2, 0.10 (1.000) |
2, 0.69 (0.624) |
2, 0.41 (0.726) |
2, -0.69 (0.535) |
0, 16.22 (1.000) |
|
>35–40 |
30 (30.6%) |
6, 0.54 (0.600) |
4, 0.13 (0.901) |
4, 0.83 (0.519) |
1, 1.66 (0.207) |
1, -1.95 (0.129) |
3, 0.15 (0.889) |
|
Breastfeeding ≤5 months |
72 (73.5%) |
12, -0.52 (0.393) |
7, 0.04 (0.964) |
7, 0.44 (0.626) |
6, 15.71 (0.992) |
12, 0.98 (0.262) |
5, 15.71 (0.993) |
|
DSD |
10 (10.2%) |
2, -0.210 (0.824) |
1, 14.590 (0.991) |
4, 15.001 (0.100) |
1, 0.78 (0.437) |
0, -0.245 (0.802) |
0, -14.604 (0.994) |
|
Stress |
70 (71.4%) |
15, 0.52 (0.454) |
6, 1.03 (0.206) |
5, 1.21 (0.147) |
3, 2.13 (0.033) |
11, 0.14 (0.868) |
5, 13.01 (0.983) |
|
Hormone |
15 (15.3%) |
3, -0.36 (0.654) |
0, -15.2 (0.991) |
2, 0.18 (0.846) |
1, -0.18 (0.885) |
3, 0.14 (0.868) |
0, 15.27 (0.994) |
|
Smoking |
69 (70.4%) |
13, 0.33 (0.599) |
6, -0.41 (0.600) |
6, -0.12 (0.886) |
4, -0.12 (0.903) |
12, 0.98 (0.262) |
1, 0.58 (0.630) |
|
Positive heredo-genetic history |
47 (48.0%) |
8, -0.64 (0.284) |
6, 0.25 (0.740) |
3, -0.85 (0.295) |
1, -1.76 (0.130) |
7, -0.15 (0.816) |
3, 0.25 (0.800) |
|
Abnormal birth weight |
25 (25.5%) |
4, -0.64 (0.363) |
4, 0.41 (0.600) |
2, -0.44 (0.626) |
1, -0.80 (0.497) |
4, -0.11 (0.885) |
0, -14.79 (0.989) |
|
Residence area (rural) |
59 (60.2%) |
10, -0.91 (0.137) |
6, -0.41 (0.600) |
4, -1.03 (0.193) |
5, 0.80 (0.497) |
10, 0.11 (0.885) |
2, -1.22 (0.227) |
|
Pollution |
70 (71.4%) |
10, 1.8 (0.010) |
6, 1.30 (0.124) |
6, 1.01 (0.257) |
5, 0.10 (0.938) |
13, 0.86 (0.463) |
3, 1.30 (0.221) |
|
Rh positive |
81 (82.7%) |
8, 2.97 (0.001) |
10, 15.00 (1.000) |
9, 14.60 (0.993) |
6, 13.70 (1.000) |
14, 14.00 (0.992) |
5, 14.64 (0.995) |
|
No postnatal vitamin D |
20 (20.4%) |
0, -15.28 (0.985) |
2, 0.18 (0.842) |
2, -0.05 (0.958) |
3, 1.20 (0.200) |
6, 0.92 (0.199) |
1, -0.18 (0.880 |
|
No postnatal vaccination |
18 (18.4%) |
8, 0.72 (0.267) |
1, -1.00 (0.389) |
1, -0.88 (0.450) |
1, -0.41 (0.733) |
0, -15.40 (0.988) |
0, -15.30 (1.000) |
|
Menstrual disorders |
25 (25.5%) |
5, 0.87 (0.275) |
1, -0.16 (0.895) |
5, 2.26 (0.013) |
4, 2.73 (0.010) |
2, 0.25 (0.802) |
2, 1.63 (0.138) |
|
Malformations |
77 (78.6%) |
18, 0.36 (0.655) |
7, -0.59 (0.490) |
8, 0.64 (0.582) |
4, -0.74 (0.457) |
9, 0.85 (0.257) |
5, 13.52 (0.986) |
|
UTI |
45 (45.9%) |
11, -0.06 (0.920) |
4, -0.56 (0.459) |
3, -0.85 (0.295) |
2, -0.85 (0.373) |
9, 0.43 (0.525) |
1, -1.54 (0.194) |
|
Multivariate |
|||||||
|
Stress |
70 (71.4%) |
15, 1.39 (0.114) |
6, 0.91 (0.276) |
5, 1.2 (0.180) |
3, 2.10 (0.048) |
11, 0.08 (0.926) |
5, 14.42 (0.990) |
|
Pollution |
70 (71.4%) |
10, 1.75 (0.041) |
6,1.27 (0.138) |
6, 0.82 (0.388) |
5, 0.12 (0.930) |
13, 0.88 (0.452) |
3, 1.19 (0.277) |
|
Rh positive |
81 (82.7%) |
8, 3.41 (0.001) |
10, 15.07 (0.100) |
9, 14.45 (0.994) |
6, 13.62 (0.100) |
14, 14.92 (0.993) |
5, 15.00 (0.900) |
|
Menstrual disorders |
25 (25.5%) |
5, 0.95 (0.343) |
1, -0.25 (0.840) |
5, 2.22 (0.017) |
4, 2.80 (0.013) |
2, 0.26 (0.794) |
2, 1.46 (0.196) |
AC: Adenocarcinoma; CI: Confidence interval; DSD: Disorder of sex development; DYS: Dysgerminoma; GCT: Germ cell tumor; GrCT: Granulosa cell tumor; IT: Immature teratoma; n: No. of cases; Rh: Rhesus; RMS: Rhabdomyosarcoma; UTI: Urinary tract infection; YST: Yolk sac tumor
Table 8.
Multinomial logistic regression of estimation coefficient (β), 95% CIs and p-values of risk factors found in our study among patients with benign tumors, using patients with cysts as the dependent variable
|
Risks |
n |
SCA total n , β coefficient ( p -value) |
MCA total n , β coefficient ( p -value) |
MT total n , β coefficient ( p -value) |
|
Univariate |
||||
|
Obesity |
37 |
4, 0.55 (0.426) |
7, 2.58 (0.001) |
10, 1.39 (0.014) |
|
Mother’s age [years] |
||||
|
≤20 |
7 |
Constant |
||
|
>20–25 |
36 |
5, -0.236 (0.851) |
3, 15.688 (0.997) |
7, 0.10 (0.935) |
|
>25–30 |
45 |
5, -0.47 (0.708) |
4, 15.743 (0.997) |
10, 0.22 (0.854) |
|
>30–35 |
14 |
3, 0.811 (0.558) |
3, 17.246 (0.996) |
3, 0.81 (0.558) |
|
>35–40 |
10 |
3, 1.098 (0.437) |
0, 0.428 (1.000) |
3, 1.10 (0.437) |
|
Breastfeeding ≤5 months |
28 |
7, 2.46 (0.001) |
3, 1.97 (0.031) |
11, 2.65 (0.001) |
|
DSD |
7 |
2, 1.94 (0.124) |
2, 11.15 (0.985) |
0, -1.55 (0.214) |
|
Stress |
27 |
4, 1.33 (0.086) |
3, 1.66 (0.055) |
12, 2.51 (0.000) |
|
Hormone |
6 |
2, 1.94 (0.124) |
0, -11.15 (0.100) |
2, 1.55 (0.214) |
|
Smoking |
27 |
6, 1.12 (0.078) |
2, 0.34 (0.698) |
9, 1.22 (0.033) |
|
Positive heredo-genetic history |
16 |
3, 1.70 (0.077) |
3, 2.39 (0.017) |
4, 1.63 (0.072) |
|
Abnormal birth weight |
9 |
3, 2.41 (0.043) |
2, 2.57 (0.045) |
3, 2.01 (0.090) |
|
Residence area (rural) |
38 |
6, 0.32 (0.585) |
3, 0.08 (0.913) |
10, 0.59 (0.249) |
|
Pollution |
30 |
7, 1.91 (0.005) |
3, 1.41 (0.090) |
9, 1.75 (0.006) |
|
Rh positive |
78 |
11, 0.49 (0.393) |
9, 2.01 (0.056) |
24, 18.37 (0.993) |
|
No postnatal vitamin D |
27 |
7, 0.77 (0.192) |
4, 0.72 (0.319) |
0, -15.14 (0.983) |
|
No postnatal vaccination |
24 |
4, 0.55 (0.426) |
8, 3.11 (0.001) |
4, 0.12 (0.860) |
|
Menstrual disorders |
11 |
2, 0.49 (0.592) |
3, 1.66 (0.055) |
1, -0.63 (0.583) |
|
Malformations |
25 |
7, 2.15 (0.003) |
3, 1.66 (0.055) |
8, 1.81 (0.007) |
|
UTI |
40 |
5, -0.21 (0.728) |
6, 1.07 (0.130) |
7, -0.22 (0.678) |
|
Multivariate |
||||
|
Breastfeeding ≤5 months |
28 |
7, 2.53 (0.005) |
3, 1.68 (0.209) |
11, 1.85 (0.045) |
|
Obesity |
37 |
4, 0.27 (0.760) |
7, 2.84 (0.013) |
10, 1.55 (0.070) |
|
Stress |
27 |
4, 0.91 (0.382) |
3, 2.18 (0.100) |
12, 2.35 (0.008) |
|
No postnatal vaccination |
24 |
4, 0.89 (0.301) |
8, 3.71 (0.002) |
4, 1.08 (0.276) |
|
Malformations |
25 |
7, 1.96 (0.032) |
3, 1.44 (0.274) |
8, 2.18 (0.044) |
CI: Confidence interval; DSD: Disorder of sex development; MCA: Mucinous cystadenoma; MT: Mature teratoma; n: No. of cases; Rh: Rhesus; SCA: Serous cystadenoma; UTI: Urinary tract infection
Table 9.
Genetic and non-genetic related malformations observed in our study, comparing the malignant, and benign tumor groups
|
Congenital malformations |
Total ( n =102) |
Malignant tumors 77 (75%) |
Benign tumors 25 (25%) |
|
Non-genetic malformations |
87 |
67 (77%) |
20 (23%) |
|
Ureter and kidney: ureterocele, ureteral obstruction, hydronephrosis |
14 |
10 (71%) |
4 (29%) |
|
Face–eye: myopia, nystagmus, astigmatism strabismus, blindness |
28 |
18 (64%) |
10 (36%) |
|
Other facial defects: cleft lip, cleft palate, ear, nasal septal defect, micrognathism, retrognathia |
11 |
11 (100%) |
0 (0%) |
|
Gonadal: hydrocele, cryptorchidism, micro-penis |
18 |
17 (94%) |
1 (6%) |
|
Spleen: accessory spleen |
6 |
4 (67%) |
2 (33%) |
|
Heart: atrial and ventricular septal defect, mitral valve anomalies |
18 |
13 (72%) |
5 (28%) |
|
Integumentary–muscular: umbilical, inguinal, and diaphragmatic hernia, abdominal wall, neural tube defect |
11 |
10 (91%) |
1 (9%) |
|
Skeletal: scoliosis, sacral, coccygeal, foot, forefoot and knee deformity |
18 |
17 (94%) |
1 (6%) |
|
Ano-rectal: anorectal agenesis, fistulas, anal stenosis |
9 |
9 (100%) |
0 (0%) |
|
Thyroid dysgenesis |
4 |
4 (100%) |
0 (0%) |
|
Hydrocephalus |
1 |
1 (100%) |
0 (0%) |
|
Lung and tracheal: bronchogenic cysts, tracheomalacia, bronchopulmonary sequestrations |
5 |
5 (100%) |
0 (0%) |
|
Pancreas: annular pancreas and pancreaticobiliary malformation |
2 |
2 (100%) |
0 (0%) |
|
Liver: choledochal cysts |
2 |
2 (100%) |
0 (0%) |
|
Genetic-related malformations |
28 |
21 (75%) |
7 (25%) |
|
Down syndrome |
11 |
10 (91%) |
1 (9%) |
|
Prader–Willi syndrome |
3 |
3 (100%) |
0 (0%) |
|
Disorders of sexual development |
17 |
10 (59%) |
7 (41%) |
|
Dwarfism |
5 |
4 (80%) |
1 (20%) |
|
Osteogenesis imperfecta |
3 |
3 (100%) |
0 (0%) |
Rare cases
Case No. 1
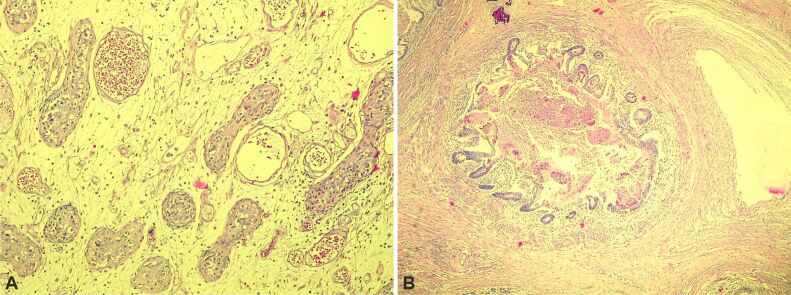
A 1-year-old boy presented with a right testicular mass, palpated during physical examination, confirmed by ultrasound (US), with AFP (100 U/L) and β-HCG (420 mU/mL). We excised the mass, and HP examination revealed intratubular germ cell neoplasia (ITGCN). Macroscopic: 1/1/0.3 cm and 2/1/1 cm tissues, caffeine-white and elastic. Microscopic: testicular parenchyma with seminiferous tubules, Periodic Acid Schiff (PAS-positive) and p53, voluminous nuclei, hyperchromatic nucleolus, cells proliferated with cytoplasm forming (dis)continuing layers with immature portions of juvenile Sertoli cells. He was discharged but returned at age three with tumor recurrence on the same testis. An orchiectomy was performed, and an HP examination showed a MT. Macroscopic: 3/2/2 cm and 3/2/1 cm nodes, grayish-translucent irregular tissue of various consistency and non-homogenous areas. Microscopic: testicular parenchyma with seminiferous tubules lined by Sertoli and germinal cells, squamous epithelium, acini seromucinous, pseudostratified ciliated epithelium of intestinal type, and muscular tissue components (Figure 4, A and B).
Figure 4.
Microscopic description of Case No. 1: (A) A section of the testis tumor with an area of intratubular neoplasm; (B) A section of the testis tumor containing mature teratoma with intestinal epithelium, muscle tissue. Hematoxylin–Eosin (HE) staining: (A and B) ×100
Case No. 2
A girl aged 17 presented with abdominal pain, and her physical and radiological examination confirmed a left ovarian mass. The tumor was excised, and the HP result revealed sclerosing stromal tumor. Macroscopic: irregular fragmented tissue 4/3/3cm, whitish gray and elastic. Microscopic: parenchyma with solid tumoral proliferation and pseudolobular pattern separates cells, having hypocellular areas through dense bands of collagen stroma tissues. Hemangiopericytoma-like areas, thin wall vessels, fusiform, round cells with clear cytoplasm, and various stages of follicles were observed (Figure 5A).
Case No. 3
A 15-year-old girl presented with bleeding and abdominal pain. Physical examination and US confirmed left ovarian mass, thick septations, and solid areas with flow. The tumor was excised, and HP examination results suggested fibroma. Macroscopic: white and elastic tissue fragments (1/1/0.3 cm and 1/1/0.5 cm). Microscopic: benign tumor proliferation with solid patterns containing fascicles, fusiform cell bands, dispose of storiform and oval nuclei, curly, pointy end, few eosinophile cytoplasm, and discreet collagen bands (Figure 5B).
Case No. 4
A boy aged 15 presented with a left testicular mass (4/5 cm), not painful at palpation, and US confirmed tumoral formation; hence, tumor excision was performed, and HP results reported MT. AFP (5 U/L), β-HCG (3 mU/mL). Macroscopic: 2/3/3.5 cm fragment with multicystic aspects, elastic, coffee white. Microscopic: proliferated tumor of testicular tissues containing squamous epithelium, muscular tissue, seromucinous acini epithelium, ciliated epithelium, intestinal, and respiratory type. The patient returned in two months with tumor recurrence on the same testes, still not painful at palpation. Computed tomography (CT) scan confirmed a 4/4.5 cm mass, orchidectomy was performed, chemotherapy was administered, and the HP results showed RMS (embryonal). Macroscopic: 2/3/4 cm fragments, grayish white, encapsulated, and elastic. Microscopic: tumoral proliferation with solid pattern, pseudocapsular fibrosis, mesenchymal, fusiform, and syncytiotrophoblast cells, alternate hyper-hypocellular areas, storiform-myxoid areas, few cytoplasm, oval nuclei. IHC staining: positive vimentin, desmin, α-SMA, Myf4, Ki67 (20%) (Figure 5, C–E).
Case No. 5
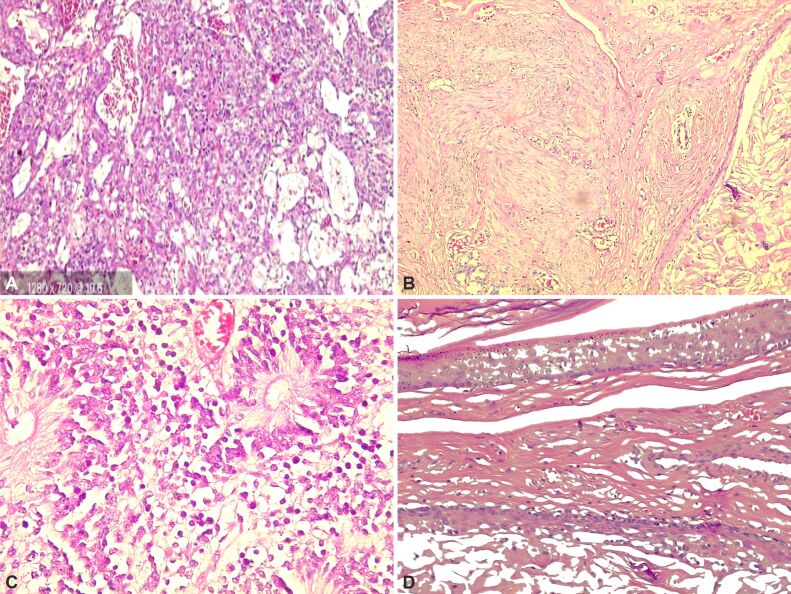
An 11-year-old girl presented with abdominal distention and pain. US and CT scan revealed an abdominal tumor of 30/22/21 cm, painful at palpation, 11/10 cm mass on the left lung, and solid nodules on the diaphragm left hepatic duct, bladder, kidney, right pleural base, noted pleuritis, pericarditis, and right perihilar renal adenopathy. AFP (8543 U/L), β-HCG (24611 mU/mL), CA-125 (258 U/mL), NSE (76 ng/mL). Stage IVB gigantic GCT from both ovaries was suspected. Chemotherapy [Maligne Keimzelltumoren (MAKEI) 2005] with three PEI (Cisplatin, Etoposide and Ifosfamide) cycles was administered; afterward, adenopathies were absent, reduced pericardial fluids and tumor by 8%, revealing a feeding artery from the left posterior tract and two external iliac arteries. Oophorectomy, salpingectomy, and excision of four hepatic and two peritoneal metastases were performed, tumor markers normalized, and the patient was discharged and still lives healthy. HP examination showed macroscopic: 3/2/1 cm, 6/2/1 cm, 9/5/1 cm irregular tissue, 20/18/6 cm encapsulated tissue, polylobulated and non-homogenous, yellow and brown cystic space, with jelly content and elastic. Microscopic: tumor proliferation with polymorphic contents, areas of embryonal carcinoma (10%), mature and IT (50%), YST (35%), and choriocarcinoma (5%). Cells had variable cytoplasm, irregular nuclei, hyaline globule, calcification, and hemosiderin pigment. Liver, kidney, and bladder metastases with fibrotic proliferation, as in the tumor described above, and hemosiderin pigment was observed (Figure 6, A–E).
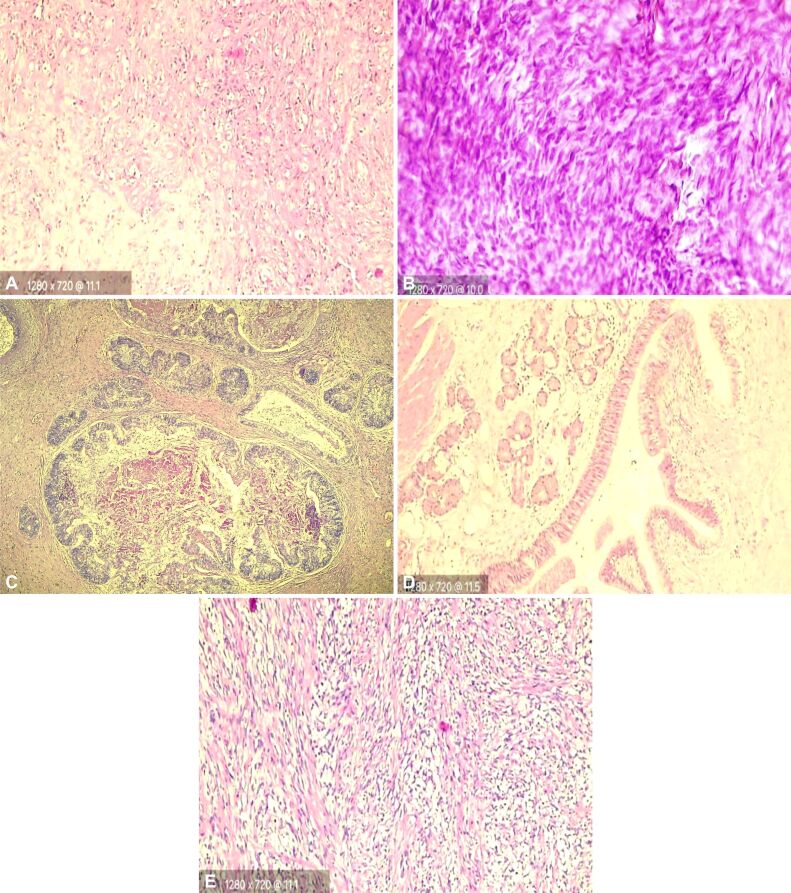
Figure 5.
HE-stained histopathology slides of patients with rare gonadal tumors in our study: (A) Sclerosing stromal tumor – hypocellular areas composed of dense collagen; (B) Fibroma – bland spindled to ovoid nuclei with pointy ends and scant eosinophilic cytoplasm; (C) MT with intestinal and respiratory epithelium, muscle tissue; (D) Testicular MT – respiratory epithelium seromucinous acini, muscle tissue; (E) Rhabdomyosarcoma – fascicles of spindle cells. HE staining: (A and B) ×400; (C) ×50; (D and E) ×200. MT: Mature teratoma
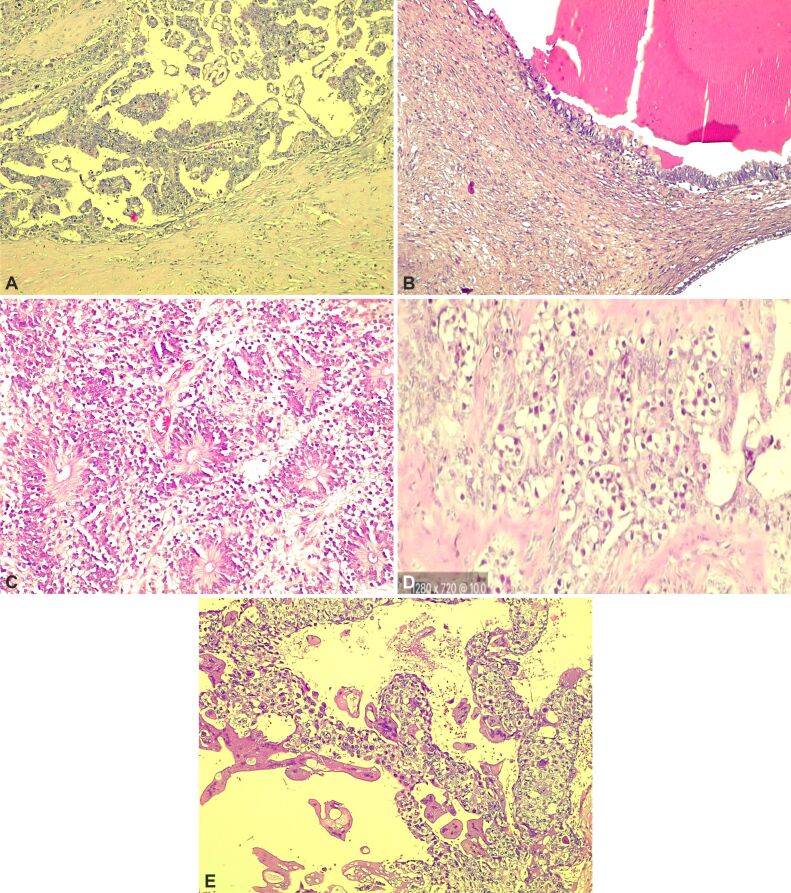
Figure 6.
Histological sections of a mixed GCT: (A) Yolk sac tumor; (B) Mature teratoma with respiratory epithelium; (C) Mixed GCT composed of immature teratoma; (D) Embryonal carcinoma – sheets and nests of large primitive cells; (E) Choriocarcinoma – multi-nucleated, large syncytiotrophoblasts surrounded by mononucleated trophoblasts. HE staining: (A, B and E) ×100; (C) ×200; (D) ×400. GCT: Germ cell tumor
Case No. 6
A 16-year-old girl presented with pleurisy, abdominal pain, and distention. US and CT scan showed a left ovarian mass; hence, a midline laparotomy and oophorectomy were performed, the right ovary was autoamputated, and chemotherapy three PEI cycles were administered. HP examination revealed stage III mixed GCT. Macroscopic: 19/14/10 cm, 3/2/1 cm, 5/10 cm fragments with nodular formations, encapsulated with multicystic aspect, containing solid and gelatinous greyish-red content, ununiformed areas, elastic and predominantly tough consistency, greyish-caffeine zone. Microscopic: tumoral formation of MT (25%) with tissues from ectoderm, endoderm, and mesoderm layers; immature tissue aspects from three embryonic layers with grade III IT (60%) predominantly neuroepithelium subform rosette; YST areas (15%), microcystic, papillary and glandular forms, and necrohemorrhagic areas. IHC staining: positive AFP, PAS, vimentin, Ki67 (10%) in solid areas. Markers were normal post-chemotherapy. The patient returned two years later, presenting with abdominal pain towards the upper quadrant, we suspected tumor metastasis requiring chemotherapy. The CT scan examination revealed multiple perihepatic tumor formations; hence, we performed exploratory laparotomy, tumor excision, biopsy, and HP examination, which revealed a MT, pointing to growing teratoma syndrome. The patient was later discharged, and to date, the tumor has never reoccurred. Macroscopic: irregular tissues 3/2/1 cm, 4/3/1 cm, and 2/1/1 cm, with caffeine-grayish zones. First, an unencapsulated fragment, mildly non-homogenous, elastic, with zones of adipose cartilage; the second fragment was cystic with 0.2–0.4 cm wall thickness and translucent liquid; and a third multi-cystic fragment. Microscopic: proliferated tumor tissue had components of mesoderm, endoderm, and ectoderm layers (Figure 7, A–D).
Figure 7.
Histopathology slides and description of Case No. 6: (A) Yolk sac tumor – anastomosing channels and variably sized cysts lined by primitive tumor cells with various amounts of clear to eosinophilic cytoplasm; (B) Germ cell tumor metastasis – after therapy was metastasized the MT, squamous epithelium, muscle tissue; (C) High-power view shows the immature teratoma component, with the presence of immature neural tissue; (D) Metastasis – MT with the presence of squamous epithelium. HE staining: (A and D) ×200; (B) ×100; (C) ×400. MT: Mature teratoma
Discussions
There are risk factors for gonadal tumors, identifying and avoiding them could lead to early tumor detection and treatment, and also prevent tumor occurrence. Smoking is known to cause gonadal malignant tumors in children by mutating germ cells; in contrast, Wang et al. reported that exposure to smoking causes a high risk of malignancy in adulthood, suggesting a progression process of carcinogenesis from childhood [15, 16]. Studies have also confirmed that hepatitis, herpesvirus, and HIV are oncoviruses [17]. Furthermore, menstrual disorders or bleeding are recognized risk factors for epithelial and SCSTs [18].
In our study, the highest estimation coefficient (β) was stress and menstrual disorders as a risk of developing GrCTs (Table 7), while pollution and Rh positive for ITs. For benign tumors, obesity and no postnatal vaccination were risks for MCA; stress and malformations were for MT, and breastfeeding ≤5 months for SCA. A group of studies showed smoking is a risk factor for MCA, obesity for SCA, chronic menstrual irregularities, infertility, congenital malformation for MT, obesity, DSD, positive heredo-genetic history, and hormones intake for GrCT, and for IT risk factors included advanced mother’s age, low birthweight, irregular menstrual cycles, cryptorchidism, hypospadias, and a very high number of patients had ear, face and neck malformation [19, 20, 21]. From the previous study, an extremely high risk [odds ratio (OR) 93.70] of developing GCT was observed only in children with facial malformations [21]. Similar to these results, in our study, facial malformations were the highest type of malformation among patients (Table 9). This is important to note, as malformation is a high-risk factor of childhood malignancy, knowing the mechanism leading to malformation also leads to malignancy; genetic-related malformation also has a higher GCT risk [22].
Biro & Deardorff explain that due to numerous developmental transformations at puberty, there are more possibilities during this period for abnormal tumor cell development [23]; likewise, in our study, 73% of patients were in puberty.
One of our patients had a recurrence of benign pre-pubertal pure MT after a 2-year history of ITGCN. Contrary to our finding, Skakkebæk reported that most GCTs originate from ITGCN as a precursor, having a malignancy risk of 50% in five years, except for pediatric YSTs and MTs [24, 25]. Additionally, in our study, we observed a malignant transformation of prepubertal MT into RMS. Literature shows that the malignant transformation of a GCT into a non-GCT is rare, with 1–3% occurrence in MT [26]. Research which supports our findings reports that large tumors (11.4 cm mean), elevated CA-125, aging, and menopause are risk factors for malignant transformation of MT, which can occur 15–20 years after MT diagnoses [27]. In terms of environmental risk factors, other studies have obtained results similar to our findings, showing that fertilizer’s nitrate, metals mining facilities, and insecticide chemicals (i.e., Dichlorodiphenyltrichloroethane) increase gonadal malignancy risk in a child, and even in that child’s offspring [28, 29, 30]. Goff et al. showed a reoccurrence of loss of appetite, distended abdomen, UTI, and abdomino-pelvic pain within a month, even before a one-year period may be a sign of malignancy [6]. In our study, only eight patients were prenatally diagnosed, suggesting that other cases may have been missing due to numerous developmental activities. This may also point to postnatal tumorigenesis in most patients or external risk factors. Similar to our study, other researchers have recognized hormones, especially estrogens, as a risk factor for gonadal malignant tumor, if the exposure length is >5 years [30, 31].
Furthermore, studies reported that high-income countries have higher tumor incidence but also higher access to treatment, hence reducing mortality rate or tumor progression, unlike patients in rural areas [14, 32]. Our study confirms this, as 75% of patients with stage IV tumors were from the rural area. Also, meta-analysis confirms obesity before menopause as a malignancy risk, and adults who were obese in childhood were at a higher risk of having malignant tumors, pointing to childhood as the start of tumorigenesis [33, 34].
Our study showed that children breastfed ≤5 months had a risk (RR: 1.11) of gonadal malignancy, this agrees with a study which showed that long-term breastfeeding reduces malignancy in mothers and children (leukemia) [35]. In our study, 71.4% of malignant patients were exposed to stress before diagnosis. A review had a similar result to ours, indicating that long-term stress, anxiety, suppressed anger, and depression promote tumorigenesis, quickening its progression through inflammation and damage of the tumor protein gene and deoxyribonucleic acid (DNA) [36, 37].
Study’s limits
Firstly, it is a retrospective study; also, being a pediatric population and a rare disease, we could not perform preoperative biopsies in >90%, but the intraoperative staging and postoperative HP examination for diagnoses were performed, being a standard, reliable method. We had an unequal population per tumor type; however, in total we had a good population size, and could present substantial results. A more extensive study to confirm our results will be helpful.
Conclusions
Malformations, smoking, pollution, obesity, and stress are the top five risk factors of pediatric malignant gonadal tumors, especially DYS, IT, and GrCT. Avoiding these risk factors will help reduce childhood (para)gonadal tumors. ≥70% of children who present with bleeding, (multilocular) solid (para)gonadal tumors, viruses, elevated markers, and weight loss have a high chance of having gonadal malignancy, especially mixed GCT, IT, and DYS.
Conflict of interests
The authors declare no conflict of interests.
Source of funding
No funding or financial support was received for making and publishing this article.
Ethics approval
This study was performed in compliance with all institutional policies, according to the Helsinki Declaration’s tenets, Romania’s regulations, Medical Research Council Guidelines for Good Clinical Practice in Clinical Trials, WMA, WHO Guidelines, approved by the Ethical Committees of the Emergency Children Hospitals of Timişoara, Iaşi, Arad and Bacău (Approval No. 124/2020-16922 on 11.12.2020) and the Scientific Research Ethics Commission (Approval No. 59/12.12.2018).
References
- 1.Baroni T, Arato I, Mancuso F, Calafiore R, Luca G. On the origin of testicular germ cell tumors: from gonocytes to testicular cancer. Front Endocrinol (Lausanne) 2019;10:343–343. doi: 10.3389/fendo.2019.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giona S . In: Urologic cancers . Barber N , et al., editors. Brisbane, Australia : Exon Publications ; 2022 . Chapter 9: The epidemiology of testicular cancer ; pp. 107 – 116 . [PubMed] [Google Scholar]
- 3.van Heerden, Tjalma WA. The multidisciplinary approach to ovarian tumours in children and adolescents. Eur J Obstet Gynecol Reprod Biol. 2019;243:103–110. doi: 10.1016/j.ejogrb.2019.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Chan WC, Ngai CH, Lok V, Zhang L, Lucero-Prisno DE, Xu W, Zheng ZJ, Elcarte E, Withers M, Wong MCS; Worldwide burden, risk factors, and temporal trends of ovarian cancer: a global study. Cancers (Basel) 2022;14(9):2230–2230. doi: 10.3390/cancers14092230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Childhood Cancer Registry (NCCR) Explorer . An interactive website for NCCR Cancer Statistics . Rockville, MD, USA : National Cancer Institute (NCI) ; 2023 . Compare cancer sites 5-year age-adjusted incidence rates, and incidence rates by age at diagnosis, 2016-2020, ages < 20 . [Google Scholar]
- 6.Goff BA, Mandel LS, Drescher CW, Urban N, Gough S, Schurman KM, Patras J, Mahony BS, Andersen MR. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109(2):221–227. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 7. Price RA , Stranges E , Elixhauser A . Healthcare Cost and Utilization Project (HCUP) Statistical Briefs . Rockville, MD, USA : Agency for Healthcare Research and Quality, US Department of Health & Human Services ; 2012 . Pediatric cancer hospitalizations, 2009 . [PubMed] [Google Scholar]
- 8.McGarvey N, Gitlin M, Fadli E, Chung KC. Increased healthcare costs by later stage cancer diagnosis. BMC Health Serv Res. 2022;22(1):1155–1155. doi: 10.1186/s12913-022-08457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romanian Society of Pediatric Onco-Hematology . Executive Report 2010-2021 . Cluj-Napoca, Romania : National Registry of Pediatric Onco-Hematology ; 2023 . pp. 1 – 68 . [Google Scholar]
- 10. National Registry of Pediatric Onco-Hematology (NRPOH) . Survival in pediatric cancers in Romania . Bucharest, Romania : NRPOH ; 2023 . pp. 1 – 25 . [Google Scholar]
- 11.Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sánchez-Pérez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R; Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5 - a population-based study. Lancet Oncol. 2014;15(1):35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 12. American Cancer Society (ACS) . Cancer Facts & Figures 2024 . ACS . Atlanta, GA, USA : ACS ; 2024 . pp. 1 – 82 . [Google Scholar]
- 13.Le Cornet, Lortet-Tieulent J, Forman D, Béranger R, Flechon A, Fervers B, Schüz J, Bray F. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer. 2014;50(4):831–839. doi: 10.1016/j.ejca.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization (WHO) . Childhood cancer inequalities in the WHO European Region . Copenhagen, Denmark : WHO Regional Office for Europe ; 2022 . [Google Scholar]
- 15.Beal MA, Yauk CL, Marchetti F. From sperm to offspring: assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat Res Rev Mutat Res. 2017;773:26–50. doi: 10.1016/j.mrrev.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Townsend MK, Vinci C, Jake-Schoffman DE, Tworoger SS. Early life exposure to tobacco smoke and ovarian cancer risk in adulthood. Int J Epidemiol. 2021;50(3):965–974. doi: 10.1093/ije/dyab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller JT, Lowy DR. An introduction to virus infections and human cancer. Recent Results Cancer Res. 2021;217:1–11. doi: 10.1007/978-3-030-57362-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirillo PM, Wang ET, Cedars MI, Chen LM, Cohn BA. Irregular menses predicts ovarian cancer: prospective evidence from the child health and development studies. Int J Cancer. 2016;139(5):1009–1017. doi: 10.1002/ijc.30144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan SJ, Green AC, Whiteman DC, Webb PM. Risk factors for benign serous and mucinous epithelial ovarian tumors. Obstet Gynecol. 2007;109(3):647–654. doi: 10.1097/01.AOG.0000254159.75977.fa. [DOI] [PubMed] [Google Scholar]
- 20.Shamsudeen S, Dunton CJ. Granulosa theca cell tumors of the ovary. StatPearls [Internet] 2024 [PubMed] [Google Scholar]
- 21.Hall C, Ritz B, Cockburn M, Davidson TB, Heck JE. Risk of malignant childhood germ cell tumors in relation to demographic, gestational, and perinatal characteristics. Cancer Epidemiol. 2017;46:42–49. doi: 10.1016/j.canep.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norwood MS, Lupo PJ, Chow EJ, Scheurer ME, Plon SE, Danysh HE, Spector LG, Carozza SE, Doody DR, Mueller BA. Childhood cancer risk in those with chromosomal and non-chromosomal congenital anomalies in Washington State: 1984-2013. PLoS One. 2017;12(6):0179006–0179006. doi: 10.1371/journal.pone.0179006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biro FM, Deardorff J. Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility. J Adolesc Health. 2013;52(5 Suppl):S15–S20. doi: 10.1016/j.jadohealth.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skakkebæk NE. Atypical germ cells in the adjacent "normal" tissue of testicular tumours. Acta Pathol Microbiol Scand A. 1975;83(1):127–130. doi: 10.1111/j.1699-0463.1975.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 25.Risk MC, Masterson TA. Intratubular germ cell neoplasms of the testis and bilateral testicular tumors: clinical significance and management options. Indian J Urol. 2010;26(1):64–71. doi: 10.4103/0970-1591.60454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin L, Zhao T, Liu X, Wang H, Gu X, Chen D, Wang Z, He D. Malignant transformation arising from mature ovarian cystic teratoma: a case series. Medicine. 2021;100(13):e24726–e24726. doi: 10.1097/MD.0000000000024726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CH, Jung MH, Ji YI. Risk factors for malignant transformation of mature cystic teratoma. Obstet Gynecol Sci. 2015;58(6):475–480. doi: 10.5468/ogs.2015.58.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donham KJ . In: Agricultural medicine: rural occupational and environmental health, safety, and prevention . 2nd . Donham KJ , et al., editors. Hoboken, NJ, USA : Wiley-Blackwell ; 2016 . Chapter 14: International agricultural safety and health ; pp. 465 – 502 . [Google Scholar]
- 29.Fernández-Navarro P, García-Pérez J, Ramis R, Boldo E, López-Abente G. Proximity to mining industry and cancer mortality. Sci Total Environ. 2012;435-436:66–73. doi: 10.1016/j.scitotenv.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Liu Y, Wang Y, Zhao X, Qi X. Hormone therapy for ovarian cancer: emphasis on mechanisms and applications (Review) Oncol Rep. 2021;46(4):223–223. doi: 10.3892/or.2021.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fénichel P, Chevalier N. Is testicular germ cell cancer estrogen dependent? The role of endocrine disrupting chemicals. Endocrinology. 2019;160(12):2981–2989. doi: 10.1210/en.2019-00486. [DOI] [PubMed] [Google Scholar]
- 32.Weeks KS, Lynch CF, West M, McDonald M, Carnahan R, Stewart SL; Impact of rurality on stage IV ovarian cancer at diagnosis: a Midwest Cancer Registry cohort study. J Rural Health. 2020;36(4):468–475. doi: 10.1111/jrh.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Zhang TT, Zhao JJ, Qi SF, Du P, Liu DW, Tian QB. The association between overweight, obesity and ovarian cancer: a meta-analysis. Jpn J Clin Oncol. 2015;45(12):1107–1115. doi: 10.1093/jjco/hyv150. [DOI] [PubMed] [Google Scholar]
- 34.Weihe P, Spielmann J, Kielstein H, Henning-Klusmann J, Weihrauch-Blüher S. Childhood obesity and cancer risk in adulthood. Curr Obes Rep. 2020;9(3):204–212. doi: 10.1007/s13679-020-00387-w. [DOI] [PubMed] [Google Scholar]
- 35.Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2(4):222–231. [PMC free article] [PubMed] [Google Scholar]
- 36.Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, Li X, Li Y, Xiong W, Li G, Guo C, Zeng Z. Chronic stress promotes cancer development. Front Oncol. 2020;10:1492–1492. doi: 10.3389/fonc.2020.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas SP, Groer M, Davis M, Droppleman P, Mozingo J, Pierce M. Anger and cancer: an analysis of the linkages. Cancer Nurs. 2000;23(5):344–349. doi: 10.1097/00002820-200010000-00003. [DOI] [PubMed] [Google Scholar]