Abstract
This paper explores the integral role of metallic nanomaterials in drug delivery, specifically focusing on their unique characteristics and applications. Exhibiting unique size, shape, and surface features, metallic nanoparticles (MNPs) (e.g., gold, iron oxide, and silver NPs) present possibilities for improving medication efficacy while minimizing side effects. Their demonstrated success in improving drug solubility, bioavailability, and targeted release makes them promising carriers for treating a variety of diseases, including inflammation and cancer, which has one of the highest rates of mortality in the world. Furthermore, it is crucial to acknowledge some limitations of MNPs in drug delivery before successfully incorporating them into standard medical procedures. Thus, challenges such as potential toxicity, issues related to long-term safety, and the need for standardized production methods will also be addressed.
Keywords: drug delivery , gold nanoparticles , silver nanoparticles , iron oxide nanoparticles , cancer therapeutics , anti-inflammatory agents
Introduction
Nanotechnology is an emerging field that explores the unique characteristics of materials at the nanoscale for applications, including precise medication administration, sensitive imaging agents based on nanoparticles (NPs) for diagnostics, and novel treatments like gene therapy and regenerative medicine. Nanotechnology provides accuracy and efficiency in medical interventions by operating at the molecular and cellular levels, promising improvements in healthcare diagnosis and treatment. Drug delivery systems (DDS) have evolved significantly following recent advancements in materials science. Scientists have been very interested in the development of nanomedicine in the recent few decades, as it falls under the broader category of nanotechnology. The increased attention comes from the need to create novel medication delivery systems that may successfully overcome the fundamental limitations of conventional approaches [1, 2]. By administering medicines without a delivery mechanism, low-solubility drugs face many difficulties, such as poor oral absorption, limited diffusion across cell membranes, higher doses needed for intravenous administration, and adverse reactions in conventional techniques. The poor solubility makes it more difficult for drugs to be delivered successfully, which affects bioavailability and requires different methods to maximize therapeutic efficacy and minimize side effects [2, 3].
To reach their full potential in medicine, NPs stability and biocompatibility must be ensured. By attaching ligands to NPs, specific targeting can be achieved by enabling the particles to bind selectively to target cells, such as cancer cells. Peptides and antibodies (Abs) are examples of ligands that bind selectively to cell surface receptors to allow for targeted administration. With fewer adverse effects and an enhanced therapeutic impact, this targeted approach makes NPs ideal for drug delivery and medical imaging applications [4].
Nanomaterials can be classified as organic and inorganic. Organic nanomaterials are made of organic compounds, such as polymers and lipids, providing flexibility and compatibility with biological systems. They find applications in drug delivery, sensors, and biomaterials [5]. Inorganic NPs include metals, metal oxides, or other inorganic compounds [4].
Researchers have studied the potential of several polymeric, lipidic, and metallic nanocarrier systems for specific drug delivery over the past few years. Metallic nanoparticles (MNPs) are one of the most effective and researched types of nanocarriers [4]. Manufacturing MNPs is a growing subject, with continuous studies focusing on their potential use in medication administration and imaging. MNPs, like gold NPs (AuNPs) and silver NPs (AgNPs), have unique physicochemical characteristics that make them suitable for therapeutic interventions, targeted drug administration, and imaging. Their effectiveness in supporting innovations in medical applications can be attributed to their small size, tunable surface properties, and compatibility with biological systems [1, 5, 6].
This paper addresses the synthesis of MNPs and their significant applications in the biomedical field, especially for drug delivery. Apart from reviewing the synthesis techniques, the discussion also includes an evaluation of their toxicity, limitations, and considerations for safe implementation. Lastly, a prospective outlook detailing possible advancements and applications will be provided.
Conventional versus targeted drug delivery
In the last several decades, traditional methods for administering drugs have been widely used. Drug formulations like tablets, pills, capsules, creams, liquids, aerosols, suppositories, injectables, or ointments have been prescribed for the treatment of chronic or severe disorders. However, because of their unpredictable biodistribution and imprecise control over the drug’s release characteristics, these popular dosage forms frequently result in undesired side effects. Overcoming these limitations has been an important focus for researchers [7].
When comparing conventional drug administration and targeted drug delivery, there are some limitations that the conventional approaches hold. In conventional drug administration, some medications have a short half-life, which means the body metabolizes or eliminates them quickly. Consequently, the duration of their therapeutic effects is limited, requiring repeated administration to maintain the desired drug concentration and therapeutic efficacy. By using targeted drug delivery, the administration frequency decreases while patient compliance increases [8].
Improving the efficacy of treatments is not the only goal of selectively delivering medications to the proper location. Reducing the possibility of adverse effects from high dosages is also essential. Finding solutions to the problems associated with conventional medication delivery methods requires appropriate targeting. Some medications are not effective when administered as tablets, some creams and ointments only work in specific locations, and injecting pharmaceuticals directly into the body can be very intrusive. The side effects are diminished by administering the drugs at the specific site while the therapeutic efficacy is improved [8, 9].
The bioavailability of conventional medicines is low. Its therapeutic effects are limited since only a tiny portion of the provided dose reaches the systemic circulation. A number of factors, such as ineffective absorption, quick metabolism, or significant elimination during the absorption process, can cause poor bioavailability. On the other hand, controlled drug delivery has an improved bioavailability, meaning that a larger proportion of the drug is absorbed into the bloodstream and may be used for the desired pharmacological effects. Medications with high bioavailability frequently show more consistent responses from person to person and can be used at lower dosages to achieve therapeutic objectives [10]. For clarity, Table 1 presents the characteristics of conventional and controlled DDS in a comparative manner.
Table 1.
|
Conventional DDS |
Controlled DDS |
|
No target specificity |
Controlled drug release |
|
Rapid elimination from the body |
Long residence of drug |
|
Repeated dosing |
Low dosing frequency |
|
Low cost |
High cost |
|
Better patient compliance |
Patient uncertainty |
|
Side effects |
Possible toxicity |
|
Accommodate different patients |
Tailored treatment for individuals |
DDS: Drug delivery systems
Drug delivery systems
DDS comprise a variety of technologies and carriers that are used to deliver medications to particular tissues, organs, cells, and subcellular structures. The principal aim is to enable regulated medication release and absorption while overcoming issues such as restricted solubility, drug aggregation, low bioavailability, improper biodistribution, limited selectivity, and possible side effect prevention. DDS aims to improve the pharmacological activities of therapeutic drugs to provide more efficient and targeted delivery for optimal therapeutic results [11]. In recent years, NPs as carriers have demonstrated significant potential among current drug delivery technologies.
Numerous groundbreaking advancements in the field of nanotechnology have been made. Different materials were manufactured at the nanoscale level by nanotechnology. A broad class of materials smaller than 100 nm is known as NPs. NPs are complex structures that can be made of three different layers that work together to determine their structure and functionality [12]. The NP’s layers are described in Table 2 .
Table 2.
|
Layer |
Role |
|
Surface layer |
▪ The outermost layer that can be functionalized to allow the attachment of different entities ( i.e. , metal ions, small molecules, surfactants); ▪ NP’s interactions with its environment are mostly determined by the functionalized surface. |
|
Shell layer |
▪ Lying below the surface layer; ▪ It affects the NP’s general characteristics, acting as a barrier, and also affecting the stability, biocompatibility, and interactions of the NP with its surroundings. |
|
Core |
▪ Vital section of the NP located in its center; ▪ Determines the basic characteristics of the NP and is frequently used interchangeably with the particle itself; ▪ Influences the optical, magnetic, or catalytic properties of the NP. |
DDS: Drug delivery systems; NP: Nanoparticle
The sizes of cells, bacteria, NPs, and other biological components are all extremely small. They may interact closely and successfully with each other due to their comparable sizes. This close connection creates opportunities for critical processes like controlled drug delivery, adhesion to body tissues and cells, and detection of disease agent concentrations in bodily fluids like blood, saliva, and urine. Few clinical studies have examined how NPs, administered through various methods, are completely removed from the human body, despite numerous laboratory and animal studies showing that NPs, both natural and engineered, work well with biological systems. It is necessary to understand these interactions to expand the use of NPs in medical and diagnostic applications [14, 15]. Different routes of drug-loaded NPs administration are available and presented in Table 3 , along with their dosage form and their advantages and drawbacks.
Table 3.
|
Route of administration |
Examples of dosage forms |
Advantages |
Disadvantages |
|
Oral |
Tablets, capsules, emulsions, medicated gums, etc. |
Easy administration; high patient compliance |
Limited penetration across the intestine membrane; variable absorption |
|
Nasal |
Sprays, drops, etc. |
Large surface area for absorption; non-invasive |
Fast elimination; mucosal and enzymatic barriers; barriers to large hydrophilic molecules |
|
Ocular |
Drops, contact lenses, ointments, etc. |
Easy dosing; easy access; rapid rate of systemic absorption |
Presence of ocular enzymes and barriers; local irritation; poor retention |
|
Dermal |
Creams, transdermal patches, sprays, etc. |
Easy administration; non-invasive; large surface area for drug absorption |
Low bioavailability; difficult permeation of large hydrophilic molecules |
|
Parenteral |
Intramuscular, intravenous, intradermal, subcutaneous |
Fast systemic delivery; good biodistribution; high availability |
Painful; swift clearance (kidney, liver, reticuloendothelial system) |
|
Pulmonary |
Aerosol, powders, nebulized solutions, etc. |
Large surface area for drug absorption; good systemic delivery; high bioavailability |
Complex clearance systems; only small particles reach the alveoli |
NPs: Nanoparticles
Nanomaterials classification
The current chemotherapeutic drug administration approach involves targeting specific locations with novel nanocarrier-mediated DDS. Nanomaterials can be divided into organic and inorganic (Figure 1). In this context, the most known nanocarriers include carbon nanotubes, dendrimers, polymeric NPs, liposomes, magnetic NPs, solid lipid NPs, and quantum dots. A few important nanocarriers and their contributions to advancing targeted medication delivery will be addressed in detail in the following subsections [17].
Figure 1.
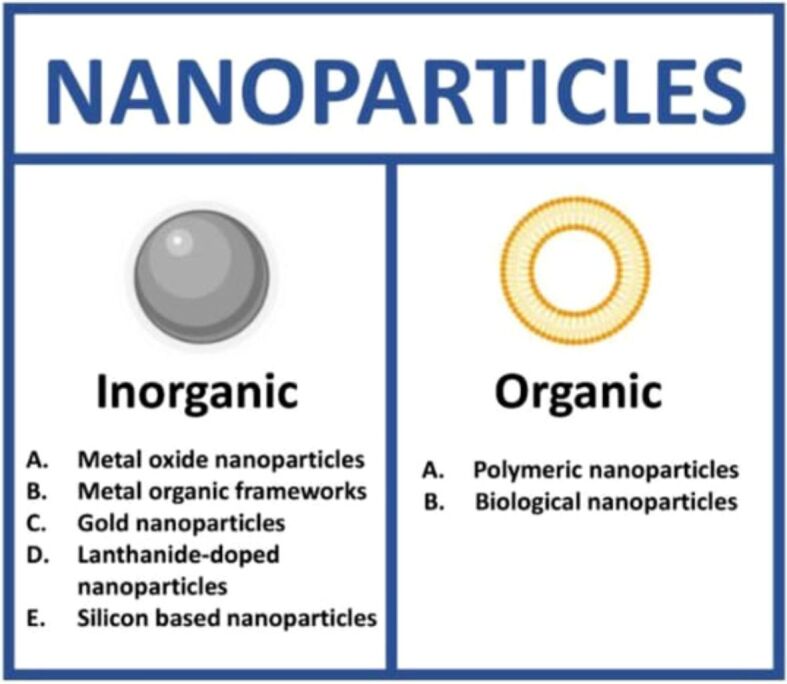
Types of nanomaterials: inorganic and organic. Reprinted from an open-access source [18]
Metallic nanomaterials
Nanomaterials can be made of organic or inorganic compounds. Both can be used in drug delivery, sensors, and biomaterials [5]. Organic materials exhibit natural compatibility with biological systems, encompassing a wide range of nanomaterials, such as liposomes, which are highly adaptable to DDS because of their structural similarity to biological membranes [18]. Other examples include micelles, polymeric NPs, or dendrimers, all of them being essential in delivering therapeutic substances to the intended sites and showing promise in gene therapy, cancer treatment, diagnosis, and other biomedical applications [19, 20, 21, 22].
Inorganic NPs comprise metals, metal oxides, or other inorganic compounds. It is well recognized that inorganic NPs have beneficial properties such as hydrophilicity, non-toxicity, and biocompatibility with biological organisms. These characteristics make them suitable for various biomedical uses, including medication administration, imaging, and diagnostics [23]. While inorganic NPs have many benefits, it is important to understand some of their drawbacks. Inorganic NPs have the potential to cause cytotoxicity and negatively impact blood-related parameters. These effects can be attributed to the size, concentration, and duration of exposure of the NPs. Researchers are investigating the synergistic synthesis of inorganic and bioactive elements in nanocarriers to solve these disadvantages. This strategy addresses long-standing issues in the pharmaceutical industry, where typical medications frequently have low solubility, poor specificity, low bioavailability, and lower therapeutic efficacy. Bioactive substances can be integrated into NPs to improve the performance of inorganic NPs and open the door to more efficient and focused DDS in biomedical applications [24, 25].
Inorganic nanomaterials also fall into two categories: non-metallic and metallic. Two non-metallic elements that are particularly significant and have wide-ranging applications are silicon and carbon. Given their fundamental chemical and physical characteristics, as well as their low cost and excellent biocompatibility, silicon and carbon stand out among potential options for the manufacturing of nanocarriers, especially when they involve the detection and treatment of cancer [26]. MNPs play a crucial role in advancing the field of theranostics. They are well-suited for drug delivery because of their properties, such as their small size. MNPs can exhibit magnetic characteristics. This is extremely beneficial when developing systems that respond to magnetic fields. By adding magnetic components, researchers can use external magnetic fields to control the movement and localization of these NPs within the body. The precision of delivering drugs to particular tissues or organs is improved by this control over the behavior of the NPs [27]. Over the years, different types of MNPs and their applications have been studied, which are presented in Table 4 .
Table 4.
Types of metallic nanoparticles and their applications in medicine
|
NPs type |
Application |
|
Gold NPs |
|
|
Silver NPs |
Cancer therapeutics [30], antimicrobial activity [29], wound healing [31] |
|
Copper NPs |
|
|
Iron oxide NPs |
Anemia treatment [34], cancer therapeutics [35], contrast agents [36] |
|
Titanium dioxide NPs |
Antimicrobial activity, antifungal activity, cancer therapeutics [37] |
|
Zinc oxide NPs |
|
|
Aluminum oxide NPs |
Cancer therapeutics, antimicrobial activity [40] |
NPs: Nanoparticles
Iron oxide nanoparticles
Iron oxide nanoparticles (IONPs) are leading the way in nanotechnology and advancing many different areas. Because of the technological importance of IONPs, especially Fe3O4, and γ-Fe2O3, their production has been thoroughly investigated. These iron oxides occur in the following phases: wustite, magnetite, hematite, and maghemite. The “quantum size effect”, which is caused by the transition to the nanoscale, provides materials with special optical, magnetic, and electrical properties that are not present in their bulk form. The behavior of IONPs is influenced differently at the nanoscale by individual atoms or molecules as compared to bulk qualities, which are determined by the average of quantum forces acting on all atoms. Magnetic IONPs, for example, show superparamagnetic behavior below 20 nm, and as size lowers, the magnetization shifts towards paramagnetic or superparamagnetic. This shrinkage reduces ferromagnetic behavior while enhancing superparamagnetic properties [41, 42].
IONPs include manufactured minerals such as magnetite and maghemite, with core diameters ranging from 10 to 100 nm. This category also includes IONPs mixed with nickel, copper, and cobalt, among other transition metals. These NPs exhibit a special characteristic known as super-paramagnetism when reduced to a size of 10–20 nm. In this state, when exposed to a magnetic field, they become magnetized; at this point, when the field is withdrawn, they lose all of their magnetism. They are useful in improving contrast for magnetic resonance imaging (MRI) because of their unique quality [43].
Similarly, the magnetic and catalytic properties of γ-Fe2O3 NPs are making them increasingly popular. They show promise for separation and biological applications due to their high magnetization and hysteretic heating, and they facilitate catalytic activities like photocatalysis due to their semiconducting property and chemically active surface [44].
IONPs offer two ways of targeting: passive and active. In passive targeting, also known as the enhanced permeability and retention (EPR) effect, IONPs exploit the abnormal vasculature resulting from tumor angiogenesis. They passively diffuse through the holes in these vessels, facilitating their accumulation in the tumor. This mechanism capitalizes on the EPR characteristic of tumor vasculature. On the other hand, active targeting involves using IONPs conjugated with a targeting ligand (TL). Without a magnetic field application, these ligand-conjugated IONPs specifically target receptors overexpressed on the surface of cancer cells. This approach ensures a more targeted and selective delivery of IONPs to cancerous tissues, enhancing their therapeutic efficacy [45].
Due to their magnetic properties, IONPs are mostly used in therapeutics such as DDS, MRI contrast agents, and hyperthermia [46]. They also have other applications in the biomedical field, as represented in Figure 2.
Figure 2.
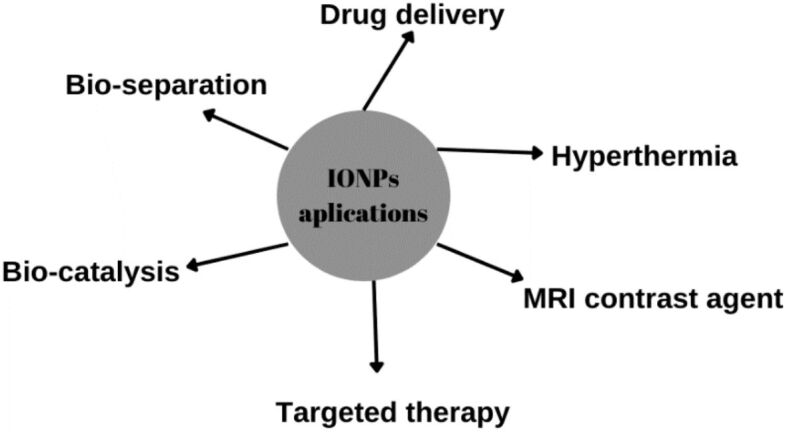
Biomedical applications of IONPs. Created based on information from [46]. IONPs: Iron oxide nanoparticles; MRI: Magnetic resonance imaging
Gold NPs
AuNPs are safer and less toxic than other MNPs because they are made of an exterior layer of organic ligands encasing an Au core. Their remarkable optical, plasmonic, and magnetic characteristics and vast surface area add to their versatility. Among MNPs, AuNPs are the safest and most appreciated for their special qualities. They are unique in that they are easy to manipulate, enabling surface functionalization with ligands or biomolecules, which makes them ideal for a wide range of uses, particularly in drug administration. AuNPs can be loaded with medicines, which increases their effectiveness as drug carriers [47].
Moreover, AuNPs’ surface can be modified to facilitate effective medication encapsulation and distribution. Targeted drug delivery is possible by adding particular modifiers, such as Abs, aptamers, sugars, and other ligands made to bind tumor-associated markers. Remarkably, several modifiers react to external factors like pH or enzymes, offering a regulated approach to drug release. This responsive activity improves the accuracy of medication administration to the targeted location. Furthermore, temperature variations can be induced by AuNPs’ effective photothermal conversion capacity, which will help with in situ medication release and tumor ablation. This dual functionality highlights the adaptability of AuNPs in enhancing treatment techniques by combining targeted drug administration with the ability for regulated release by external stimuli and photothermal conversion [48]. AuNPs applications are illustrated in Figure 3.
Figure 3.
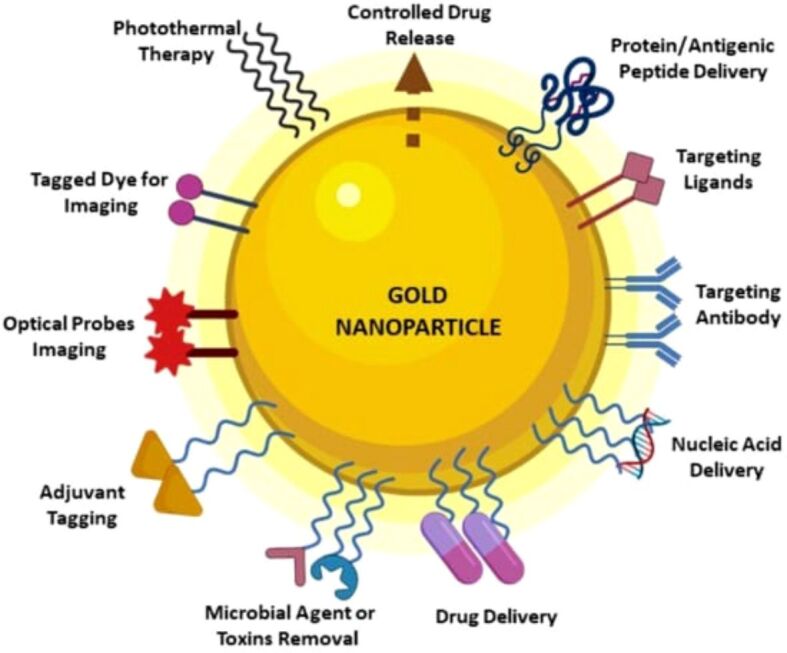
Possible applications for AuNPs in the biomedical field. Reprinted from an open-access source [49]. AuNPs: Gold nanoparticles
Silver NPs
The properties and applications of AgNPs are numerous and include optical, biological, and electrical properties. AgNPs’ distinctive qualities and excellent electrical conductivity make them useful tools in a broad range of applications. Interestingly, AgNPs have shown to be quite beneficial in industrial and medical products. Their uses include coating medical equipment, acting as antibacterial, antifungal, antiparasitic, antiviral, and anticancer drugs, as well as biosensors for bioimaging. AgNPs’ versatility in therapeutics is further demonstrated by their use as drug carriers. AgNPs are generally considered biocompatible, and their surface can be easily modified, allowing for functionalization with various molecules. This feature enhances their utility in biomedical applications, drug delivery, and targeted therapies. The stability of AgNPs is influenced by factors like size, shape, and environmental conditions, emphasizing the need for proper stabilization to prevent aggregation and maintain their unique properties [2, 50].
Applications and limitations
Cancer therapeutics
Cancer remains a global health challenge, with millions of new cases diagnosed each year. It is the second leading cause of death worldwide, coming after heart disease. Cancer is a complex category of diseases marked by uncontrolled division of cells and expansion, which frequently results in the development of cancerous tumors [51, 52].
Conventional cancer treatments include surgical resection, chemotherapy, radiotherapy, and biological therapy. Chemotherapy is a proven treatment for a variety of cancers [53]. Nevertheless, its wide-ranging cytotoxic effect causes undesirable adverse reactions. The distribution of the medications employed is heterogeneous, which results in inconsistent cancer cell targeting. The development of multidrug resistance (MDR) and this variability impedes chemotherapy’s overall effectiveness [54]. MDR has serious side effects, including limited therapy options for patients, recurrence of the disease, and treatment failures. In order to overcome these limitations, current research explores novel approaches such immunotherapies, targeted therapies, and DDS based on nanotechnology. By reducing side effects and enhancing treatment specificity, these strategies aim to increase the overall efficacy of cancer therapy [55].
The search for targeted active molecules with reduced side effects, cost-effectiveness, increased therapeutic potential, and a lower chance of developing disease resistance has become necessary in order to treat cancer more successfully. The focus has switched to natural chemicals, which are acknowledged for their variety of structures, modes of action, and prospective applications in the treatment of serious illnesses such as cancer. The increasing use of natural products highlights their potential to improve physiological pathways that are essential for successful cancer treatment [56]. Table 5 lists a few examples of synthetic analogs of anticancer drugs that have been demonstrated efficient and are derived from plants.
Table 5.
Natural antitumor drugs and their analogs. Adapted from an open-access source [56]
|
Antitumor drug |
Natural source/Analog |
|
Paclitaxel |
Taxus brevifolia Nutt. |
|
Docetaxel | |
|
Cabazitaxel | |
|
Camptothecin |
Camptotheca acuminata Decne. |
|
Belotecan | |
|
Topotecan | |
|
Irinotecan | |
|
Vinblastine |
Vinca rosea L. |
|
Vincristine | |
|
Vindesine | |
|
Vinorelbine | |
|
Podophyllotoxin |
Podophyllum spp. |
|
Dactinomycin |
Streptomyces spp. |
|
Doxorubicin |
Streptomyces peucetius var. caesius |
|
Daunorubicin |
Streptomyces spp. |
Studies have shown that other drugs have shown effects on MDR. Curcumin, a chemical found in turmeric that is mostly obtained from the root of Curcuma longa, has gained recognition for its several medicinal applications. In addition to its anti-inflammatory, anticancer, and antioxidant properties, it also exhibits modulatory effects on MDR. Research shows that Curcumin improves the efficacy of Doxorubicin (DOX) and Paclitaxel, even in resistant cell lines such MCF-7 cells that are resistant to Adriamycin and A549 cells that are resistant to Taxol. Nevertheless, Curcumin encounters difficulties like poor pharmacokinetics (PK), low water solubility, and chemical instability. Researchers have developed Curcumin analogs with improved chemical stability in order to overcome these drawbacks. Other strategies, such as liposomes, polymeric micelles, nanodrug systems, and polymer NPs have been developed in addition to chemical alterations to address the drug’s poor PK profile [54].
Combining NPs with active substances is very beneficial in cancer treatment, revolutionizing drug delivery by enhancing precision, efficacy, and bioavailability. NPs serve as targeted carriers, delivering therapeutic agents directly to tumors while minimizing damage to healthy tissues.
IONPs in cancer treatment
Because of their strong reactivity, IONPs have a lot of potential applications. This is especially true when IONPs are strategically placed into tumor cells. Important strategies in this endeavor include conjugate synthesis with targeted anticancer drugs, regulated delivery using an applied magnetic field, modification with specific Abs, and functionalization with pH-dependent groups. The majority of approved anti-cancer nanodrugs are parenterally delivered conjugates of small molecular weight chemotherapeutic medicines, such as DOX, with nanocarriers. When used as nanoenhancers, IONPs selectively accumulate in the vascularized region of solid tumors, exhibiting targeted activity. Studies using a variety of cancer models, such as brain tumors, liver, prostate, and breast cancer, provide evidence for IONPs effectiveness. The accumulation of NPs within tumors is facilitated by the EPR effect [57, 58].
Daviu et al. synthetized IONPs with Dimercaptosuccinic acid (DMSA) coating and investigated their effect on various cancer cells [59]. Results showed that IONPs–DMSA induce metabolic alterations and oxidative stress in cancer cells, which have an impact on mitochondrial function in particular. In highly metastatic cancer cells, DMSA–NPs cause mitophagy, decrease oxidative phosphorylation (OXPHOS) activity, and initiate mitochondrial fusion. Furthermore, exposure to DMSA–NPs causes cell cycle arrest, which influences long-term cancer cell proliferation. In another study by Attri et al. [60], lactoferrin (LF)-coated iron oxide nanospheres were created for targeted hyperthermia in gastric cancer. Due to LF’s interaction with chemicals on the surface of cancer cells, it was possible to deliver LF specifically to gastric cancer cells by conjugating it with IONPs (LF–IONPs). LF–IONPs were quickly internalized by cancer cells and had strong synergistic anticancer activity at low doses. These nanospheres showed significant thermal and magnetic properties as well, opening up a wide range of potential uses in biomedical research. PEGylated (polyethylene glycol, PEG) starch-coated IONPs demonstrated their efficacy in photothermal therapy (PTT) of cancer in a study conducted by Amatya et al. [61]. The experiments were produced in vitro and in vivo. At a certain laser power, the tumor temperature rose above 45°C, enough to kill the tumor cells, when exposed to the PEG–starch–IONPs. These coated NPs aggregated more in tumors and remained in the bloodstream longer compared to other coated NPs. This increased the results of heat therapy.
However, using IONPs as DDS can hold some drawbacks, such toxicity and immunological reaction. These are dependent on a number of variables. Significant influences include the NPs’ size, shape, and surface coating. Higher toxicity can result from specific coatings and smaller particles. Another important factor is the dose, or amount, of NPs; larger concentrations may be potentially harmful. Certain chemicals in the body can be released by IONPs, which can initiate immune system reactions. For example, in experiments with mice, specific sizes of IONPs caused inflammation [62]. To overcome these challenges, strategies like optimal surface functionalization, size and shape modification, and coating selection should be addressed [63].
AuNPs for cancer treatment
Because of their special qualities, AuNPs have become one of the most promising options for drug administration in cancer treatment. AuNPs provide versatility in drug encapsulation, targeting, and controlled release because of their easily modifiable sizes and surface treatment capabilities. To enable specific binding to cancer cells, the surface of AuNPs can be functionalized with various ligands, such as aptamers, Abs, or other targeting components, as presented in Figure 4.
Figure 4.
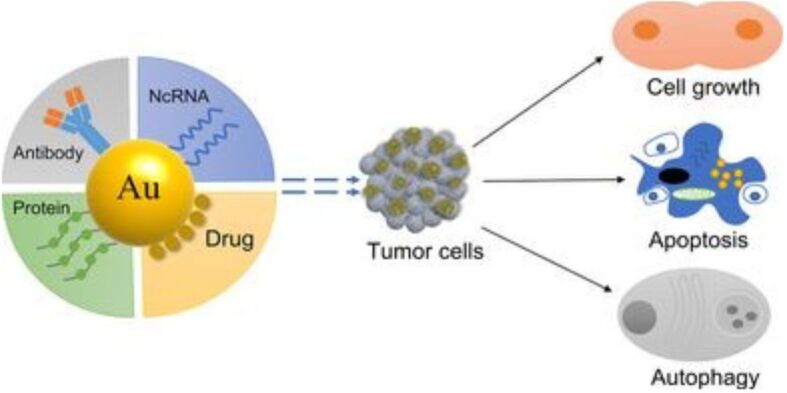
AuNPs delivery for cancer treatment. Reprinted from an open-access source [66]. AuNPs: Gold nanoparticles; NcRNA: Non-coding ribonucleic acid
Furthermore, the non-toxic, non-immunological, highly permeable, and retention effects of AuNPs make them more likely to infiltrate the tumor and enhance therapeutic effects [43]. Studies have shown that Methotrexate (MTX), an anticancer drug, has increased efficacy when combined with AuNPs. Compared to the administration of free MTX, this novel strategy has been demonstrated to increase cytotoxicity against various tumor cell lines. The enhanced cytotoxic effects are a result of the faster and greater accumulation of MTX inside tumor cells, which is made possible by AuNPs’ carrier properties. This approach can potentially improve drug delivery issues by increasing drug bioavailability and reducing off-target effects, as well as increasing the therapeutic benefit of MTX. The effective conjugation of MTX with AuNPs demonstrates how nanotechnology can be used to enhance the effectiveness of currently available chemotherapeutic drugs [64]. In a study by Ferreira-Gonçalves et al. [64], they synthetized AuNPs using different concentrations of rosmarinic acid in order to evaluate their efficacy in cancer treatment. The PTT effect could be improved by combining near-infrared (NIR) irradiation with AuNPs. The absorbance spectra of the AuNPs shifted towards the NIR region when the concentration of rosmarinic acid increased. When exposed to NIR laser radiation, these AuNPs showed the capacity to increase the temperature of the surrounding medium. The safety of the AuNPs was demonstrated by in vitro experiments using human and murine cell lines and human red blood cells.
In another study, researchers have created AuNPs by using seaweed. The phenolic extracts in the seaweed stabilized the AuNPs. Cytotoxicity and apoptosis induction were investigated against human lung cancer cells (H460). Results showed that anti-apoptotic gene expression was inhibited, and pro-apoptotic gene expression was enhanced. Overall, it was demonstrated that synthesized AuNPs have the potential to induce apoptosis in cancer cells by modulating gene expression and triggering nuclear damage, offering a promising alternative for cancer treatment [65].
Using AuNPs linked by an acid-labile linker in combination with DOX is an important advance in the control of MDR in cancer therapy. Specifically, this novel formulation showed a remarkable increase in toxicity when tested against the MDR MCF-7/ADR breast cancer cell line. An essential element is the acid-labile linker, which enables the conjugation of DOX to AuNPs in an approach that promotes responsive drug release in the cellular microenvironment. This individualized strategy works particularly well in combating MDR, a frequent cancer treatment problem. The improved uptake of the AuNPs-tethered DOX by the cancer cells contributes to the reported increased toxicity [64].
By specifically targeting tumor areas inside the complex tumor microenvironment (TME), AuNPs show promise in the treatment of cancer. Nevertheless, limitations restrict their effectiveness. The TME makes it difficult to maintain the ideal plasmonic characteristics of AuNPs since it is both hypoxic and hypertonic. There is a lack of research on the hypertonic tumor medium in solid tumors, which indicates a knowledge gap and needs investigation. NP penetration is limited by the fibrotic tissue and dense extracellular matrix, which block access to specific tumoral regions. AuNPs-based nanomedicines show promise, but their translation to clinical trials is still limited, and they are still only at the preclinical stage. In order to fill this gap, more studies are needed to understand the related pathways and replicate real-world tumor environments [67].
AgNPs for cancer treatment
AgNPs’ unique characteristics have drawn interest in their potential for cancer therapy. AgNPs cause oxidative stress, deoxyribonucleic acid (DNA) damage, and apoptosis in cancer cells. By focusing on different signaling pathways involved in the development of cancer, they can also prevent tumor growth and metastasis. Furthermore, when combined with radiation and chemotherapy, AgNPs can improve their effectiveness. Because of their small size, anticancer medications can be delivered to tumor locations with precision due to their efficient cellular uptake [68].
Using a chemical method, researchers produced AgNPs and examined their characteristics. Afterwards, they examined the effects of these AgNPs at various doses on the cancer cell lines HepG2 and MCF-7. They discovered that the dose of AgNPs had an impact on the cancer cells. Cell growth and appearance were minimally affected at low dosages. At higher dosages, however, the ability of cells to grow was severely compromised and their viability decreased to 10%. This implies that AgNPs may be able to eradicate specific types of cancer cells. Additionally, the study showed that AgNPs cause damage and cell death in cancer cells through specific pathways [69]. AgNPs can also be used in combination with other treatments or methods. This was demonstrated in a study conducted by Shipunova et al. [70]. The researchers conjugated the AgNPs with an affibody that specifically recognizes a protein found in cancer cells, called human epidermal growth factor receptor 2 (HER2). The conjugation was mediated by PEG, and they obtained AgNPs–PEG–HER2 system. These NPs demonstrated effective hyperthermic characteristics by heating up and killing cancer cells when exposed to PTT. AgNPs–PEG–HER2 particles bind to HER2-overexpressing cells with success, according to experiments conducted using flow cytometry. These NPs caused tumor regression during in vivo experiments, and they also prevented the spread of cancer.
When using AgNPs in cancer treatment, there are some possible challenges that need to be taken into consideration. For example, AgNPs alone could be filtered out from the circulation via reticuloendothelial system (RES), before they can reach the tumor. This can be overcome by different substances or molecules, such as PEG, as it can inhibit immune clearance [71]. Moreover, high doses of AgNPs have been found to cause toxic effects in in vivo studies [72], so there is still a need for precaution and more research needs to be performed, in order to ensure the safety of AgNPs in cancer treatment.
Antimicrobial and antibacterial activity
Being one of the first organisms on Earth, bacteria have proven to be remarkably adaptive over time. The discovery of antibiotics in the 20th century was a significant advancement in medicine and had an important impact on public health [73]. Antibiotic resistance has, however, emerged as a result of antibiotic overuse and misuse. This is due to the emergence, spread, and persistence of MDR bacteria. Bacteria develop resistance over time, and these resistances appear spontaneously in microorganisms due to genetic modifications. This phenomenon leads to prolonged illnesses, increased healthcare costs, and heightened mortality rates. This emphasizes how urgently new antibacterial compounds are needed. These guidelines’ main goal is to encourage scientists to focus their research and development efforts on creating novel antibiotics [73, 74].
Bacteria with negatively charged surfaces are classified as both Gram-positive and Gram-negative. Electrostatic interactions are promoted by positively charged NPs being drawn to negatively charged bacterial cell walls. Strong connections are formed between positively charged metal-based NPs and bacterial membranes, causing cell walls to break down and increase permeability. These NPs have the ability to release metal ions, which causes oxidative stress and the generation of reactive oxygen species (ROS). Antioxidant defenses of the bacteria are compromised by this stress, which allows metal ions to interact with cellular structures and generally inhibits bacterial functions [73, 75].
AgNPs as antimicrobial and antibacterial agents
AgNPs exhibit strong antibacterial effects, making them promising agents against bacterial infections. AgNPs cause oxidative stress and rupture cell membranes in Gram-positive and Gram-negative bacteria, which ultimately results in bacterial cell death. When administered by oral, cutaneous, pulmonary, or intravenous routes, they provide a high degree of adaptability in terms of targeting infections. Their topical treatment, which shows effectiveness against surface infections, is especially interesting. Comprehending the PK impacted by AgNPs’ physicochemical attributes, such as size and shape, is imperative for their advancement as treatments. The antimicrobial action of AgNPs stems from four primary mechanisms: first, their attraction to the bacterial surface; second, their ability to destabilize the bacterial cell wall and membrane, altering permeability; third, the induction of toxicity and oxidative stress through the generation of ROS and free radicals; and fourth, the modulation of signal transduction pathways. AgNPs interact with bacteria through two different methods after adhering to their surfaces. While larger AgNPs stay outside the cell but release Ag+ ions, smaller AgNPs enter the cell directly. Bacterial permeability increases as a result of the instability of cell membranes caused by these ions. Once inside, proteins, lipids, and DNA interact with AgNPs and Ag+ ions, leading to cellular malfunction. These NPs overpower the defense mechanisms of bacteria by producing free radicals such as hydrogen peroxide and ROS. Elevated amounts of Ag+ ions cause severe oxidative stress even in the presence of antioxidant enzyme activity. In short, AgNPs can penetrate the cells and inhibit enzymatic systems in the respiratory chain of bacteria, thus affecting their DNA synthesis [75].
Studies have assessed the antimicrobial potential of AgNPs through different methods. In a specific study, AgNPs were synthetized using aqueous Citrus limon zest extract in order to investigate their antimicrobial activity. Against the strains that were evaluated, the C. limon extract exhibited no antibacterial activity. On the other hand, strong bactericidal effects against both Gram-positive and Gram-negative bacteria were demonstrated by AgNPs. Further evidence of AgNPs’ potential as antibacterial agents comes from their comparable efficacy to the common antibiotic, Gentamicin [76]. In a study by Kota et al. [77], researchers have also used green synthesis in order to produce AgNPs from an aqueous leaf extract of Rumex acetosa. Its bactericidal effect was examined against 16 human pathogenic clinical isolates. These AgNPs have demonstrated high antimicrobial and antioxidant activity. Another study was performed using the disk diffusion method, where a 15 μL solution of AgNPs, along with 5 μg Ciprofloxacin, was impregnated on 6-mm discs, investigating potential synergies with the antibiotic. Control discs contained only 5 μg Ciprofloxacin. These discs were placed on bacterial cultures (Escherichia coli and Pseudomonas aeruginosa), and after 24 hours of incubation, the inhibition diameter was measured. There was a clear synergistic effect of the antibiotic and AgNPs, which was more evident when treating E. coli as opposed to P. aeruginosa. Furthermore, at higher concentrations of the precursory solution, there is an evident increase in the antibacterial effect [78].
AgNPs have demonstrated their efficacy in these applications; however, they also have some limitations. One main concern that needs to be addressed is their cytotoxicity. Their physicochemical characteristics, such as size, shape, solubility, and surface charge, are closely related to toxicity. There is a greater chance of potentially dangerous occurrences because of their high surface area and reactivity in biological media. AgNPs with a negative charge are typically less toxic than those with a positive charge. AgNPs become more toxic when they disintegrate or lose their spherical shape. More research is necessary to determine if the discharged Ag+ ions or the NPs cause the toxicity. Nanosphere-like forms are more toxic. AgNPs have been shown in multiple investigations to exhibit non-selective toxicity, affecting cells including hepatocytes and brain cells, despite the initial assumption that they were less harmful to mammalian cells than bacterial cells [73].
AuNPs as antimicrobial and antibacterial agents
AuNPs have been shown in the literature to have broad-spectrum antibacterial action against a variety of pathogens, including both Gram-positive and Gram-negative bacteria. Furthermore, AuNPs are effective against a number of viruses, such as the herpes simplex virus, hepatitis B virus, influenza virus, and human immunodeficiency virus-1 (HIV-1). The ability of AuNPs to target a variety of microorganisms demonstrates their promise as a strong antibacterial agent that can be used to treat viral and bacterial infections. The size-dependent response and local-field interaction between AuNPs and the virus cell wall are linked to the antiviral capabilities of AuNPs. According to research, AuNPs have size-dependent (7–70 nm) antiviral efficacy against influenza virus A/FM/1/47 (H1N1) in Madin–Darby canine kidney (MDCK) cell line. According to the experimental results, AuNPs up to 30 nm in size exhibit the highest level of antiviral activity, while particles larger than 30 nm exhibit very little or no antiviral activity. This suggests that smaller NPs, less than 30 nm in size, interacted strongly with the viral capsid, ultimately causing the virus to be destroyed [79].
Studies have shown that when Ampicillin binds to the surfaces of AuNPs, those NPs have antibacterial abilities. Broad-spectrum fungicidal activities of Ampicillin-functionalized AuNPs are efficient against both Gram-positive and Gram-negative bacteria. Flavonoid–glycerin was used by Su et al. [80] to reduce and cap synthesized AuNPs. The synthesized AuNPs have been shown to exhibit broad-spectrum antibacterial properties against opportunistic bacterial pathogens that cause respiratory diseases. The impact of flavonoid-coated gold nanoparticles (FAuNPs) on Enterococcus faecalis colonization in mouse liver and kidney was studied by Riaz et al. There was a considerable decrease in the number of bacteria in mouse organs as compared to when free flavonoids were used. The results highlight the capabilities of functionalized AuNPs, especially in conjunction with antibiotics, and promote the development of AuNPs for combating a variety of Gram-positive and Gram-negative bacterial strains and overcome MDR [80].
AuNPs can also be used in film formation with antimicrobial properties. In a particular study, a nanocomposite film was created with AuNPs, chitosan and (3-Aminopropyl) trimethoxysilane (APTMS). These positively charged elements interact with the negatively charged Salmonella typhimurium bacteria cell membrane when combined. This interaction disrupts the membrane integrity, leading to cell death. The combination of chitosan, APTMS, and AuNPs improves the antibacterial activity of the film [81].
This DDS also has its limitations. Potential cytotoxicity, difficulties achieving the optimal drug loading, and concerns about the long-term effects are some of the challenges. Other factors to consider are the stability of AuNPs under physiological environments and the body’s ability to eliminate them. It is difficult to achieve good targeting and controlled release, and resistance development may occur, thus close observation is required. Furthermore, the expense of producing on a large scale could prevent broader use. For AuNPs to be further developed in antibacterial and antimicrobial DDS, these limitations must be addressed [79, 80].
IONPs as antimicrobial and antibacterial agents
Because IONPs can produce ROS when they come in contact with bacteria, they have strong antibacterial activity. These ROS cause oxidative stress, which harms the DNA and membranes of bacterial cells and ultimately kills the cells. In addition, because IONPs have the ability to break down bacterial biofilms, they hold great promise in combating antibiotic-resistant illnesses as well as in enhancing sanitization procedures [82]. Because of their capacity to both denature and limit enzyme activity, IONPs have further demonstrated antibacterial action. Enzymes, for instance, can bind to IONPs directly, changing their three-dimensional structure and, consequently, how they function [83].
Several studies have demonstrated the antimicrobial effect of IONPs. A particular study examined the antibacterial activities of IONPs and leaf extract of Strobilanthes crispus, against a range of human pathogens. Only E. coli was suppressed by the leaf extract, but IONP had strong antibacterial action against every pathogen tested, with Staphylococcus aureus showing the most significant suppression. Examination of IONP’s characteristics verified its potential as an antibacterial agent in the medical field [84]. Al-Badr et al. [85] investigated the antimicrobial activity of IONPs functionalized with glycine and coated by chitosan as root canal irrigation. The system was tested against different pathogens, such as Candida albicans, E. faecalis and Streptococcus mutans. In regard to E. faecalis, the chitosan–IONP group showed inhibition that was comparable to that of 5.25% Sodium hypochlorite (NaOCl) irrigant, which is recognized as the most effective antibacterial agent. In contrast, C. albicans was extremely sensitive to chitosan–IONP, showing an average inhibition zone of 29.3 mm as opposed to 25.5 mm for 5.25% NaOCl. Overall, IONPs exhibit great potential in antimicrobial treatments, demonstrating their effectiveness against many types of bacteria.
Anti-inflammatory treatment
The immune system’s reaction to dangerous stimuli, such as infections, injured cells, poisonous substances, or radiation, is inflammation. Inflammation serves to eliminate harmful stimuli and start the healing process. Therefore, inflammation is an essential defense mechanism for good health. Cellular and molecular processes and interactions reduce the risk of harm or infection during acute inflammatory reactions. This reducing procedure aids in the resolution of acute inflammation and the return of tissue homeostasis. On the other hand, uncontrolled acute inflammation can develop into a chronic condition and lead to a number of chronic inflammatory illnesses. Inflammation can be caused by a number of pathogenic events that damage tissue, including infection, myocardial infarction, and tissue damage. Examples of the causes for inflammation are presented in Table 6 [86].
Table 6.
|
Inflammation trigger category |
Examples |
|
Physical |
Burns, injuries, foreign bodies, trauma, frostbites |
|
Chemical |
Alcohol, glucose, toxins, fats |
|
Biological |
Damaged cells |
|
Lifestyle factors |
Lack of sleep, unhealthy diet, chronic stress, physical inactivity |
|
Infectious factors |
Bacteria, viruses, other microorganisms |
Many diseases originate from the inflammatory response, which is a key pathogenic component. A strong focus on hybrid materials with multiple functions – anti-inflammatory properties combined with effective DDS – has been demonstrated by recent research. Overproduction of inflammatory mediators has been linked to the development of a number of diseases, including sepsis, rheumatoid arthritis (RA), osteoarthritis, and inflammatory bowel disease (IBD). Strategies based on NPs are showing promise as tools with anti-inflammatory potential. By doing this, the effectiveness of the treatment is increased, and the negative effects are reduced. Glucocorticoids (GCs) are a common and effective treatment for RA, a chronic inflammatory disease. However, repeated use of GC therapy leads to systemic effects and significant adverse responses due to its non-specific character. Here, a promising approach is provided by ongoing research into DDS based on NPs. By delivering medication in a localized way, these devices may be able to reduce the systemic side effects of prolonged GC therapy [88, 89].
AgNPs for anti-inflammatory treatment
Over the years, it has been reported that AgNPs have shown anti-inflammatory potential. Because they suppress pro-inflammatory cytokines and lower inflammatory mediators, AgNPs have natural anti-inflammatory qualities that impact immune responses. Their interactions with immune cells, antioxidant activity, and immunomodulatory activities all help to decrease inflammation. AgNPs can be engineered for the regulated release of anti-inflammatory medicines in the delivery of drugs, improving therapeutic efficacy while minimizing side effects. Cytotoxicity is reduced by optimizing AgNP properties, including size and surface modification. This multidimensional approach presents AgNPs as promising agents for anti-inflammatory drug delivery. However, to allow safe and successful usage, a comprehensive assessment of possible risks is necessary [90].
In a study conducted by Singh et al. [91], researchers produced AgNPs through green synthesis from Prunus serrulata, to test their anti-inflammatory potential in vitro. The 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay demonstrated that Prunus–AgNPs were safe for use in additional studies because they did not display any cytotoxic effects at the doses that were examined. Research indicates that illnesses including cancer and inflammatory bowel syndrome may be minimized by blocking the inflammatory enzymes cycloxogigenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). The system inhibited the production of NO and prostaglandin E2 (PGE2), key inflammatory mediators. Another particular study examined the effects of Cotyledon–AgNPs on cellular inflammation. The NPs contained C. orbiculata plant leaf extract. They measured the amount of specific proteins (cytokines) produced by immune cells (macrophages) using a technique known as enzyme-linked immunosorbent assay (ELISA). Following exposure to bacteria-like substances (lipopolysaccharides, LPS), these cells produced much less inflammatory proteins when treated with Cotyledon–AgNPs. The fact that Cotyledon–AgNPs decreased the amounts of proteins that typically rise during inflammation indicates that they have anti-inflammatory properties. In earlier research, AgNPs derived from Curcumin and Asparagus racemosus root extract showed comparable benefits [92].
One of the main contributing factors to the development of RA is the invasion of inflammatory cells, particularly M1 macrophages. M1 macrophages play a major role in the inflammatory processes seen in RA by releasing a variety of cytokines, which are chemicals that cause inflammation. Another study focused on recognizing and attacking these immune cells, particularly M1 macrophages, using drug-loaded AgNPs. They coated the AgNPs with PEG to make them stable and added folic acid (FA) to target the M1 macrophages. These modified AgNPs, known as FA–AgNPs, demonstrated promising results in laboratory tests and research using RA mice. The purpose of the AgNPs was to release Ag+ ions, which led to the M1 macrophages’ apoptosis and assisted in the removal of ROS. A favorable transition from M1 to M2 macrophages was the result of this method. Due to a phenomenon called the “ELVIS” effect (extravasation via leaky vasculature and inflammatory cell-mediated sequestration), the modified AgNPs were also intended to passively accumulate in the RA-affected joints. FA supplementation additionally aided in the active delivery of the NPs to M1 macrophages, therefore decreasing joint inflammation in RA mice. These FA–AgNPs proved to be safer and more effective than MTX, the standard of care RA medication [93].
AgNPs have proven their effectiveness in anti-inflammatory treatment. However, like other DDS, they also have some disadvantages. Specific studies found that the smallest particles (20 nm) were more harmful than larger ones (80 and 113 nm). Regardless of size, these particles caused the release of inflammation-causing markers. In animal studies, AgNPs affected gut bacteria and led to behavioral changes. High doses of orally administered AgNPs were linked to issues in the biliary system in animals. Therefore, AgNPs have useful properties, it’s important to be aware of their potential downsides [94].
IONPs for anti-inflammatory treatment
Research has demonstrated that IONPs possess anti-inflammatory capacities. They can have an impact on T-cells, which are cells that produce anti-inflammatory mediators to decrease inflammation or release pro-inflammatory cytokines to enhance it [95]. In a study investigating how IONPs affected Jurkat T-cells, scientists found that although the NPs blocked a signaling channel in the cells, they did not cause any damage to their integrity. Certain intracellular molecules showed significant changes in response to the presence of IONPs, particularly an increase in nicotinamide adenine dinucleotide phosphate (NADP+) and a decrease in reduced NADP+ (NADPH). Moreover, the presence of IONPs inhibited the production of genes linked to inflammation, including interferon gamma (IFNγ), interleukin (IL)-2, and IFN response genes. All of these results indicate the possibility that IONPs have an anti-inflammatory effect on T-cells, which suggests that they may be useful in reducing inflammatory responses in the immune system [96]. Another study used Madhuca indica for the manufacture of IONPs, in order to evaluate their anti-inflammatory effect. This plant has medicinal properties that can possess health benefits, including hepatoprotection, anti-inflammatory, and antidiabetic properties. The study used egg albumin protein in order to assess the anti-inflammatory properties of IONPs. Egg albumin protein denaturation is a common model used in experiments to study anti-inflammatory properties. Inflammation often involves changes in protein structures and substances that inhibit these changes may have anti-inflammatory effects. The denaturation of egg albumin protein and a plant extract from Madhuca indica were evaluated for the inhibitory effects of IONPs. A process known as denaturation causes a protein to lose both its structure and its function. The findings demonstrated that IONPs had a greater inhibitory impact (96.2%) on egg albumin protein’s denaturation than the plant extract (71.2%). Acetylsalicylic acid was used as a control, and an anti-inflammatory medication was widely used. These results suggest that IONPs may have beneficial effects in managing diabetes and reducing inflammation. However, there are still concerns about toxicity, biodistribution, and the complex process of translating laboratory findings into clinical applications. Further research, particularly in clinical trials, is essential to validate safety and efficacy in human use [97].
AuNPs for anti-inflammatory treatment
The anti-inflammatory capabilities of AuNPs stem from their capacity to regulate many cellular signaling pathways implicated in inflammation. These NPs have the ability to suppress the release of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and ILs, while simultaneously promoting the release of anti-inflammatory cytokines like IL-10. Furthermore, AuNPs can reduce the expression of adhesion molecules involved in leukocyte migration to inflammatory tissues and inhibit the activation of inflammatory cells, such as neutrophils and macrophages. They can also inhibit oxidative stress [98, 99].
In a study, AuNPs were synthetized from Isodon excisus leaf tissue (Isodon–AuNPs) and tested for treating skin inflammation. Human keratinocytes were used in this experiment. Isodon–AuNPs blocked the production of ROS, reduced the expression of chemokines and anti-inflammatory cytokines, and activated autophagy indicators. These results highlight the anti-inflammatory potential of AuNPs, which can be further researched in order to achieve novel treatments for different skin conditions or inflammatory diseases [100]. Xu et al. [101] conducted a study wherein they extracted bioactive compounds from Hibiscus syriacus callus (HSC). Utilizing HSC, they synthesized AuNPs (HSC–AuNPs) and examined their anti-inflammatory effects in macrophages. The outcomes demonstrated that HSC–AuNPs enhanced mitochondrial function and decreased inflammation. Additionally, they stimulated autophagy, which enhanced mitochondrial function and further decreased inflammation. These results imply that HSC–AuNPs may improve cellular functions and therefore represent a promising treatment for disorders associated with inflammation. In another research study by Uchiyama et al., they conjugated AuNPs with biomolecules. In both in vitro and in vivo settings, the study showed that AuNP bioconjugates exhibited minimal or non-existent toxicity. It demonstrated the in vivo anti-inflammatory characteristics of AuNP bioconjugates. Leukotriene B4 (LTB4) activation was shown to decrease leukocyte–endothelium interactions and leukocyte influx into surrounding tissues. The substantial reduction in chemotaxis and oxidative burst activation of rat neutrophils treated with AuNP–immunoglobulin G (IgG) following stimulation with LTB4 or phorbol myristate acetate (PMA) supported these findings in vitro [102].
Conclusions
Investigating MNPs in drug delivery is an intriguing and promising field with significant potential for use in medicine. The distinct physicochemical characteristics of MNPs, including their dimensions, shape, and surface characteristics, present unique prospects for targeted and controlled delivery of medicines. These NPs have proven to be very effective at increasing drug solubility, improving bioavailability, and facilitating targeted release at the desired location, all of which help to reduce undesired side effects. MNPs are used in imaging, diagnostics, pharmacological therapy, and drug delivery. Several preclinical investigations have shown the remarkable effectiveness of AuNPs, AgNPs, and IONPs, among others, indicating their potential for clinical application as DDS. However, challenges still need to be resolved, such as concerns about long-term toxicity, biodistribution, and the requirement for standardized manufacturing procedures. Future treatment approaches are anticipated to be more effective, accurate, and customized, using MNPs for improving medical procedures. The significant potential of MNPs to revolutionize healthcare practices is becoming increasingly apparent as time passes, offering the possibility of a future with novel treatment methods and enhanced patient outcomes.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1. Ahmad MZ , Ahmad J , Warsi MH , Abdel-Wahab BA , Akhter S . In: Nanoengineered biomaterials for advanced drug delivery . Mozafari M , et al., editors. Oxford, UK : Elsevier ; 2020 . 13 - Metallic nanoparticulate delivery systems ; pp. 279 – 328 . [Google Scholar]
- 2.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71–71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;5(6):1593–1615. doi: 10.1007/s42247-021-00335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neha Desai, Momin M, Khan T, Gharat S, Ningthoujam RS, Omri A. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin Drug Deliv. 2021;18(9):1261–1290. doi: 10.1080/17425247.2021.1912008. [DOI] [PubMed] [Google Scholar]
- 5.Alshammari BH, Lashin MMA, Mahmood MA, Al-Mubaddel FS, Ilyas N, Rahman N, Sohail M, Khan A, Abdullaev SS, Khan R. Organic and inorganic nanomaterials: fabrication, properties and applications. RSC Adv. 2023;13(20):13735–13785. doi: 10.1039/d3ra01421e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts. 2021;11(8):902–902. [Google Scholar]
- 7.Laffleur F, Keckeis V. Advances in drug delivery systems: work in progress still needed. Int J Pharm. 2020;590:119912–119912. doi: 10.1016/j.ijpharm.2020.119912. [DOI] [PubMed] [Google Scholar]
- 8.Khizar S, Alrushaid N, Alam Khan, Zine N, Jaffrezic-Renault N, Errachid A, Elaissari A. Nanocarriers based novel and effective drug delivery system. Int J Pharm. 2023;632:122570–122570. doi: 10.1016/j.ijpharm.2022.122570. [DOI] [PubMed] [Google Scholar]
- 9.Tewabe A, Abate A, Tamrie M, Seyfu A, Abdela Siraj. Targeted drug delivery - from magic bullet to nanomedicine: principles, challenges, and future perspectives. J Multidiscip Healthc. 2021;14:1711–1724. doi: 10.2147/JMDH.S313968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sultana A, Zare M, Thomas V, Sampath Kumar, Ramakrishna S. Nano-based drug delivery systems: conventional drug delivery routes, recent developments and future prospects. Med Drug Discov. 2022;15:100134–100134. [Google Scholar]
- 11.Li C, Wang J, Wang Y, Gao H, Wei G, Huang Y, Yu H, Gan Y, Wang Y, Mei L, Chen H, Hu H, Zhang Z, Jin Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9(6):1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. [Google Scholar]
- 13.Kumari S, Sarkar L. A review on nanoparticles: structure, classification, synthesis & applications. J Sci Res. 2021;65(8):42–46. [Google Scholar]
- 14.Moradi Kashkooli, Soltani M, Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies. J Control Release. 2020;327:316–349. doi: 10.1016/j.jconrel.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Sábio RM, Bagliotti Meneguin, Martins dos, Monteiro AS, Chorilli M. Exploiting mesoporous silica nanoparticles as versatile drug carriers for several routes of administration. Microporous Mesoporous Mater. 2021;312:110774–110774. [Google Scholar]
- 16.Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26(19):5905–5905. doi: 10.3390/molecules26195905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edis Z, Wang J, Waqas MK, Ijaz M, Ijaz M. Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int J Nanomedicine. 2021;16:1313–1330. doi: 10.2147/IJN.S289443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrese B, Sanità G, Lamberti A. Nanoparticles design for theranostic approach in cancer disease. Cancers (Basel) 2022;14(19):4654–4654. doi: 10.3390/cancers14194654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma D, Ali AAE, Trivedi LR. An updated review on: liposomes as drug delivery system. PharmaTutor. 2018;6(2):50–62. [Google Scholar]
- 20.Atanase LI. Micellar drug delivery systems based on natural biopolymers. Polymers (Basel) 2021;13(3):477–477. doi: 10.3390/polym13030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, Awasthi V, Cosco D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol. 2021;12:601626–601626. doi: 10.3389/fphar.2021.601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias AP, da Silva, da Silva, Parise-Filho R, Igne Ferreira, Seoud OE, Giarolla J. Dendrimers in the context of nanomedicine. Int J Pharm. 2020;573:118814–118814. doi: 10.1016/j.ijpharm.2019.118814. [DOI] [PubMed] [Google Scholar]
- 23.Choi Y, Lee SY. Biosynthesis of inorganic nanomaterials using microbial cells and bacteriophages. Nat Rev Chem. 2020;4(12):638–656. doi: 10.1038/s41570-020-00221-w. [DOI] [PubMed] [Google Scholar]
- 24.Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, Qoronfleh MW. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):20–20. doi: 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maus A, Strait L, Zhu D. Nanoparticles as delivery vehicles for antiviral therapeutic drugs. Eng Regen. 2021;2:31–46. doi: 10.1016/j.engreg.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Yang L, Chen G, Xu F, Yang F, Yu H, Li L, Dong X, Han J, Cao C, Qi J, Su J, Xu X, Li X, Li B. A review on drug delivery system for tumor therapy. Front Pharmacol. 2021;12:735446–735446. doi: 10.3389/fphar.2021.735446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Moreno P, Ortega-Vinuesa JL, Peula-Garcia JM, Marchal JA, Boulaiz H. Smart drug-delivery systems for cancer nanotherapy. Curr Drug Targets. 2018;19(4):339–359. doi: 10.2174/1389450117666160527142544. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Zheng X, Chen L, Gong X, Yang H, Duan X, Zhu Y. Multifunctional gold nanoparticles in cancer diagnosis and treatment. Int J Nanomedicine. 2022;17:2041–2067. doi: 10.2147/IJN.S355142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sher N, Alkhalifah DHM, Ahmed M, Mushtaq N, Shah F, Fozia F, Khan RA, Hozzein WN, Aboul-Soud MAM. Comparative study of antimicrobial activity of silver, gold, and silver/gold bimetallic nanoparticles synthesized by green approach. Molecules. 2022;27(22):7895–7895. doi: 10.3390/molecules27227895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovács D, Igaz N, Gopisetty MK, Kiricsi M. Cancer therapy by silver nanoparticles: fiction or reality. Int J Mol Sci. 2022;23(2):839–839. doi: 10.3390/ijms23020839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh M, Thakur V, Kumar V, Raj M, Gupta S, Devi N, Upadhyay SK, Macho M, Banerjee A, Ewe D, Saurav K. Silver nanoparticles and its mechanistic insight for chronic wound healing: review on recent progress. Molecules. 2022;27(17):5587–5587. doi: 10.3390/molecules27175587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvo J, Sandoval C. Role of copper nanoparticles in wound healing for chronic wounds: literature review. Burns Trauma. 2022;10:tkab047–tkab047. doi: 10.1093/burnst/tkab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu VW, Nizami MZI, Yin IX, Yu OY, Lung CYK, Chu CH. Application of copper nanoparticles in dentistry. Nanomaterials (Basel) 2022;12(5):805–805. doi: 10.3390/nano12050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumari A, Chauhan AK. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: a review. J Food Sci Technol. 2022;59(9):3319–3335. doi: 10.1007/s13197-021-05184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseinkazemi H, Samani S, O’Neill A, Soezi M, Moghoofei M, Azhdari MH, Aavani F, Nazbar A, Keshel SH, Doroudian M. Applications of iron oxide nanoparticles against breast cancer. J Nanomater. 2022;2022:6493458–6493458. [Google Scholar]
- 36.Montiel Schneider, Martín MJ, Otarola J, Vakarelska E, Simeonov V, Lassalle V, Nedyalkova M. Biomedical applications of iron oxide nanoparticles: current insights progress and perspectives. Pharmaceutics. 2022;14(1):204–204. doi: 10.3390/pharmaceutics14010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagadevan S, Imteyaz S, Murugan B, Anita Lett, Sridewi N, Weldegebrieal GK, Fatimah I, Oh WC. A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process Synth. 2022;11(1):44–63. [Google Scholar]
- 38.Lyu W, Qian M, Yang F. Nanoparticles in sunscreen: exploration of the effect and harm of titanium oxide and zinc oxide. Highl Sci Eng Technol. 2022;13:155–162. [Google Scholar]
- 39.Siddiqi KS, Ur Rahman, Tajuddin undefined, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):141–141. doi: 10.1186/s11671-018-2532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassanpour P, Panahi Y, Ebrahimi-Kalan A, Akbarzadeh A, Davaran S, Nasibova AN, Khalilov R, Kavetskyy T. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018;13(9):1227–1231. [Google Scholar]
- 41.Hernández-Hernández AA, Aguirre-Álvarez G, Cariño-Cortés R, Mendoza-Huizar LH, Jiménez-Alvarado R. Iron oxide nanoparticles: synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chem Pap. 2020;74(11):3809–3824. [Google Scholar]
- 42.Niculescu AG, Chircov C, Grumezescu AM. Magnetite nanoparticles: synthesis methods - a comparative review. Methods. 2022;199:16–27. doi: 10.1016/j.ymeth.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Sun L, Liu H, Ye Y, Lei Y, Islam R, Tan S, Tong R, Miao YB, Cai L. Smart nanoparticles for cancer therapy. Signal Transduct Target Ther. 2023;8(1):418–418. doi: 10.1038/s41392-023-01642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajinkya N, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: present and future. Materials (Basel) 2020;13(20):4644–4644. doi: 10.3390/ma13204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alphandéry E. Iron oxide nanoparticles for therapeutic applications. Drug Discov Today. 2020;25(1):141–149. doi: 10.1016/j.drudis.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 46. Jackson MJ . In: Micro and Nanomanufacturing . Jackson MJ , et al., editors. Boston, MA, USA : Springer ; 2007 . 12. Micro- and nanomanufacturing ; pp. 635 – 685 . [Google Scholar]
- 47.Yafout M, Ousaid A, Khayati Y, El Otmani. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: a new lead for targeted pharmacological cancer treatments. Sci Afr. 2021;11:e00685–e00685. [Google Scholar]
- 48.Huang H, Liu R, Yang J, Dai J, Fan S, Pi J, Wei Y, Guo X. Gold nanoparticles: construction for drug delivery and application in cancer immunotherapy. Pharmaceutics. 2023;15(7):1868–1868. doi: 10.3390/pharmaceutics15071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengupta A, Azharuddin M, Al-Otaibi N, Hinkula J. Efficacy and immune response elicited by gold nanoparticle-based nanovaccines against infectious diseases. Vaccines (Basel) 2022;10(4):505–505. doi: 10.3390/vaccines10040505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomes HIO, Martins CSM, Prior JAV. Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials (Basel) 2021;11(4):964–964. doi: 10.3390/nano11040964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Zhang YB, Pan XF, Chen J, Cao A, Zhang YG, Xia L, Wang J, Li H, Liu G, Pan A. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. 2020;122(7):1085–1093. doi: 10.1038/s41416-020-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85–85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dallavalle S, Dobričić V, Lazzarato L, Gazzano E, Machuqueiro M, Pajeva I, Tsakovska I, Zidar N, Fruttero R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist Updat. 2020;50:100682–100682. doi: 10.1016/j.drup.2020.100682. [DOI] [PubMed] [Google Scholar]
- 55.Zhao CY, Cheng R, Yang Z, Tian ZM. Nanotechnology for cancer therapy based on chemotherapy. Molecules. 2018;23(4):826–826. doi: 10.3390/molecules23040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharifi-Rad J, Ozleyen A, Boyunegmez Tumer, Oluwaseun Adetunji, El Omari, Balahbib A, Taheri Y, Bouyahya A, Martorell M, Martins N, Cho WC. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules. 2019;9(11):679–679. doi: 10.3390/biom9110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darwesh R, Elbialy NS. Iron oxide nanoparticles conjugated curcumin to promote high therapeutic efficacy of curcumin against hepatocellular carcinoma. Inorg Chem Commun. 2021;126:108482–108482. [Google Scholar]
- 58.Shestovskaya MV, Luss AL, Bezborodova OA, Makarov VV, Keskinov AA. Iron oxide nanoparticles in cancer treatment: cell responses and the potency to improve radiosensitivity. Pharmaceutics. 2023;15(10):2406–2406. doi: 10.3390/pharmaceutics15102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daviu N, Portilla Y, Gómez de, Ramírez de, Barber DF. DMSA-coated IONPs trigger oxidative stress, mitochondrial metabolic reprograming and changes in mitochondrial disposition, hindering cell cycle progression of cancer cells. Biomaterials. 2024;304:122409–122409. doi: 10.1016/j.biomaterials.2023.122409. [DOI] [PubMed] [Google Scholar]
- 60.Attri K, Chudasama B, Mahajan RL, Choudhury D. Therapeutic potential of lactoferrin-coated iron oxide nanospheres for targeted hyperthermia in gastric cancer. Sci Rep. 2023;13(1):17875–17875. doi: 10.1038/s41598-023-43725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amatya R, Hwang S, Park T, Min KA, Shin MC. In vitro and in vivo evaluation of PEGylated starch-coated iron oxide nanoparticles for enhanced photothermal cancer therapy. Pharmaceutics. 2021;13(6):871–871. doi: 10.3390/pharmaceutics13060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soetaert F, Korangath P, Serantes D, Fiering S, Ivkov R. Cancer therapy with iron oxide nanoparticles: agents of thermal and immune therapies. Adv Drug Deliv Rev. 2020;163-164:65–83. doi: 10.1016/j.addr.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gambhir RP, Rohiwal SS, Tiwari AP. Multifunctional surface functionalized magnetic iron oxide nanoparticles for biomedical applications: a review. Appl Surf Sci Adv. 2022;11:100303–100303. [Google Scholar]
- 64.Ferreira-Gonçalves T, Gaspar MM, Coelho JMP, Marques V, Viana AS, Ascensão L, Carvalho L, Rodrigues CMP, Ferreira HA, Ferreira D, Reis CP. The role of rosmarinic acid on the bioproduction of gold nanoparticles as part of a photothermal approach for breast cancer treatment. Biomolecules. 2022;12(1):71–71. doi: 10.3390/biom12010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pitchai P, Subramani P, Selvarajan R, Sankar R, Vilwanathan R, Sibanda T. Green synthesis of gold nanoparticles (AuNPs) using Caulerpa racemosa and evaluation of its antibacterial and cytotoxic activity against human lung cancer cell line. Arab J Basic Appl Sci. 2022;29(1):351–362. [Google Scholar]
- 66.Yang Z, Wang D, Zhang C, Liu H, Hao M, Kan S, Liu D, Liu W. The applications of gold nanoparticles in the diagnosis and treatment of gastrointestinal cancer. Front Oncol. 2022;11:819329–819329. doi: 10.3389/fonc.2021.819329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Q, Zhang J, Gao J, Zhang Z, Zhu H, Wang D. Gold nanoparticles in cancer theranostics. Front Bioeng Biotechnol. 2021;9:647905–647905. doi: 10.3389/fbioe.2021.647905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jabeen S, Qureshi R, Munazir M, Maqsood M, Munir M, Shah SSH, Rahim BZ. Application of green synthesized silver nanoparticles in cancer treatment - a critical review. Mater Res Express. 2021;8(9):092001–092001. [Google Scholar]
- 69.Al-Khedhairy AA, Wahab R. Silver nanoparticles: an instantaneous solution for anticancer activity against human liver (HepG2) and breast (MCF-7) cancer cells. Metals. 2022;12(1):148–148. [Google Scholar]
- 70.Shipunova VO, Belova MM, Kotelnikova PA, Shilova ON, Mirkasymov AB, Danilova NV, Komedchikova EN, Popovtzer R, Deyev SM, Nikitin MP. Photothermal therapy with HER2-targeted silver nanoparticles leading to cancer remission. Pharmaceutics. 2022;14(5):1013–1013. doi: 10.3390/pharmaceutics14051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, Liu P, Ma J, Li D, Yang H, Chen W, Jiang Y. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. Int J Nanomedicine. 2019;14:9483–9496. doi: 10.2147/IJN.S224160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Aerle, Lange A, Moorhouse A, Paszkiewicz K, Ball K, Johnston BD, de-Bastos E, Booth T, Tyler CR, Santos EM. Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environ Sci Technol. 2013;47(14):8005–8014. doi: 10.1021/es401758d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, Cano A, Espina M, Ettcheto M, Camins A, Silva AM, Durazzo A, Santini A, Garcia ML, Souto EB. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials (Basel) 2020;10(2):292–292. doi: 10.3390/nano10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat MKF, Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan P, Ding X, Yang YY, Xu QH. Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv Healthc Mater. 2018;7(13):e1701392–e1701392. doi: 10.1002/adhm.201701392. [DOI] [PubMed] [Google Scholar]
- 76.Khane Y, Benouis K, Albukhaty S, Sulaiman GM, Abomughaid MM, Al Ali, Aouf D, Fenniche F, Khane S, Chaibi W, Henni A, Bouras HD, Dizge N. Green synthesis of silver nanoparticles using aqueous Citrus limon zest extract: characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials (Basel) 2022;12(12):2013–2013. doi: 10.3390/nano12122013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kota S, Dumpala P, Anantha RK, Verma MK, Kandepu S. Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Sci Rep. 2017;7(1):11566–11566. doi: 10.1038/s41598-017-11853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ghiuţă I , Cristea D . In: Nanoengineered biomaterials for advanced drug delivery . Mozafari M , et al., editors. Oxford, UK : Elsevier ; 2020 . 15 - Silver nanoparticles for delivery purposes ; pp. 347 – 371 . [Google Scholar]
- 79.Pradhan D, Biswasroy P, Goyal A, Ghosh G, Rath G. Recent advancement in nanotechnology-based drug delivery system against viral infections. AAPS PharmSciTech. 2021;22(1):47–47. doi: 10.1208/s12249-020-01908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su C, Huang K, Li HH, Lu YG, Zheng DL. Antibacterial properties of functionalized gold nanoparticles and their application in oral biology. J Nanomater. 2020;2020:5616379–5616379. [Google Scholar]
- 81.Virgili AH, Laranja DC, Malheiros PS, Pereira MB, Costa TMH, de Menezes. Nanocomposite film with antimicrobial activity based on gold nanoparticles, chitosan and aminopropylsilane. Surf Coat Technol. 2021;415:127086–127086. [Google Scholar]
- 82.Gudkov SV, Burmistrov DE, Serov DA, Rebezov MB, Semenova AA, Lisitsyn AB. Do iron oxide nanoparticles have significant antibacterial properties. Antibiotics (Basel) 2021;10(7):884–884. doi: 10.3390/antibiotics10070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zúñiga-Miranda J, Guerra J, Mueller A, Mayorga-Ramos A, Carrera-Pacheco SE, Barba-Ostria C, Heredia-Moya J, Guamán LP. Iron oxide nanoparticles: green synthesis and their antimicrobial activity. Nanomaterials (Basel) 2023;13(22):2919–2919. doi: 10.3390/nano13222919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suboh SF, Mahat AM, Yusof MH, Abdul Razak. Antimicrobial activity of Strobilanthes crispus leaves aqueous extract and green biosynthesis iron oxide nanoparticles against selected human pathogens. Asia Pac J Mol Biol Biotechnol. 2022;30(4):20–32. [Google Scholar]
- 85.Al-Badr RJ, Al-Huwaizi HF. Antimicrobial evaluation for novel solution of iron oxide nanoparticles functionalized with glycine and coated by chitosan as root canal final irrigation. Syst Rev Pharm. 2020;11(6):633–642. [Google Scholar]
- 86.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y, Xie F, Rao P, Qian H, Chen R, Chen H, Li D, Mu D, Zhang L, Lv P, Shi G, Zheng L, Liu G. TRAIL-expressing cell membrane nanovesicles as an anti-inflammatory platform for rheumatoid arthritis therapy. J Control Release. 2020;320:304–313. doi: 10.1016/j.jconrel.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Zhou Y, Sun Q, Zhou C, Hu S, Lenahan C, Xu W, Deng Y, Li G, Tao S. Update on nanoparticle-based drug delivery system for anti-inflammatory treatment. Front Bioeng Biotechnol. 2021;9:630352–630352. doi: 10.3389/fbioe.2021.630352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naveed M, Batool H, Rehman Su, Javed A, Makhdoom SI, Aziz T, Mohamed AA, Sameeh MY, Alruways MW, Dablool AS, Almalki AA, Alamri AS, Alhomrani M. Characterization and evaluation of the antioxidant, antidiabetic, anti-inflammatory, and cytotoxic activities of silver nanoparticles synthesized using Brachychiton populneus leaf extract. Processes. 2022;10(8):1521–1521. [Google Scholar]
- 91.Singh P, Ahn S, Kang JP, Veronika S, Huo Y, Singh H, Chokkaligam M, El-Agamy Farh, Aceituno VC, Kim YJ, Yang DC. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: a green synthetic approach. Artif Cells Nanomed Biotechnol. 2018;46(8):2022–2032. doi: 10.1080/21691401.2017.1408117. [DOI] [PubMed] [Google Scholar]
- 92.Tyavambiza C, Elbagory AM, Madiehe AM, Meyer M, Meyer S. The antimicrobial and anti-inflammatory effects of silver nanoparticles synthesised from Cotyledon orbiculata aqueous extract. Nanomaterials (Basel) 2021;11(5):1343–1343. doi: 10.3390/nano11051343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Y, Guo L, Wang Z, Liu P, Liu X, Ding J, Zhou W. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and re-polarization. Biomaterials. 2021;264:120390–120390. doi: 10.1016/j.biomaterials.2020.120390. [DOI] [PubMed] [Google Scholar]
- 94.Krajewska JB, Długosz O, Sałaga M, Banach M, Fichna J. Silver nanoparticles based on blackcurrant extract show potent anti-inflammatory effect in vitro and in DSS-induced colitis in mice. Int J Pharm. 2020;585:119549–119549. doi: 10.1016/j.ijpharm.2020.119549. [DOI] [PubMed] [Google Scholar]
- 95.Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. 2023;8(1):235–235. doi: 10.1038/s41392-023-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan L, Liu X, Liu WX, Tan XQ, Xiong F, Gu N, Hao W, Gao X, Cao JM. Fe2O3 nanoparticles suppress Kv1.3 channels via affecting the redox activity of Kvβ2 subunit in Jurkat T cells. Nanotechnology. 2015;26(50):505103–505103. doi: 10.1088/0957-4484/26/50/505103. [DOI] [PubMed] [Google Scholar]
- 97.Shabbir MA, Naveed M, Rehman SU, Ain NU, Aziz T, Alharbi M, Alsahammari A, Alasmari AF. Synthesis of iron oxide nanoparticles from Madhuca indica plant extract and assessment of their cytotoxic, antioxidant, anti-inflammatory, and anti-diabetic properties via different nanoinformatics approaches. ACS Omega. 2023;8(37):33358–33366. doi: 10.1021/acsomega.3c02744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pinho RA, Haupenthal DPS, Fauser PE, Thirupathi A, Silveira PCL. Gold nanoparticle-based therapy for muscle inflammation and oxidative stress. J Inflamm Res. 2022;15:3219–3234. doi: 10.2147/JIR.S327292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aili M, Zhou K, Zhan J, Zheng H, Luo F. Anti-inflammatory role of gold nanoparticles in the prevention and treatment of Alzheimer’s disease. J Mater Chem B. 2023;11(36):8605–8621. doi: 10.1039/d3tb01023f. [DOI] [PubMed] [Google Scholar]
- 100.Dhandapani S, Wang R, Hwang KC, Kim H, Kim YJ. Exploring the potential anti-inflammatory effect of biosynthesized gold nanoparticles using Isodon excisus leaf tissue in human keratinocytes. Arab J Chem. 2023;16(10):105113–105113. [Google Scholar]
- 101.Xu XY, Tran THM, Perumalsamy H, Sanjeevram D, Kim YJ. Biosynthetic gold nanoparticles of Hibiscus syriacus L. callus potentiates anti-inflammation efficacy via an autophagy-dependent mechanism. Mater Sci Eng C Mater Biol Appl. 2021;124:112035–112035. doi: 10.1016/j.msec.2021.112035. [DOI] [PubMed] [Google Scholar]
- 102.Uchiyama MK, Deda DK, Rodrigues SF, Drewes CC, Bolonheis SM, Kiyohara PK, Toledo SP, Colli W, Araki K, Farsky SH. In vivo and in vitro toxicity and anti-inflammatory properties of gold nanoparticle bioconjugates to the vascular system. Toxicol Sci. 2014;142(2):497–507. doi: 10.1093/toxsci/kfu202. [DOI] [PubMed] [Google Scholar]


