Summary
Intrinsically photosensitive retinal ganglion cells (ipRGCs) play a crucial role in several physiological light responses. In this study, we generate an improved Opn4cre knockin allele (Opn4cre(DSO)), which faithfully reproduces endogenous Opn4 expression and improves compatibility with widely used reporters. We evaluated the efficacy and sensitivity of Opn4cre(DSO) for labeling in retina and brain and provide an in-depth comparison with the extensively utilized Opn4cre(Saha) line. Through this characterization, Opn4cre(DSO) demonstrated higher specificity in labeling ipRGCs with minimal recombination escape. Leveraging a combination of electrophysiological, molecular, and morphological analyses, we confirmed its sensitivity in detecting all ipRGC types (M1–M6) and defined their unique topographical distribution across the retina. In the brain, the Opn4cre(DSO) line labels ipRGC projections with minimal labeling of cell bodies. Overall, the Opn4cre(DSO) mouse line represents an improved tool for studying ipRGC function and distribution, offering a means to selectively target these cells to study light-regulated behaviors and physiology.
Keywords: retina, ipRGC, recombinase, mouse
Graphical abstract

Highlights
-
•
An improved Opn4cre knockin mouse line better captures endogenous expression patterns
-
•
Opn4cre(DSO) is compatible with sensitive Cre-dependent reporter lines (Ai9 and Ai14)
-
•
IpRGC distributions are easily defined within the retina using the Opn4cre(DSO) line
-
•
Opn4cre(DSO) has limited off-target recombination within the central nervous system
Motivation
There is a critical need for more specific genetic tools to label intrinsically photosensitive retinal ganglion cells (ipRGCs) that express melanopsin. Our aim was to design a Cre recombinase mouse line that was compatible with sensitive Cre-dependent reporters and to use this genetic tool to assess the local distribution of ipRGCs within the retina.
Photosensitive retinal ganglion cells play important roles in light-regulated physiology. Dyer et al. develop an Opn4cre(DSO) mouse line that displays minimal off-target recombination with sensitive reporter lines and use it to uncover the distribution of melanopsin-expressing cells in the retina and brain.
Introduction
Intrinsically photosensitive retinal ganglion cells (ipRGCs) represent a diverse class of retinal output neuron with 6 types (M1–M6) classified by melanopsin content, dendritic stratification, gene expression, and electrophysiological response characteristics.1,2 A key shared feature of these cells is their expression of melanopsin (encoded by Opn4) and thus their ability to autonomously detect light that causes the cell to depolarize.1 While some types, like the M1–M3 ipRGCs, display strong photocurrents and express sufficient photopigment to be detected by typical antibody staining approaches,3 M4–M6 have limited melanopsin immunoreactivity and correspondingly weak photocurrents.4 Functionally, ipRGCs convey photic information to diverse brain regions, serving as a major conduit for the effects of light on circadian entrainment,5,6,7 the pupillary light response,8,9 mood,10,11 sleep,12 learning,10,11 and pattern vision.13,14
The discovery of ipRGCs and their functions has been driven by the development of genetic tools that express recombinases (Cre),14 toxins (DTA and attnDTA),9,15 or reporter genes (GFP, tauLacZ, and tdTomato) from the Opn4 locus.6,15,16 However, due to variable expression of melanopsin within each ipRGC type, these lines vary considerably in their specificity and selectivity. For example, Opn4tauLacZ primarily labels M1 ipRGCs due to their high expression of melanopsin,6 while the Opn4-GFP transgenic line tends to label types with higher melanopsin content.16 The most sensitive of these methods, labeling all ipRGC types, utilizes Cre recombinase expressed from the Opn4 locus (Opn4cre(Saha)) in conjunction with a Cre-dependent reporter, and it has provided insight into ipRGCs diversity.14 Additionally, when coupled to loss-of-function alleles (floxed genes) or Cre-dependent intersectional ablation lines (Pou4f2zDTA), the use of Opn4cre(Saha) has uncovered several type-specific and functional roles for ipRGCs in light-regulated behavior and physiology11,17,18,19
When crossed with sensitive Cre-dependent reporters (Ai9 and Ai14),20 Opn4cre(Saha) labels mostly non-ipRGC cell types within the retina and across the brain.21 Conversely, when crossed to less sensitive reporters such as Z/EG, in addition to recombination in outer retinal photoreceptors,14 it has been reported that ∼30% of melanopsin immunoreactive cells are not labeled,21,22 suggesting the specificity and sensitivity of this line are highly reporter dependent. One explanation for this may be the design of the Opn4cre(Saha) line (Figure 1A). In this line, Cre recombinase was knocked into the Opn4 locus, replacing most of the gene, and a human beta globin intron was incorporated upstream of the Cre coding sequence. This was likely motivated by the desire for robust expression and efficient deletion of floxed alleles in weakly expressing cells such as M4–M6 ipRGCs.4 However, given that only a few molecules of Cre recombinase may be sufficient to achieve recombination in the genome,23 this additional regulatory element could drive off-target effects depending on the type of reporter.
Figure 1.
The design of Opn4cre alleles
(A) Opn4 gene structure in the mouse (top). Design of the Opn4cre(DSO) line (middle). Design of the Opn4cre(Saha) line (bottom). In this allele, the majority of the Opn4 gene is deleted and replaced with a Cre cassette comprising an upstream human beta globin intron (HBBi) and a nuclear localization signal (NLS). UTR, untranslated region.
(B) Melanopsin immunostaining in whole mount retina of heterozygous (left) and homozygous (right) Cre mice for both recombinase lines. Animal age = postnatal day 30 (P30).
In this study, we describe and characterize an improved knockin Opn4cre line (termed Opn4cre(DSO)) where Cre is knocked in immediately downstream of the Opn4 start codon, leading to disruption of the gene but preservation of the downstream gene structure. We compared this line to Opn4cre(Saha) by crossing them to widely used reporters and assessing their labeling efficiency within the retina and brain. We find that Opn4cre(DSO) specifically labels ipRGCs within the retina and has low recombination escape for M1–M3 ipRGCs. Additionally, using a combination of electrophysiology, molecular markers, and morphology, we find that this line is sensitive, capable of detecting all ipRGC types previously described (M1–M6). Using Opn4cre(DSO), we describe the topographical distribution of these types across the retina from adult mice, showing the M5 and M6 ipRGCs are spatially biased toward the central-ventronasal retina, unlike their M1–M4 counterparts.24,25,26 In addition, we catalog the labeled cells within the brain using both Cre lines. We find that Opn4cre(Saha) labels multiple brain regions at high density, while Opn4cre(DSO) shows low-density sporadic activity with no specific pattern. Finally, using a Cre-dependent reporter with larger inter-loxP distances (mTmG) but identical genomic accessibility to Ai14, we find that both Opn4cre lines have reduced efficiency of labeling ipRGCs. These data define the characteristics of Opn4cre(Saha) and Opn4cre(DSO) and inform decisions about their use.
Results
Opn4cre(DSO) mouse line generation
To generate a mouse line with specific expression of Cre from the Opn4 locus, we employed a CRISPR-Cas9 targeting approach in C57BL/6J embryos (STAR Methods) that introduced a cut upstream of the Opn4 start codon. A co-injected long single-stranded DNA (ssDNA) containing the coding sequence of Cre recombinase, the SV40 polyA element, and homology arms to the 5′ UTR and exon 1 served as a donor. This design places expression of Cre under the control of the endogenous Opn4 promoter. In this design, the downstream coding sequence is disrupted by a frameshift, but the gene elements (exons 1–9 and introns) remain unchanged (Figure 1A). Using this approach, we generated a single founder male that was used to propagate the Opn4cre(DSO) genetic line. Breeding the Opn4cre(DSO) allele to homozygosity led to lack of detectable melanopsin in the retina, similar to results obtained from the Opn4cre(Saha) line (Figure 1B). Thus, it is anticipated that mice carrying this Cre allele will display hetero- and homozygous phenotypes observed in Opn4 loss-of-function animals.
Opn4cre ipRGC efficiency, sensitivity, and off-target labeling when crossed to a sensitive reporter
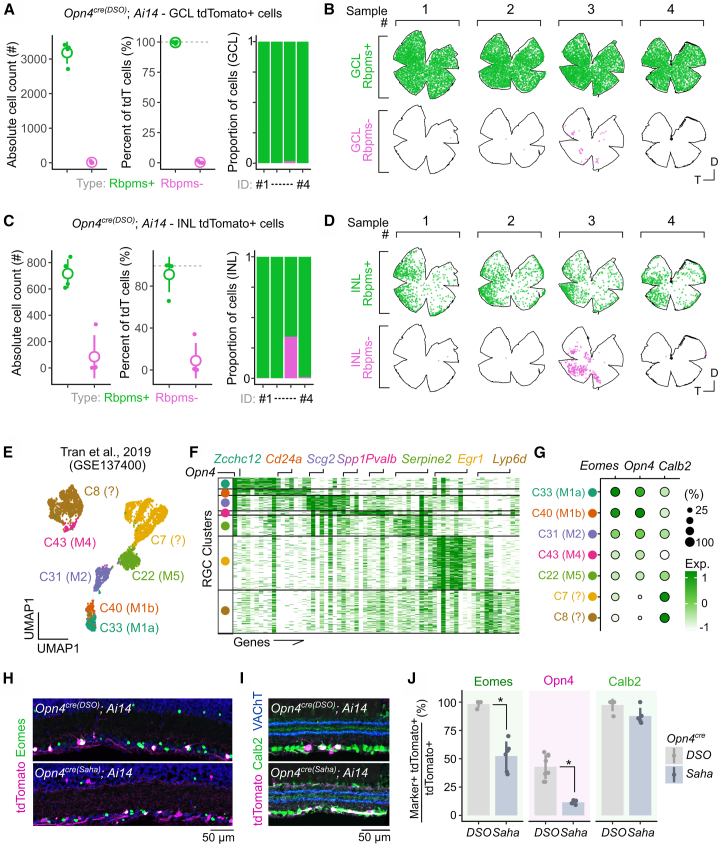
To compare the targeting efficiencies of both Opn4cre lines, we crossed them to Ai14 mice,20 one of the most widely used Cre-dependent reporters. This line harbors a loxP-3xSTOP-polyA-loxP cassette upstream of a tdTomato fluorescent reporter located in the highly accessible ROSA26 locus. Upon Cre-mediated excision of the stop cassette, strong tdTomato expression is driven by an upstream CAG promoter. From these crosses, we analyzed expression patterns of tdTomato within the inner retina of whole-mount samples while simultaneously immunolabeling ipRGCs for Opn4 (melanopsin). When crossed to Ai14, the Opn4cre(DSO) line expressed tdTomato primarily in the ganglion cell layer (GCL; 3,264 ± 170 cells, n = 8) and within the inner nuclear layer (INL; 800 ± 47 cells, n = 4), where Opn4-expressing ipRGCs reside (Figure 2A). In comparison, tdTomato+ cells were far more abundant in the Opn4cre(Saha); Ai14 retina, with 10,737 ± 148 cells in the GCL (n = 4) and 1,795 ± 186 cells within the INL (n = 2) (Figure 2C). In contrast, it was previously reported that only ∼2,000 cells per retina were labeled in the Opn4cre(Saha); Z/EG mouse line.14,21
Figure 2.
ipRGC labeling efficiency within the inner retina of each Opn4cre line
(A) Representative flat-mount retina from Opn4cre(DSO); Ai14 mice.
(B) Reconstruction of flat-mount retina in (A) colored by combination of tdTomato expression (Ai14, magenta) and Opn4 co-expression (green) in both the GCL and INL.
(C and D) Representative flat-mount and reconstructed retina from Opn4cre(Saha); Ai14 presented across the GCL and INL as in (A) and (B).
(E–G) Analysis of Cre-driven recombination across both Cre lines crossed to Ai14. (E) Absolute cell counts of tdTomato+ Opn4+ cells in the GCL (DSOn = 8; Sahan = 4 animals) with gray box depicting min and max range of published and dashed line representing mean of published counts. (F) Same as (E) but comparing tdTomato+ Opn4− cells in the GCL; gray box and line are mirrored from (E) (∗∗p < 0.01, Wilcoxon signed-rank test). (G) Percentage of Opn4+ cells that are tdTomato− in the GCL. GCL, ganglion cell layer; INL, inner nuclear layer. D, dorsal; N, nasal; V, ventral; T, temporal. Small dots, individual samples (n); large circles, group means; error bars, standard deviation. Animal age = postnatal day 60 (P60).
To assess the efficiency of Cre-mediated recombination, we categorized cells into 3 groups following tdTomato and Opn4 labeling.: (1) those that have recombined Ai14 to express tdTomato and express Opn4, reflecting M1–M3 types (tdTomato+ Opn4+), (2) cells that have recombined Ai14 but lack Opn4 immunoreactivity (tdTomato+ Opn4−), comprising the M4–M6 types and/or off-target labeling, and (3) cells that express Opn4 protein but had not recombined Ai14 (tdTomato− Opn4+) (Figures 2B and 2D). Across the GCL, we found a large disparity in the tdTomato+ Opn4− population between the two Opn4cre lines (DSO: 1,904 ± 217 and Saha: 9,494 ± 215 cells) (Figure 2F), but we found similar numbers of tdTomato+ Opn4+ cells (DSO: 1,360 ± 68 and Saha: 1,243 ± 109 cells) and cells that evaded recombination (DSO: 14 ± 2 and Saha: 9 ± 2 cells). In both lines, double-positive cells were within the range of previously published counts for Opn4-immunoreactive ipRGCs (Figure 2E),21,25,27,28,29,30 with ∼1% or less that escaped (Figure 2G). These data argue that both lines, even with differing strategies for driving Cre expression, are efficient at recombining a sensitive reporter in Opn4+ cells with minimal escape rates.
Given the nearly 5-fold difference in tdTomato+ Opn4− cells between the Opn4cre lines, we sought to better understand whether the abundance in Opn4cre(Saha) cells reflected higher sensitivity or off-target labeling. To this end, we assessed tdTomato+ cell distributions in retinal cross-sections, which allowed for a more detailed, layer-specific analysis of labeled cells (Figures S1A and S1B). As observed in whole-mount analysis, Opn4cre(DSO) retina contained fewer tdTomato+ cells in both the GCL and INL. We also found that Opn4cre(DSO) lacked any cell labeling in the outer nuclear layer (ONL), where rod and cone photoreceptors reside (Figures S1A and S1B). By contrast, Opn4cre(Saha) produced abundant cell labeling in the ONL, with the majority of labeled cells within the retina (312.6 ± 44.3 cells mm−2). Recombination in the outer retina is not an artifact of Ai14, as the Opn4cre(Saha); Z/EG line also shows abundant labeling in outer retinal photoreceptors.14 Given that there is limited data to suggest rod and cone photoreceptors express melanopsin, and there is no evidence of outer photoreceptor labeling in the Opn4cre(DSO) line, it is likely this reflects line-specific off-target labeling.
Molecular fingerprinting of Opn4cre-labeled cells
Melanopsin expression is thought to be restricted to RGCs, which are located primarily in the GCL with a small fraction displaced in the INL.5,22,31 Given that 11.6% (Saha) to 41.6% (DSO) of tdTomato cells express Opn4, we set out to determine whether the remaining inner retinal cells represent RGCs or other cell types. To this end, we stained retina for Rbpms, a selective marker of RGCs,32 and determined, in both Opn4cre lines, the extent of colocalization with tdTomato. In the Opn4cre(DSO) line, nearly all tdTomato+ cells were RGCs across the GCL and INL (Figures S1C and S1D). By contrast, in the Opn4cre(Saha) line, 73.8% of tdTomato+ cells in the GCL and 5.6% in the INL appear to be RGCs (Figures S1C and S1D). Considering that >10,000 cells were labeled within the GCL of the Opn4cre(Saha) line (Figure 2), ∼2,800 cells are likely to be non-RGCs.
Due to low density of cells in cross-section, we also performed Rbpms immunolabeling in flat mounts from Opn4cre(DSO); Ai14 mice. Whole-mount analysis of tdTomato/Rbpms overlap across the inner retina (Figures 3A–3D) revealed that nearly all cells across animals were RGCs (tdTomato+ Rbpms+ = 99.6% ± 0.37%, n = 4). Spatially, and consistent with previous reports, INL-localized ipRGCs appeared to be enriched in the periphery of the temporal retina28 and the ciliary marginal zone of the nasal retina30 (Figure 3D, INL tdTomato+ Rbpms+ = 91.1% ± 8.48%, n = 4). With the exception of one example of sporadic and spatially restricted recombination within the INL (Figures 3B and 3D; sample #3, n = 1/18 animals assessed), these results suggest this Opn4cre(DSO) specifically marks RGCs, consistent with the known expression patterns of Opn4 and spatial distribution of the ipRGC population.
Figure 3.
Molecular characterization of cells labeled in the Opn4cre(DSO) mouse line
(A) Colocalization analysis of Rbpms in tdTomato+ cells across the GCL in the Opn4cre(DSO) line as absolute counts (left), percent of tdTomato+ cells (middle), and individual sample proportion plots (right) (n = 4 animals).
(B) Representative spatial analysis of Rbpms and tdTomato colocalized cells in the GCL.
(C) Same samples and analysis as in (A) but within the INL.
(D) Same samples and analysis as in (B) but within the INL. (D and E) Reanalysis of Tran and Shekhar et al. (2019) to identify shared and unique genes between ipRGC (C22–C43) and putatively ipRGC (C7 and C8) clusters. (D) 2D UMAP embedding of clusters from Tran and Shekhar et al. (2019). (E) Heatmap depiction of significantly differentially expressed genes (DEGs) between each cluster, ordered by cells as rows and genes as columns.
(F) Dotplot of shared ipRGC genes Eomes, Opn4, and Calb2. Expression scale bar is identical between (E) and (F).
(G–I) Validation and comparison of marker expression in tdTomato+ cells from both Cre lines crossed to Ai14. (G and H) Representative confocal images of Eomes staining (G) and Calb2 staining (H). (I) Comparison of tdTomato+ colocalization with different markers between both Cre lines (EomesDSO vs Saha∗p < 0.05; Opn4DSO vs Saha ∗p < 0.05; Calb2DSO vs Sahap > 0.05; Wilcoxon signed-rank test with FDR correction). Bar height, mean; error bars, standard deviation. Animal age = postnatal day 60 (P60).
We sought more specific markers of ipRGCs to determine the specificity of each line and reanalyzed an existing single-cell RNA-seq atlas of RGC types in the adult mouse33 (Figures 3E and 3F). In this analysis, we included clusters C33 (M1a), C40 (M1b), C31 (M2), C43 (M4), C22 (M5), C7 (novel), and C8 (novel). These RGC clusters share expression of the transcription factor Eomes, which is expressed in all ipRGC types and displaced amacrine cells.34 The novel clusters, C7 and C8, lack Opn4 expression and, though considered Eomes+ RGCs, express the least amount of Eomes (Figures 3G and S2A). Thus, it is currently unclear if they represent ipRGCs (M1–M6) or whether they represent transcriptionally related non-ipRGC types.34 Assessment of general and type-specific markers of ipRGCs surveyed from the literature revealed the original cluster assignments to be consistent with known markers of each ipRGC type (Figure S2A)22,28,35 but also revealed that cluster C7 likely represents M6s that have been shown to express Cdh3 and Tbx20.22
Using this dataset, we found consistent and shared expression of Eomes and Calb2 in ipRGCs (Calb2 encodes calretinin, which is expressed broadly by most RGCs and types of amacrine cells36). We labeled retina from Opn4cre(DSO); Ai14 and Opn4cre(Saha); Ai14 against Eomes (Figure 3H) and calretinin (Calb2, Figure 3I). Almost all labeled cells in the Opn4cre(DSO) line expressed Eomes (98.2% ± 1.8% Eomes+). This compared with 52.4% ± 5.2% overlap in the Opn4cre(Saha) line (Figure 3J). Calretinin co-labeling did not vary between different lines (Figure 3I). Together, these data suggest that when crossed to Ai14, the Opn4cre(DSO) line primarily labels ipRGCs, while Opn4cre(Saha) labeling also includes a distribution of different RGC and amacrine types in the inner retina. Leveraging the increased specificity of the Opn4cre(DSO) line, we validated that M1 ipRGCs strongly and specifically express Prkcd (protein kinase C delta), which was detected in previous bulk transcriptomic analyses of ipRGCs22 and is one of the top differentially expressed M1 ipRGC genes in the reanalyzed scRNA-seq dataset (Figures S2A–S2E). We labeled retina of Opn4cre(DSO); Ai14 against Prkcd and found ∼25% of ipRGCs co-labeled (Figure S2D). These cells exclusively stratify their dendrites in S1 of the inner plexiform layer (S1) suggesting they represent M1 type. Thus, coupling prior transcriptomic analysis with the Opn4cre(DSO) line can be used to uncover markers of ipRGCs.
Consistent with the specificity of each mouse line, analysis of tdTomato+ dendrite stratification in the inner plexiform layer (IPL) revealed spatially restricted signal in the Opn4cre(DSO) line that was highest at the inner (S5) and outer (S1) boundaries of the IPL, where ipRGC dendrites stratify (Figures S3A–S3C).37 In contrast, dendritic signals were much more diffuse in the Opn4cre(Saha) line, consistent with previous reports, with a notable peak at the outer boundary of the IPL (S1) (Figures S3D–S3F). Taken together, the Opn4cre(DSO) line specifically labels ipRGCs within the inner retina.
Electrophysiological profiling of RGCs in the Opn4cre(DSO) line
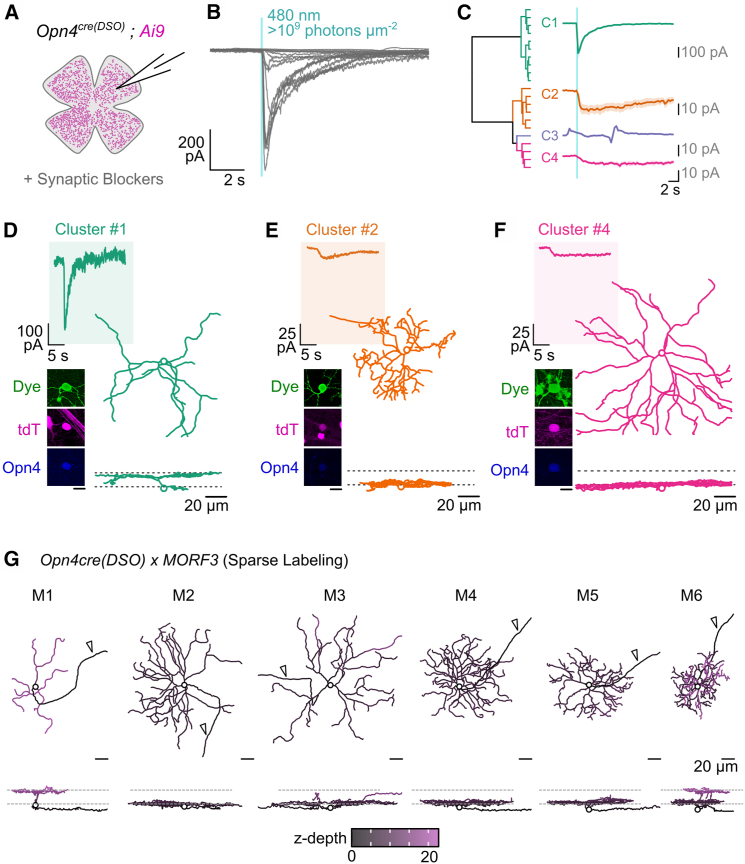
A hallmark of ipRGCs is their light-evoked intrinsic photocurrent in the absence of synaptic input from rod and cone pathways.5,16 Though intrinsic responses to light vary in both amplitude and kinetics,4 all ipRGCs respond to a full-field flash of light with an inward current, even if their melanopsin content is extremely low.4 Given that the Opn4cre(DSO) line primarily labels ipRGCs based on molecular analyses, we assessed their intrinsic responses to a full-field flash of 480-nm light (Figures 4A and 4B). Whole-cell voltage-clamp recordings revealed photocurrents in 95.2% of cells (20/21 tdTomato+) patched in the Opn4cre(DSO) line crossed to Ai9 (a Cre-dependent reporter nearly identical to Ai14). Peak inward photocurrents varied from 10 to 500 pA, consistent with intrinsic responses from M1 to M6 ipRGCs.3,13,38,39,40,41 Due to the heterogeneity of responses, we clustered photocurrents based on their temporal properties. We identified 3 responsive clusters (C1, -2, and -4) and a cluster that contained a non-responsive cell, which appeared to have current fluctuations prior to stimulus onset (C3) (Figure 4C). Cluster C1 represents cells with the strongest photocurrents (average peak inward current = 367.8 pA), while clusters C2 and C4 had substantially weaker intrinsic responses to light (peak inward current, C2 = 36.6 pA; C4 = 11.4 pA). A subset of cells was dye filled following recordings, allowing us to reconstruct morphologies and match them to their intrinsic responses (Figures S4A–S4C). A cell that mapped to cluster C1 (peak inward current of 339.8 pA) had sparsely branched dendrites stratified deep in the IPL, consistent with the response and morphology of M1s (Figure 4D).3,5,8,27 Cells that mapped to C2 (Figure 4E) and C4 (Figure 4F) had weaker photocurrents and had more branched monostratified dendrites localized to the inner boundary of the IPL (S5). These features suggest these cells may represent the M526,40,41 (C2), and M23,27 (C4) type. Thus, we find a wide range of intrinsic photocurrents and morphological types in the Opn4cre(DSO) line, consistent with existing data describing ipRGCs.
Figure 4.
Electrophysiological and morphological assessment of ipRGCs in the Opn4cre(DSO) line
(A) Schematic depicting whole-cell recording of Opn4cre(DSO); Ai9 cells from the retina.
(B) Current traces of all recorded cells held at −60mV and given a 100-ms full-field flash of >9 log photons μm−2 sec−1; cyan region, light pulse.
(C) Hierarchical clustering dendrogram and cluster averaged responses to light; bold line, mean response; shaded region, standard error.
(D–F) Representative responses, confocal images, and 3D morphological reconstructions of cells from cluster C1 (D), C2 (E), and C4 (F). (G) Representative 3D reconstructions of ipRGC types from Opn4cre(DSO); MORF3 retina (n = 5) with XY (top) and XZ profile (bottom) with z-depth coding. S, seconds. Arrowhead, axon. Animal age = P60 (A–F); P30 (G).
Morphological diversity of ipRGCs in the Opn4cre(DSO) line
The finding that majority of cells in the Opn4cre(DSO) line lack melanopsin immunoreactivity but still represent ipRGCs due to their expression of Rbpms and Eomes (Figure 2), coupled with their diverse electrophysiological responses and morphologies, prompted a more robust characterization of ipRGC diversity. M1–M6 ipRGCs are readily distinguished morphologically as they vary in their dendritic stratification, branching, and soma size.2,4
However, to unequivocally assign cells to specific types requires reconstructing their full dendritic arbors with minimal overlap from other cells in close proximity. Thus, to genetically and sparsely label ipRGCs, we crossed the Opn4cre(DSO) line to MORF3 mice.42 This Cre-dependent reporter utilizes mononucleotide repeat frameshift (MORF) as a switch for sparse labeling. For cells to express smV5 (spaghetti monster V5) epitopes, two events must take place: cells must express Cre to excise a loxP-flanked STOP cassette (as in Ai14) and must have undergone a spontaneous frameshift mutation in the polycytosine (C22) MORF switch upstream of V5. In the cells that these events do occur, V5 is detectable via antibody labeling and can be coupled with other markers.42 Our prior work using this method estimated ∼3% of ipRGCs were labeled in this approach,43 and thus we implemented a similar strategy to study the range of ipRGC types targeted by our line.
We surveyed 356 cells (n = 5 animals) and performed 3D reconstruction on a subset of neurons with representative morphologies (Figure 4G). We found that cells labeled in the Opn4cre(DSO) line match the morphological characteristics of all known ipRGC types, with non-ipRGCs representing only 5 amacrine cells in this assessment (all labeled in the same retina).
-
•
M1 types that laminate their dendrites in S1 (outer boundary of the IPL) had sparsely branching monostratified dendritic trees3,5,6,8,27 (10 reconstructed; 84 surveyed).
-
•
M2 types had more branched dendritic trees than the M1s that laminated in S5, close to their somas3,27 (5 reconstructed; 54 surveyed).
-
•
M3 types were rare, but the few we found had similar branching densities to the M2 but stratified its dendrites in both S1 and S527,38 (3 reconstructed; 10 surveyed).
-
•
M4 types, like M2s, were monostratified in S5 but had the largest somas of cells in this line, were more branched, and expressed the highest V5 content based on immunofluorescence13,26,39 (14 reconstructed; 96 surveyed).
-
•
M5 types were monostratified in S5 with dense, small dendritic fields with small somas14,26,40 (8 reconstructed; 60 surveyed).
-
•
M6, like the M5, had small dendritic fields and somas but were bistratified (S1 and S5), occasionally containing recurrent dendrites that returned to S5 after branching in S141 (6 reconstructed; 41 surveyed).
Topographical distributions of ipRGC types revealed by the Opn4cre(DSO) line
Nonuniformities in the spatial distribution of retinal ganglion cell types serve to enhance encoding of specific visual features.24,44 Examples include the lateral positioning of ipsilaterally projecting RGCs that allow for binocularity, to the heightened RGC densities of the primate fovea and area centralis of carnivores that enhance high-resolution vision.44,45 Such spatial anisotropies are not limited to image-forming RGC types, as many ipRGCs display spatial enrichments. The pSON-projecting M1 subtype is nearly exclusively located in the dorsal retina,46 while the remainder of the M1 population is evenly distributed across the dorsoventral axis but is enriched temporally.30,46 Similarly, the M2 type is thought to be enriched dorsally,25 while the M4 (also known as Alpha ON-sustained RGC) has the highest density in the temporal retina.24,26 However, it remains unclear if the other ipRGCs types display nonuniform distributions in retinal space. Previous work that first described the M6 type used the Cdh3-GFP mouse line that shows dynamic expression over development and strain,41,47 and thus, it cannot provide much insight into spatial distributions of the M6 ipRGC. Given that the Opn4cre(DSO) line specifically labels ipRGCs, including the weakly melanopsin-expressing M5 and M6 types (Figure 4), we set out to understand whether each type is nonuniformly distributed through the retina.
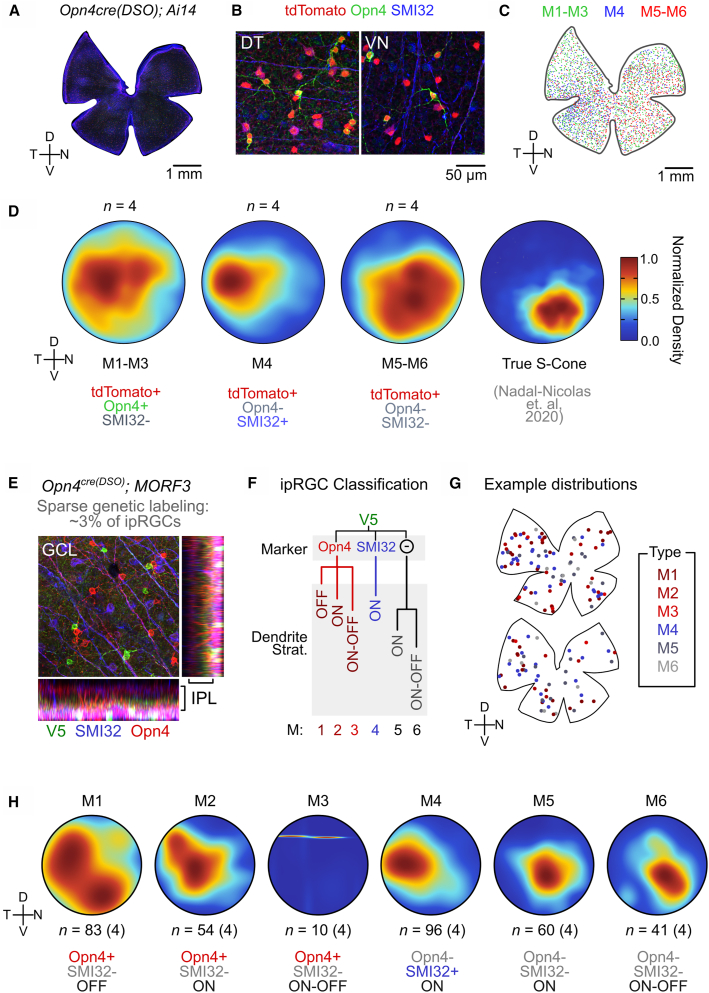
We first used an approach that coarsely defined ipRGC types in Opn4cre(DSO); Ai14 retina (Figures 5A–5C). Immunostaining of tdTomato+ cells for melanopsin (Opn4) reveals the M1–M3 types, while reactivity to SMI32 (an antibody generated against the non-phosphorylated form of neurofilament heavy chain) selectively marks M4s.13,26 The remaining cells that are defined by lack of melanopsin or SMI32 staining are considered the M5 and M6 types. As a quality control step, we surveyed the entire retina (GCL, INL, and ONL) for any off-target labeling that might lead to a false positive M5/6 assignment. We then assessed the spatial positions of each soma for three broad classes (M1–M3, M4, and M5–M6), with a representative flat mount highlighted in Figure 5C. To eliminate the spatial distortions introduced by relaxing cuts on the retina made during preparation, we reconstructed retina into a standard, polar retinal space. Beyond limiting cut-site distortions, this approach allows for a registered space to compare spatial distributions of several ipRGC and photoreceptor populations.
Figure 5.
Spatial distribution of ipRGC types in the Opn4cre(DSO) mouse line
(A) Flat-mount representative image of Opn4cre(DSO); Ai14 stained against tdTomato (red), Opn4 (green), and SMI32 (blue).
(B) Representative magnification of fields in the dorsotemporal (DT) and ventronasal (VN) locations highlighting labeling diversity.
(C) Same flat-mount retina in (A) but with computed labels for each coarse ipRGC type: M1–M3 (tdTomato+ Opn4+ SMI32−), M4 (tdTomato+ Opn4- SMI32−), and M5–M6 (tdTomato+, Opn4−, SMI32−).
(D) Normalized 2D density heatmaps from polar reconstructed retina, averaged across n = 4 per coarse ipRGC type, and analysis of data from Nadal-Nicolas et al. (2020) depicting true S-cone distributions.
(E) Representative high-magnification confocal stack of sparse labeling and multiple markers of ipRGCs.
(F) Decision tree to define each ipRGC type using a combination of markers and dendritic stratification.
(G) Representative flat-mount retinae with spatial locations of each ipRGC type used in this analysis.
(H) Similar to (D) but for each ipRGC type (total number of cells n is located below each polar plot) averaged across number of animals shown in parentheses. GCL, ganglion cell layer; IPL, inner plexiform layer; D, dorsal; N, nasal; V, ventral; T, temporal. Animal age = postnatal day 60 (P60).
Using this approach, we generated polar density maps (Figure 5D) that represent average spatial densities of each coarse ipRGC type from n = 4 animals (each polar plot can be found in Figure S5A). Consistent with previous work, we found a broad distribution of M1–M3 ipRGCs, with a slight emphasis in the temporal retina.46 Additionally, and similar to previous work on M4/Alpha ON-sustained RGCs, we find these cells in a strong nasal-to-temporal gradient, with highest density in the temporal retina.24,26 Surprisingly, we find that M5 and M6 ipRGCs, while more broadly distributed than M4s, increase in density in the ventronasal retina (Figure 5D). We performed a similar analysis on published spatial coordinates for true S-cones48 and find that the M5–M6 population is more spatially correlated to true S-cones than to the M1–M3 or M4 types (Figure S5B).
To more comprehensively define the spatial distributions of ipRGCs, we performed similar spatial analyses on sparsely labeled cells from the Opn4cre(DSO); MORF3 line. To categorize each V5-labeled ipRGC type, we used a combination of molecular markers (Figure 5E) coupled with dendritic stratification and morphology (Figures 5E and 5F). Thus, ipRGC typing had 3 criteria: Opn4 expression (+/−), SMI32 labeling (+/−), and dendritic stratification (ON, OFF, ON-OFF) (see Figure S5C for example types and reconstructions). In total, we surveyed 344 cells from n = 4 flattened retinae (Figure 5G) and analyzed their densities in polar space averaged across animals (Figure 5H). Consistent with our findings using Ai14 and markers (Figures 5A–5D), we find the following.
-
•
M1s are broadly distributed with a higher temporal density (83/344 cells).
-
•
M2s are more restricted to the dorsal retina with a higher temporal density (56/344 cells).
-
•
M3s are extremely rare (10/344 cells), and thus, we could not conclude whether they are nonuniformly distributed.
-
•
M4s are strongly temporally enriched (96/344 cells), a finding identical to using tdTomato and SMI32 as markers in Figures 5A–5D.
-
•
M5s and M6s appear to be enriched in the central-to-ventronasal retina (M5: 60/344, M6: 41/344).
Thus, from these experiments, we conclude that not only are all ipRGC types labeled in the Opn4cre(DSO) line, but the majority of types are nonuniformly distributed within the retina.
ipRGC projections and Opn4cre labeling in the brain
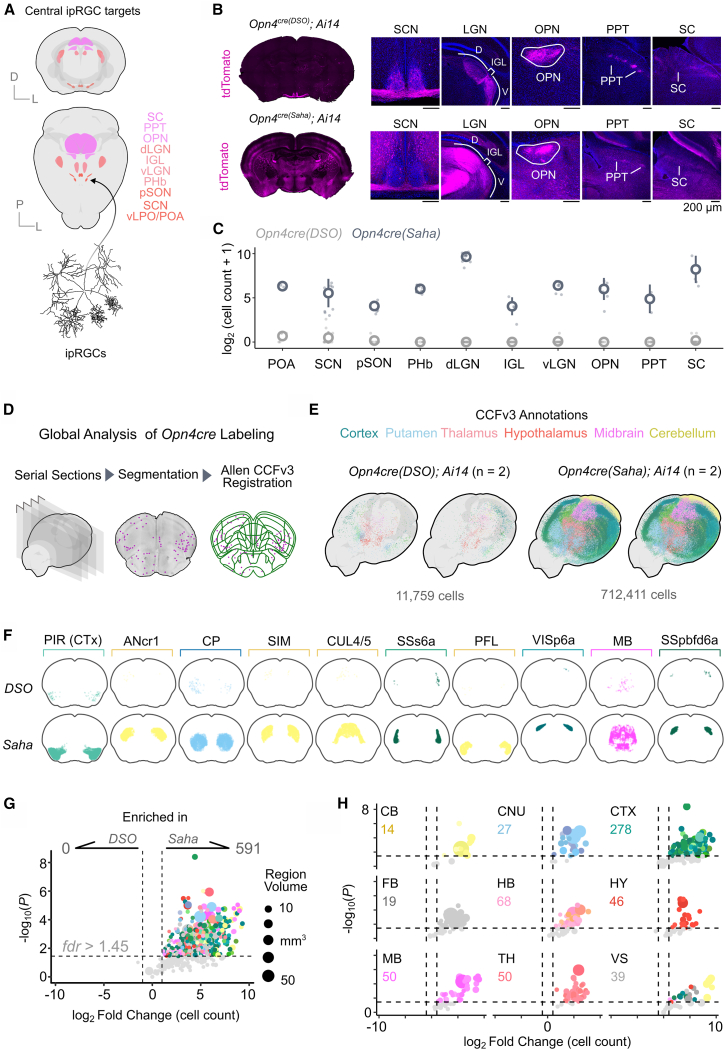
ipRGCs collectively project to several targets in the brain, the majority of which are involved in non-image-forming vision (ventrolateral preoptic area, suprachiasmatic nucleus, peri-supraoptic nucleus, peri-habenula, intergeniculate leaflet, ventral lateral geniculate nucleus, intergeniculate leaflet, olivary pretectal nucleus , and posterior pretectal nucleus), with innervation of image-forming nuclei as well (dorsal lateral geniculate nucleus and superior colliculus)49,50 (Figure 6A). Previous work has shown that when crossed to Ai14, Opn4cre(Saha) labels several regions of the brain, including the targets of ipRGC innervation, the cortex, thalamic groups, and other nuclei.14,21 While it is possible that Opn4 could be expressed by several brain regions either developmentally or in the adult, given the already described off-target effects of this Cre line in the retina, we reasoned that further analysis of Cre labeling between lines was warranted.
Figure 6.
A brain-wide analysis of labeled cells reveals divergent labeling across Cre lines
(A) Volumetric projections of ipRGC targets in the Allen Brain Atlas CCFv3.
(B) Representative sections and major ipRGC targets in the brain from Opn4cre(DSO); Ai14 (top) and Opn4cre(Saha); Ai14 (bottom) lines.
(C) Quantification of absolute cell count, displayed as log2(cell count + 1), for ipRGC targets between lines.
(D) Schematization of global cell labeling pipeline used in (E)–(H).
(E) Whole-brain point clouds of cells in both lines crossed to Ai14, colored by CCFv3 annotations.
(F) Comparison between 10 regions with highest cell counts in the Opn4cre(Saha) line.
(G) Volcano plot depicting differences in cell count per region with annotated cells. Numbers at the top depict the number of regions in the CCFv3 with significant enrichment between the lines.
(H) Same data as in (G) but segregated by 9 parent structures. Abbreviations are as follows: POA, preoptic area; SCN, suprachiasmatic nucleus; pSON, peri-supraoptic nucleus; PHb, peri-habenula; dLGN, dorsal lateral geniculate nucleus; IGL, intergeniculate leaflet; vLGN, ventral lateral geniculate nucleus; OPN, olivary pretectal nucleus; PPT, posterior pretectal nucleus; SC, superior colliculus; PIR, piriform cortex; ANcr1, crus 1; CP, caudoputamen; SIM, simple lobule; CUL4/5, lobule IV-V; SSs6a, supplemental somatosensory area layer 6a; PFL, paraflocculus; VISp6a, primary visual area layer 6a; MB, midbrain; SSpbfd6a, somatosensory barrel field associated area layer 6a; CB, cerebellum; CNU, caudoputamen; CTX, cortex; FB, fiber tracts; HB, hindbrain; HY, hypothalamus; MB, midbrain; TH, thalamus; VS, ventricle system. Animal age = postnatal day 60 (P60).
Using Ai14 as a reporter, we assessed labeling in primary targets of ipRGCs across both Cre lines. These analyses revealed strong tdTomato+ fibers in all central ipRGC targets within the Opn4cre(DSO) line, consistent with the line’s specificity in the retina (Figure 6B, top). Comparatively, the Opn4cre(Saha) line displayed major off-target cell body labeling in all targets assessed (Figure 6B, bottom). While many cells were labeled in each central target within this line, the image-forming targets dLGN and SC had the highest number (Figure 6C). By contrast, in the Opn4cre(DSO) line, while a few sporadic cells were detected unilaterally in the SCN (∼5–6 cells), no other cell bodies were located in ipRGC projection targets (Figures 6B and 6C).
To establish a global map of labeling between these two lines, we generated serial sections of brains from 2.5 to −7.5 mm AP (anterior-posterior) across both lines crossed to Ai14, performed automated cell segmentation, and serially registered slices and cell coordinates to the Allen Mouse Brain Atlas using their Closed Coordinate Framework (CCFv3) (Figure 6D). Using this approach, we captured and registered the spatial locations of >750,000 cells, and as such, we only performed this analysis on n = 2 brains from each line (each mouse was from a separate litter). To validate that registration using this approach produced reproducible results, we split the dataset into odd and even serial section series and independently registered them to the CCFv3 (Figure S6A), which showed high region-specific correlation, regardless of mouse line (Figure S6B).
Using this approach, we identified cells in over 600 unique regions of the mouse brain, totaling ∼710,000 in the Opn4cre(Saha) line. In comparison, 11,800 cells in total were detected in the Opn4cre(DSO), representing ∼1.6% of the cells detected in the Saha line. In the Opn4cre(DSO) line, cells appeared to be more enriched in the ventral brain, but they appeared unilaterally, suggesting random recombination (Figures 6E and S6D). This is in contrast to the global distribution of cells in the Opn4cre(Saha) line, which were found in all 7 parent brain structures within the CCFv3 (Figure S6C). As a comparison, we computed the regions with the top 10 cell counts within the Opn4cre(Saha) line, and a comparison between lines is presented in Figure 6F. Regions with the highest cell counts were within the cerebellum: crus 1 (2nd), simple lobule (4th), lobule IV–V (5th), and paraflocculus (7th), followed by cortical structures: piriform cortex (1st), supplemental somatosensory area layer 6a (6th), primary visual area layer 6a (8th), and supplemental somatosensory barrel field associated layer 6a (10th). The caudoputamen (1/10, 3rd) and midbrain (1/10, 9th) were large structures that contained several thousand cells. In comparison, the Opn4cre(DSO) line has minimal labeling in each of these regions, with bilateral asymmetries suggesting random recombination events (Figure 6F, top row). Across 638 regions with cell bodies surveyed, 591 were significantly enriched in the Opn4cre(Saha) line over the Opn4cre(DSO) line when crossed to Ai14 (Figure 6G). Almost half of these regions represented cortical areas (278/591), followed by hindbrain structures (68/591), midbrain (50/591), thalamus (50/591), hypothalamus (46/591), ventricular system (39/591), fiber tracts (19/591), and lastly cerebellum (14/591). No brain regions showed significant enrichment in the Opn4cre(DSO) line when tested against the Opn4cre(Saha) line. Assessment of cell bodies across the same volume from multiple litters of Opn4cre(DSO); Ai14 mice showed no consistent pattern of cell labeling, suggesting that Opn4 expression is highly restricted to RGCs in the retina (data not shown).
Altering inter-loxP distances shifts recombination efficiency of Opn4cre lines
The recombination efficiency of Cre lines depends on several factors including Cre expression level, genomic accessibility at the loxP-flanked locus, and the inter-loxP distance.23,51,52 The Ai9 and Ai14 series of reporters are sensitive because their CAG promoter-driven expression of tdTomato is within the highly accessible ROSA26 locus.20 With this genomic context, we can observe stark differences in recombination between both Opn4cre lines in both the retina and brain.
When Opn4cre(Saha) is crossed to Z/EG or Z/AP, as done for most analysis using this line, labeling is more specific within the retina than compared with Ai9 or Ai14, but the sensitivity of Cre labeling is diminished (∼30% Opn4+ cells escape recombination).21,22 Even with this enhanced specificity, alternative cell types remain labeled in the retina (rods and cones) and in the brain (Figure S4 in Ecker et al., 2010).14 These differences in labeling likely occur for 2 major reasons. The first is that the Z/EG and Z/AP alleles are both within regions of unknown accessibility in the genome.53,54 Both lines show differences in the cell types that they label under ubiquitous Cre drivers, suggesting the local genomic architecture can modify expression of their reporters.53 The second is that both transgenic lines require recombination of a cassette with large inter-loxP distances (>4.5kb), containing a lacZ-pgkNeoR-polyA sequence between loxP sites.53 The combination of these variables likely means reduced efficiency of even strong Cre drivers.
We set out to determine how altering components of a reporter system would influence recombination efficiencies in both Opn4cre lines. To this end, we utilized the mTmG line that contains a loxP-mTomato-polyA-loxP (∼2.4kb) upstream of an mGFP coding sequence (Figure S7A). While this has a larger inter-loxP distance than Ai14 (∼0.9kb), it has identical accessibility as it is in the ROSA26 locus (Figure S7A). We crossed Opn4cre(DSO) and Opn4cre(Saha) mice to the mTmG line, labeled retina for mGFP and Opn4 as in the Ai14 experiments (Figure 2), and assessed recombination efficiency. We performed these experiments on P12 mice, as M4s express higher levels of melanopsin during development (Figure S7B).55 We characterized all Opn4+ and mGFP+ cells and their overlap, categorizing them into (1) cells that escaped recombination (Opn4+ mGFP−), (2) cells that were accurately targeted (Opn4+ mGFP+), and non-Opn4 expressing cells (Opn4− mGFP+), which could be ipRGCs and/or off-target labeling (Figures S7C and S7D).
We found that, in contrast to our experiments with Ai14, the Opn4cre(DSO) line displayed significant recombination escape (Opn4+ mGFP−; 63.6% ± 6.8%), while the remaining cells were appropriately targeted (Opn4+ mGFP+; 35.0% ± 6.8%), and almost no cells contained mGFP only (Opn4-mGFP+; 1.36% ± 2.4%; Figure S7E). In contrast, in the Opn4cre(Saha) line, only 22.75 ± 10.1% of cells escaped recombination (Opn4+ mGFP−), while 58.7% ± 11.4% expressed both Opn4 and mGFP, and the remaining 18.6% ± 8.3% of cells expressed mGFP only (Figure S7E). Thus, labeling in both Opn4cre lines is influenced by increasing inter-loxP site distances of floxed constructs, even at a highly accessible locus.
Discussion
In this study, we generated an improved Opn4cre knockin mouse line (Opn4cre(DSO)) and provide an in-depth comparison between it and the most widely used ipRGC-targeting Cre line (Opn4cre(Saha)). By placing Cre immediately downstream of the Opn4 start codon and using the native Opn4 promoter to drive recombinase expression, we found that ipRGC photoreceptors within the retina can be efficiently and selectively labeled when crossed to widely used, chromatin accessible Cre-dependent reporters (Ai9, Ai14, and MORF3; Figures 2, 3, 4, 5, and 6). Owing to the enhanced specificity of the Opn4cre(DSO) line, we uncovered the spatial distributions of M1–M6 ipRGCs using both molecular markers and sparse labeling, and we found that the M2, M4, M5, and M6 types are nonuniformly distributed across the retina. In contrast, we confirmed prior reports that the Opn4cre(Saha) line labels ipRGCs, but these cells represent a small minority of the total labeled cells across the central nervous system. It is likely that the distinct design of these two alleles leads to vastly different labeling within the eye and brain.
Retinal Cre efficiency, off-target cells, and molecular markers of ipRGCs
Our assessment of both Cre lines in the retina revealed two important findings. The first is that both Cre lines, even with differing regulatory elements, are similarly efficient at labeling ipRGCs expressing detectable melanopsin protein when crossed to sensitive reporters and assessed in the adult (∼99%, Figure 2). This is surprising given that widespread recombination in non-ipRGC types occurs in the Opn4cre(Saha) line, suggesting a negligible gain in sensitivity by boosting Cre expression coupled to sensitive reporters. Secondly, while it has been noted that the Opn4cre(Saha) line labels non-ipRGCs in the outer retina, it is apparent that it labels other types of RGCs and some amacrine cells in the inner retina as well (Figure S1). This would explain why most cells lack an intrinsic photocurrent in Opn4cre(Saha); Ai14 mice.21 Importantly, this does not appear to be restricted to crosses with sensitive reporters. The original paper detailing the Opn4cre(Saha) line reported that ∼10% of recorded cells lacked an intrinsic photocurrent. This may be attributable to recombination of Z/EG in non-ipRGCs or non-RGCs within the inner retina.14
Given the variable expression of melanopsin between ipRGC types, it is difficult to estimate the true sensitivity of either Cre line. For this, we must be able to independently label each type of cell and describe the overlap between them and Cre-dependent reporters. Based on previously reported counts of Opn4+ cells from various reports, both lines appear to be sensitive enough to label the entire population (Figures 2A–2F).25,27,28,29,30 However, it is unclear how sensitive they are to label or recombine loxP-flanked cassettes in M4–M6s. Surveying the entire retina for M4s using the Opn4cre(DSO); Ai14 (tdTomato+ SMI32+, Figures 5A–5C), we found 722 ± 58 cells, representing ∼1.44% of all RGCs similar to the reported ∼840 Alpha ON-sustained cells per retina.56 However, with no alternative means to label the M5 and M6 population comprehensively and independently of Opn4cre(DSO), it remains unclear if this, or any line, captures the majority of these low-melanopsin-expressing populations. Using transcriptome data coupled with the Opn4cre(DSO) mouse line, we provide an example of how these two modalities could be used together to uncover markers of ipRGCs (Prkcd in M1s), which can be further expanded to identify selective genes expressed in the M1–M6 types (Figure 3F; see STAR Methods).
Central-to-ventronasal enrichment of M5 and M6 types
Even without metrics of absolute sensitivity, the utility of this improved Cre line is clear: even with low expression of melanopsin, it is capable of labeling M5s and M6s as a group (Figures 5A–5C) or individually and stochastically in sufficient quantities for spatial analysis (Figure 5G). We used this feature to study the nonuniform spatial distribution of each ipRGC type to find that both the M5 and M6 type are spatially enriched in the ventronasal retina. This pattern is reminiscent of developmental Cdh3-GFP labeling in the retina, a marker known to label both M5 and M6 ipRGCs.41,47 We also show empirically that their spatial density patterns strongly correlate with the true S-cones48 that are enriched in the ventronasal retina and likely serve as the outer sensory basis of M5 color opponency (UV-ON, green-OFF).40 Similarly, type 9 bipolar cells (or S-cone bipolar cells), which receive input from true S-cones and provide input to M5s, are enriched in a similar spatial gradient.40,48,57 Together, these data highlight several components of a microcircuit displaying spatial anisotropies within the retina.
In contrast, the M6 type shares spatial enrichment with both true S-cones and M5s but does not appear to encode chromatic information.41 This spatial enrichment is observed in lines such as the Cdh3-GFP and in experiments tracing RGCs projecting to the olivary pretectal nucleus, which is dominated by M6s.58 One potential explanation for this consistent spatial pattern but lack of color opponency may be the differences in bipolar inputs to M6 (type 2 and 9o) compared to M5.59 Further characterization of the microcircuits and optimal responses of M6s will be necessary to understand why they are localized to the ventronasal retina but encode different visual features than their M5 counterparts.
A case for selective expression of Opn4 in the central nervous system
Photopigments are expressed in cells beyond the neural retina and the eye.60 For example, Opn3 and Opn5 are distributed in tissues outside the eye and have had reported functions within the brain.61,62,63 Expression of Opn4 across the brain would be consistent with other photopigment expression patterns and potential function. However, given that the Opn4cre(Saha) line labels many regions, including those that are targets of the ipRGCs themselves, it is hard to accurately determine whether any bona fide expression of Opn4 takes place elsewhere in the central nervous system.
Using the Opn4cre(DSO) line, we found limited evidence for expression within the brain. While we see sporadic, often unilateral recombination of cells in different locations, this is never consistent. This likely represents a feature of the sensitivity of Ai14 as a reporter, rather than an underlying expression pattern of Opn4. Similar features have been seen with other photopigment Cre lines in the brain, with uni-hemispheric labeling in regions unrelated to normal expression.61 While our whole-brain analyses focused on brain volumes between 2.5 mm and 7.5 mm (AP), and could have limited sampling beyond these bounds, we never encountered fiber tracts that terminate within the volumes assessed. This suggests that at least no long-range projecting neuron types express Opn4. We did not, however, survey the rest of the organism for this study. Other groups have reported functions of melanopsin in the trigeminal nerve,64 iris sphincter muscle,65 vascular relaxation,66 and adipocyte physiology,67 and further work will be necessary to determine whether these tissues are labeled by the Opn4cre(DSO) line.
Implications of Cre use for conditional knockout experiments
While we performed an in-depth analysis of labeling using various reporter lines, the conclusions of these experiments have implications that reach beyond tagging cells for visualization. The Opn4cre(Saha) line likely drives strong Cre expression but at the apparent expense of specificity. This is observed across sensitive reporters such as Ai9 and Ai14, moderately sensitive reporters such as mTmG, and fairly insensitive reporters like Z/EG. Importantly, this may have an impact on experiments assessing function and behavior that utilize Opn4cre(Saha) as a driver to abolish gene expression conditionally (Slc17a6flox and Gad1/2flox)18,68 or drive suicide gene expression (Pou4f2zDTA).17 Given that both genomic accessibility and inter-loxP distances contribute to Cre recombination efficiency,23,69,70 experimental work using this line must assess off-target recombination of floxed cassettes across the eye and brain.
We argue that experiments utilizing the Opn4cre(Saha) line to abolish gene expression should assess the efficiency of deletion in the retina with methods such as RNA-scope and immunofluorescence but also characterize recombination across the eye and brain. As typical reporters and floxed alleles of genes are differentially accessible and vary in their inter-loxP distances, a reporter cannot substitute for determining if gene expression has been specifically abolished and if off-target events have occurred.71 While it is possible that the current Cre-dependent reporters oversample due to their ease of genomic access and small inter-loxP distances, certain widely used lines such as Slc17a6 (encoding Vglut2) and Gad2 (GAD65) have flox lines with short inter-loxP distances: floxed exon 1 of Slc17a6flox is <0.5 kb72 and floxed exon 1 of Gad2flox is <0.1 kb.73 Both genes are highly accessible in excitatory and inhibitory neurons, due to the very nature of the gene product, and as such will be susceptible to off-target recombination. Given that over 591 regions of the brain were labeled in the Opn4cre(Saha) line, investigators should consider the potential for confounding effects in their experiments.
Conversely, while our line is more specific with Cre-dependent reporters, because of the variable and low expression of the Opn4 locus, recombination efficiency may be limited for genes with large inter-loxP site distances or in regions of the genome with lower accessibility. Thus, for conditional loss-of-function experiments, we recommend assessing ipRGCs for successful recombination using in situ transcript detection or immunohistological approaches to verify that generalized loss-of-function has taken place. Moving forward as a field, we must continue to explore methods to genetically access ipRGC types using gene drivers that are both highly expressed and cell type specific, which would allow us to circumvent using the variably expressed Opn4 locus to interrogate ipRGC function in the eye and in behavior.
Limitations of the study
While this study comprehensively characterizes the Opn4cre(DSO) mouse line when crossed to sensitive Cre-dependent reporters that utilize the same recombination cassette (typical of the Ai series), we uncovered variable recombination with larger and less sensitive reporter types (mTmG). Thus, investigators will need to confirm the specificity and sensitivity of each Opn4 Cre-driver line, especially if the recombination cassette (or floxed allele) is large (>2 kb). This is also the case for virally delivered constructs that typically implement “double-floxed inverted open-reading frames.”
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-tdTomato (1:1000) | Sicgen | Cat#AB8181-200, polyclonal goat, RRID: AB_2722750 |
| anti-Melanopsin N15 (1:10000) | A gift from Iggy Provencio | N/A |
| anti-Melanopsin N38 (1:1000) | Advanced Targeting Systems | Cat #AB-N39, RRID:AB_1608076 |
| anti-Rbpms (1:500) | Abcam | Cat #ab152101, RRID:AB_2923082 |
| anti-VAChT (1:750) | Synaptic Systems | Cat #139103, RRID:AB_887864 |
| anti-Tbr2/Eomes (1:500) | Abcam | Cat #ab183991, RRID:AB_2721040 |
| anti-Calb2 (1:500) | Abcam | Cat #ab277631, RRID: N/A |
| anti-V5 (1:2000) | Novus Biological | Cat #NB600-379, RRID: AB_10003214 |
| SMI32 (1:500) | Enzo LifeScience | Cat #ENZ-ABS219, RRID: N/A |
| anti-GFP (1:1500) | Novus Biological | Cat #NB100-1614, RRID: AB_10001164 |
| anti-Prkcd (1:500) | Abcam | Cat #ab182126, RRID:AB_2892154 |
| Deposited data | ||
| Integrated ipRGC scRNA-Seq Seurat Object | This paper | Mendeley: https://doi.org/10.17632/36tpcd3ykb.1 |
| Differentially expressed genes between ipRGC clusters | This paper | Mendeley: https://doi.org/10.17632/36tpcd3ykb.1 |
| Tran et al., 2019 scRNA-Seq of adult RGCs | Tran et al., 201933 | https://singlecell.broadinstitute.org/single_cell/study/SCP509/mouse-retinal-ganglion-cell-adult-atlas-and-optic-nerve-crush-time-series |
| Experimental models: Organisms/strains | ||
| Opn4cre(DSO) | This study | N/A |
| Opn4cre (Opn4tm1(cre)Saha/J) | JAX | #035925 |
| Ai14 (Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) | JAX | #007914 |
| Ai9 (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) | JAX | #007909 |
| MORF3 (Gt(ROSA)26Sortm3(CAG-sfGFP∗)Xwy/J) | JAX | #035403 |
| mTmG (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) | JAX | #007676 |
| Software and algorithms | ||
| R v4.1.2 | https://www.r-project.org/ | N/A |
| Cellpose2.0 | Stringer et al., 202174 | N/A |
| Fiji (ImageJ1, v1.53t) | NIH | N/A |
| MATLAB 2021b | Mathworks | N/A |
Resource availability
Lead contact
Further information and requests for data and/or mouse lines should be directed to and will be fulfilled by the lead contact, Dr. Shane Peter D’Souza (shane.dsouza@cchmc.org).
Materials availability
The Opn4cre(DSO) mouse line is available upon request from the lead contact.
Data and code availability
-
•
Reanalysis of the ipRGC and ipRGC-proximal clusters from Tran et al., 2019 have been deposited and are available through Mendeley (https://doi.org/10.17632/36tpcd3ykb.1).
-
•
This manuscript did not generate any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Mice
Mouse line generation
The Opn4cre(DSO) mouse line was generated in-house at the Cincinnati Children’s Hospital Transgenic Core using CRISPR-Cas9 targeting and long single-strand DNA (ssDNA) knock-in technology. A single guide RNA (gRNA) targeting the region between the 5′ UTR and Exon 1 of the mouse Opn4 locus (mm10; cut site Chr14:34,599,959) was generated and validated in ES cells for cutting efficiency. A long ssDNA donor containing 5′ and 3′ homology arms (∼370 bp), followed by Cre recombinase, and SV40 polyA signal was synthesized to generate Opn4cre(DSO) knock-in mice. Pronuclear injections of gRNA, long ssDNA donor, and Cas9 were performed on fertilized C57BL/6J (JAX #000664) eggs. From this, 1 male was generated with the appropriate insertion which was confirmed via insertion-specific PCR validation and subsequently targeted sequencing of the insert.
gRNA sequence (PAM): 5′-CTGAAGGAGAGTCCATGCTC AGG-3’
Mouse lines
Mice between the ages of P30-P60 (both sexes) were used in this study, except for P12 mice in Figure S7 which is specified in text and figure. All animals were housed and maintained in a pathogen-free vivarium in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Cincinnati Children’s Hospital Medical Center and Washington State University. Other genetically modified mouse lines used in this include: Opn4cre(Saha) (Opn4tm1(cre)Saha/J; #035925), Ai9 (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; #007909), Ai14 (Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; #007914), MORF3 (Gt(ROSA)26Sortm3(CAG-sfGFP∗)Xwy/J; #035403), and mTmG (Gt(ROSA)26Sortm4(ACTB-Tomato,-EGFP)Luo/YgchJ; #037456). All mouse lines were backcrossed to C57BL/6J (#000664) at least 7 generations before experimental crosses were performed and data were acquired.
Methods details
Histology and tissue processing
Tissue processing
Retinae and brains were processed as previously described. Briefly, whole eyes were fixed in 4% paraformaldehyde (PFA) for 40 min at room temperature, followed by a PBS wash. Retinae were dissected out and cryoprotected (for sectioning) or processed for whole mount immunofluorescence. Brains were processed similarly, with fixation in 4% PFA overnight at 4C, followed by 2x washes with PBS and serial dehydration/cryoprotection. Brains were serially sectioned at 40 μm thickness, adhered directly to SuperFrost charged slides, and stored at −20C until analysis.
Histology and immunofluorescence
Retina and brain were permeabilized in 0.5% Triton X-100 in PBS (PBST), followed by blocking in 10% normal donkey serum diluted in PBST. Sections were incubated in primary antibodies (see table below) overnight, with the exception of wholemount retina, which were stained on an orbital rocker at 4°C for 2–3 days. Following primary incubation, tissue sections and wholemounts were washed up to 6 times with PBS, followed by re-permeabilization with 0.5% PBST, and a 1-h incubation with secondary antibodies (1:1000 dilution) at room temperature. Following 6+ washes with PBS, tissue sections and whole retina were cover-slipped and imaged the same day.
Primary and secondary antibodies
The following primary antibodies were used in this study: anti-tdTomato (SICGEN, #AB8181, RRID:AB_2722750), anti-Melanopsin N15 (a gift from Ignacio Provencio), anti-Melanopsin (Advanced Targeting Systems, #AB-N39, RRID:AB_1608076), anti-Rbpms (abcam, #ab152101, RRID:AB_2923082), anti-VAChT (Synaptic Systems, #139103, RRID:AB_887864), anti-Tbr2/Eomes (abcam, #ab183991, RRID:AB_2721040), anti-Calb2 (abcam, #ab277631, RRID: N/A), anti-V5 (Novus Biological, #NB600-379, RRID: AB_10003214), SMI32 (Enzo LifeScience, #ENZ-ABS219, RRID: N/A), anti-GFP (Novus, #NB100-1614, RRID: AB_10001164). The following secondary antibodies were used (Jackson ImmunoResearch): Donkey anti-rabbit 488 (#711-545-152) & 647 (#711-605-152), Donkey anti-chicken 488 (#703-545-155), Donkey anti-mouse 647 (#715-605-151), and Donkey anti-sheep 594 (#713-585-147).
Cell counting and spatial analyses
When possible, automated, and validated methods were employed to count and annotate cell positions on images (cellpose, see below). However, certain experiments with low signal-to-noise or multiple markers that did not mark the same cellular domain (tdTomato, SMI32, melanopsin) required manual annotation as downstream analytical methods could not reliably distinguish different populations computationally.
Manual cell counting and annotation
For experiments in Figure 3 & Figure 5, cells were manually annotated by importing confocal images into FIJI, followed by annotation using the CellCounter plugin. Subsequently, an XML file corresponding to the spatial coordinates and a csv file corresponding to the features (x, y, Area, etc.) of the annotated cell were exported and used from downstream analysis or further processing (density, etc.).
Automated cell segmentation
To segment cell boundaries for downstream analysis, we utilized cellpose, a convolutional neural network trained on a variety of cellular bioimage data. For tdTomato cell segmentation, we utilized a pretrained model (cyto) with parameters cell_diameter = 12.5, cellprob_threshold = −1.0 and flow_threshold = 0.4. For Opn4, we used an alternative pretrained model (cyto2) with default parameters. Each image was inspected following automated segmentation for errors and dropouts, subsequently corrected in the built-in GUI, and exported as a text file with polygon coordinates for each cell.
Cell type classification following automated segmentation
To determine if cells overlapped in marker expression, as performed in Figure 2, we used a spatial nearest neighbor approach with stringent criteria. First we imported polygons as (x,y) coordinates for each marker (tdTomato, Melanopsin) per image. Then we computed the centroid position of each cell in 2D space using their polygons. To determine if masks overlapped and thus represent double-positive cells (tdTomato+ Opn4+), we computed the nearest neighbor distances between different markers and assigned masks to be shared between cells if their centroid distances were less than 4 pixels (nndtdTomato → nndOpn4 < 4). Using this method, we estimated the total number of tdTomato+ Opn4+ cells, tdTomato+ Opn4-cells, and tdTomato- Opn4+ cells for each retina analyzed. We confirmed that this method accurately captures overlapping and non-overlapping masks by employing two different quality control measures: (1) for a random sampling of retina we manually annotated after running this analysis pipeline, and independently assessed cell type assignments projected onto each retina and found nearly identical results. (2) Following automated type assignment, we visually inspected each sample with its cognate image to be sure that masks were not spuriously assigned to each group.
Single cell transcriptome mining
Single cell transcriptomes of atlas RGCs (Tran et al., 2019) were downloaded from the Gene Expression Omnibus (GEO) under GSE137400. We performed standard preprocessing by including only genes expressed in >3 cells and cells with >200 expressed genes. Following this general preprocessing, we next performed integration using the Seurat v4 pipeline to eliminate batch effects. For this, we split the experiment by their sample id (aRGC1-9) and batch (1–3), scaled and transformed the data using the SCTransform() function while regressing mitochondrial genes. Next, we generated anchor features using SelectIntegrationFeatures() using 3000 variable features. The data was then integrated, using the default parameters, and preprocessed using a standard Seurat v4 workflow. Next, we subset the ipRGCs (and related clusters) using corresponding metadata from the original paper and performed dimensionality reduction (using the first 15 PCs) and UMAP embedding (for visualization) and differential gene expression analysis to define markers of ipRGC types.
Electrophysiology and morphology
Methods for path-clamp recordings from ipRGCs were similar to methods previously described.75 Following at least 1 h of dark adaptation, mice were euthanized by cervical dislocation after isoflurane anesthesia. Eyes were enucleated under dim red light, and the cornea was slit to remove the lens, followed by retina extraction into oxygenated, bicarbonate-buffered Ames media (US Biologicals), 3 mM kynurenic acid (Sigma), 50 μM DL-AP4 (Tocris), and 50 μM (+)-bicuculline (Sigma). The retinas were then cut into quarters, mounted on a nitrocellulose filter with a hole providing access to the retinal ganglion cell layer, which were stored in the same solution in the dark at room temperature. Retinas could be maintained for more than 4 h under these conditions before recording.
Patch-clamp recordings
Whole-mounted retinas on nitrocellulose were transferred to the recording chamber, where they were maintained in bubbled Ames’ media (35C) flowing at 2.5 mL min−1. Retinal ganglion cells were visualized with infrared Dodt gradient contrast optics through an inverted Olympus microscope (BX51WI) using a 40× water-immersion objective, a Nikon DS-QiMc camera, and NIS Elements Advanced Research Imaging software (v3.20; Nikon). The microscope was mounted on a Sutter X-Y translation stage; electrode positioning was accomplished via a micromanipulator (MP-285; Sutter). Micropipettes were fabricated from filamented borosilicate glass with resistances of 5–6 MOhm when filled with a potassium-based internal solution containing (in mM) 125 K-gluconate, 2 CaCl2, 2 MgCl2, 10 EGTA, 10 K-HEPES, 0.5 Na2-GTP, 2 Mg-ATP and 0.3% Neurobiotin (pH 7.2). Synaptic blockers, which allowed for the isolation of intrinsic melanopsin-based photocurrents, were added to Ames’ media to the following concentrations: 3 mM kynurenic acid (Sigma), 50 μM DL-AP4 (Tocris), and 50 μM (+)-bicuculline (Sigma; from a 1000X stock in DMSO). ipRGCs were identified by tdTomato fluorescence using a short exposure to 540 nm light (<1s; 1 x 106 photons cm−2 s−1; Lambda DG-4, Sutter Instruments).
For light response experiments, recordings were made in voltage-clamp mode at a holding potential of −60 mV (not corrected for a junction potential of −13 mV). Responses were low-pass filtered at 5Hz post hoc (SigmaPlot) to reduce noise and breakthrough action potentials. Maximal melanopsin-based intrinsic photocurrents were elicited with a 100 ms 480 nm blue-light stimulus delivered from a Lambda DG-4 light source (Sutter Instruments) with an intensity of 2 x 1010 photons um−2 s−1. The intensity of background illumination from the computer monitors throughout these experiments was no greater than 1 x 10−9 W cm−2.
Immunohistochemistry post recording
After patch-clamp recording from an ipRGC, the retina was fixed in 5% paraformaldehyde for 1 h and washed 3 times with 1 x PBS for 20 min before applying immunohistochemistry buffer (0.5% Triton X-1000, 1% BSA, 5% Normal Donkey Serum in PBS). Immunohistochemistry was performed using rabbit anti-Melanopsin (mOpn4)76 and streptavidin-488 (1:1000, Invitrogen). After immunostaining, the retinas were mounted and imaged using a Leica White Light Laser Confocal Microscope (TCS SP8 X).
Single ipRGC reconstructions
High resolution images of cells labeled in the Opn4cre(DSO); Morf3 retina were acquired using a 20× objective and 2x digital zoom (z-step 0.2um) and traced semi-automatically using the Neuroanatomy toolbox (formerly Simple Neurite Tracer; SNT) in ImageJ. Briefly, single channel z-stacks were imported into SNT, a root was set at the center of the soma and using the NBA∗Search tracing algorithm, positions were defined along the cell that branched from the soma root. Cells were continually assessed in both the XZ and YZ planes to ensure no artifacts were introduced during reconstruction. Reconstructed cells were saved as SWC files to plot in XY and XZ dimensions using the natverse library (v1.8.19) in R.
Spatial and polar reconstruction of whole mount retina
Polar reconstruction
To project cells from wholemount images to polar, retinotopic space, we implemented the retistruct package in R (v0.6.3). For these experiments, a deep dorsal cut was made during dissection to identify the dorsal pole and left/right retina were noted and separated for analysis. (x,y) coordinates of cells across the entire retina and the retinal outline were acquired as per Sterratt et al., and retina were reconstructed using ϕ0 = 115° (based on adult mouse ocular properties in Sterratt et al.). The newly reconstructed retina had each cell position (x,y) converted into phi and lambda polar coordinates, which were subsequently saved and used for downstream analysis.
Spatial density analysis
By performing the previous step, we can register cellular coordinates to common reference space and leveraged this to understand the spatial heterogeneities of different ipRGC types. To study the spatial densities of cells, we performed kernel density estimation (KDE) on the 2D polar coordinates using the MASS package in R. For this, we first estimated the joint range of cell type phi and lambda positions, then performed 2D-KDE using the kde2d() function with n = 100 grid points in each direction. The output of this analysis is probability densities for each cell type across a reference retinal grid (as in Figure 5).
Whole brain lineage tracing analysis
Automated cell segmentation
Similar to analysis performed in the retina, to automatically segment cells from serial sections of brain from Opn4cre(DSO); Ai14 and Opn4cre(Saha); Ai14 mice, we utilized cellpose’s cyto model with parameters flow_threshold = 0.4, cellprob_threshold = −1, and cell_diameter = 12.5. Images were batch processed using scripting and directories of text files with polygons corresponding to individual cells’ masks were used for downstream analysis. These masks were then converted to centroid (x,y) coordinates in image-space that required registration to the Allen Mouse Brain Atlas Common Coordinate Framework v3 (CCFv3).
Manual registration of sections
To register sections and their cognate cell positions to the CCFv3, we utilized a MATLAB pipeline developed by the Root lab.77 Briefly, this pipeline allows for individual sections to be registered to the 10 μm atlas in CCFv3 with flexibility in the cutting angle of the section, as the atlas volume can be sliced in 3-dimensions (AP, DV, ML). Using this method, we independently registered odd and even series of brain sections from both mouse lines to validate that this approach yields consistent and reproducible results. After slices and cell coordinates had been transformed, cells are assigned a region-specific label based on their location in atlas-space and were registered spatially to the CCFv3.
Quantification and statistical analysis
Investigator blinding
Investigators were not blinded to the genotypes and sexes of the animals used in this study. However, most analyses comparing cell counts between genetic lines utilized automated approaches that meant limited input from investigators.
IPL lamination of dendrites
To investigate the stratification of Opn4cre-labeled cells in the IPL, we imaged the inner plexiform layer (IPL) at 40x resolution, generated ∼5 55 × 35 μm ROIs centered within the IPL and aligned with the IPL-INL boundary. We then extracted mean intensity values for each channel (tdTomato, VAChT) across the IPL depth (55 μm). Each ROI channel was normalized to their maximum intensity value for downstream analysis and comparison. The analysis included 49 Opn4cre(DSO) ROIs (n = 4 animals) and 46 Opn4cre(Saha) ROIs (n = 4 animals).
Clustering ipRGC photocurrents
To cluster intrinsic photocurrents produced by cells during whole-cell recording, we limited our analysis to time points corresponding to −1 and 30 s from stimulus onset. Additionally, we down-sampled recordings to 50Hz for computational efficiency. Currents were converted to a matrix that was centered to the cell’s mean response and scaled to unit variance prior to clustering. Then, using the hclust() function with nstart = 50, we performed hierarchical clustering on photocurrents. To determine optimal clusters, we used the gap statistics method in the factoextra package (v1.0.7).
Average ipRGC spatial densities and correlation
Following KDE analysis on individual ipRGC types from each retinal sample, given that KDE produces a grid with probability densities, we leveraged this to (1) generate an average map of broad ipRGC type densities (Figure 5) and (2) use this density-coordinate grid to correlate different types of ipRGCs and other photoreceptors (True S-Cones). To generate average density maps, first each sample was scaled by subtracting their minimum spatial density followed by dividing by their new maximum density, transforming the density bounds to (0,1). This was repeated across each cell type per sample and used for subsequent analysis. Similar analyses were performed on sparsely labeled ipRGCs from the Opn4cre(DSO); MORF3 line. True S-Cone spatial distributions (x,y coordinates) were downloaded from Nadal-Nicolás et al., where a variant of polar reconstruction had been performed, and processed through our analysis pipeline. To correlate spatial densities of different cell types, we performed Spearman’s rank correlation between each of the normalized densities per sample. The results from this analysis are visualized as a clustered heatmap in Figure S5.
Statistical analysis
All statistical analyses were performed in R using the rstatix package (v0.7.0). Statistical tests and corresponding sample sizes (n) are noted in the figure legends. For all analyses utilizing multiple comparisons, or multiple independent tests of a hypothesis, p-values were corrected via false discovery rate correction (fdr in text). Sample sizes were not predetermined.
Acknowledgments
We thank Paul Speeg for his excellent colony management. We thank Allie Pendery, MD, for critically reviewing the manuscript. This work was supported by R01EY034456 to R.A.L., R01EY027202 to R.L.B., funds from the Abrahamson Pediatric Eye Institute (CCHMC) to R.A.L., the WSU-CVM Marvel Shields Autzen Fund to R.L.B., and the Albert J. Ryan Fellowship to S.P.D.
Author contributions
S.P.D. conceived and directed the study. B.D. and S.P.D. performed experiments and computational analysis. S.O.Y. and R.L.B. performed patch-clamp and dye-filling experiments. R.A.L. and S.P.D. provided project leadership. S.P.D. wrote the manuscript. B.D., S.O.Y., R.L.B., R.A.L., and S.P.D. edited the manuscript.
Declaration of interests
The authors declare no competing financial or non-financial interests.
Published: August 9, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2024.100837.
Supplemental information
References
- 1.Do M.T.H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron. 2019;104:205–226. doi: 10.1016/j.neuron.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sondereker K.B., Stabio M.E., Renna J.M. Crosstalk: The diversity of melanopsin ganglion cell types has begun to challenge the canonical divide between image-forming and non-image-forming vision. J. Comp. Neurol. 2020;528:2044–2067. doi: 10.1002/cne.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt T.M., Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J. Neurosci. 2009;29:476–482. doi: 10.1523/jneurosci.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aranda M.L., Schmidt T.M. Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cell. Mol. Life Sci. 2021;78:889–907. doi: 10.1007/s00018-020-03641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berson D.M., Dunn F.A., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 6.Hattar S., Liao H.W., Takao M., Berson D.M., Yau K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Göz D., Studholme K., Lappi D.A., Rollag M.D., Provencio I., Morin L.P. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatori M., Le H., Vollmers C., Keding S.R., Tanaka N., Buch T., Waisman A., Schmedt C., Jegla T., Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güler A.D., Ecker J.L., Lall G.S., Haq S., Altimus C.M., Liao H.W., Barnard A.R., Cahill H., Badea T.C., Zhao H., et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeGates T.A., Altimus C.M., Wang H., Lee H.K., Yang S., Zhao H., Kirkwood A., Weber E.T., Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez D.C., Fogerson P.M., Lazzerini Ospri L., Thomsen M.B., Layne R.M., Severin D., Zhan J., Singer J.H., Kirkwood A., Zhao H., et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell. 2018;175:71–84.e18. doi: 10.1016/j.cell.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altimus C.M., Güler A.D., Villa K.L., McNeill D.S., Legates T.A., Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl. Acad. Sci. USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt T.M., Alam N.M., Chen S., Kofuji P., Li W., Prusky G.T., Hattar S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron. 2014;82:781–788. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ecker J.L., Dumitrescu O.N., Wong K.Y., Alam N.M., Chen S.K., LeGates T., Renna J.M., Prusky G.T., Berson D.M., Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew K.S., Renna J.M., McNeill D.S., Fernandez D.C., Keenan W.T., Thomsen M.B., Ecker J.L., Loevinsohn G.S., VanDunk C., Vicarel D.C., et al. A subset of ipRGCs regulates both maturation of the circadian clock and segregation of retinogeniculate projections in mice. Elife. 2017;6 doi: 10.7554/eLife.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt T.M., Taniguchi K., Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J. Neurophysiol. 2008;100:371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S.K., Badea T.C., Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keenan W.T., Rupp A.C., Ross R.A., Somasundaram P., Hiriyanna S., Wu Z., Badea T.C., Robinson P.R., Lowell B.B., Hattar S.S. A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. Elife. 2016;5 doi: 10.7554/eLife.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rupp A.C., Ren M., Altimus C.M., Fernandez D.C., Richardson M., Turek F., Hattar S., Schmidt T.M. Distinct ipRGC subpopulations mediate light's acute and circadian effects on body temperature and sleep. Elife. 2019;8 doi: 10.7554/eLife.44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloney R., Quattrochi L., Yoon J., Souza R., Berson D. Efficacy and specificity of melanopsin reporters for retinal ganglion cells. J. Comp. Neurol. 2024;532 doi: 10.1002/cne.25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg D.J., Kartheiser K., Leyrer M., Saali A., Berson D.M. Transcriptomic Signatures of Postnatal and Adult Intrinsically Photosensitive Ganglion Cells. eNeuro. 2019;6 doi: 10.1523/eneuro.0022-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vooijs M., Jonkers J., Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2:292–297. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleckert A., Schwartz G.W., Turner M.H., Rieke F., Wong R.O.L. Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Curr. Biol. 2014;24:310–315. doi: 10.1016/j.cub.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes S., Watson T.S., Foster R.G., Peirson S.N., Hankins M.W. Nonuniform distribution and spectral tuning of photosensitive retinal ganglion cells of the mouse retina. Curr. Biol. 2013;23:1696–1701. doi: 10.1016/j.cub.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonoda T., Okabe Y., Schmidt T.M. Overlapping morphological and functional properties between M4 and M5 intrinsically photosensitive retinal ganglion cells. J. Comp. Neurol. 2020;528:1028–1040. doi: 10.1002/cne.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berson D.M., Castrucci A.M., Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J. Comp. Neurol. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duda S., Block C.T., Pradhan D.R., Arzhangnia Y., Greschner M., Puller C. Spatial distribution and functional integration of displaced ipRGCs. bioRxiv. 2023 doi: 10.1101/2023.09.05.556383. Preprint at. [DOI] [Google Scholar]
- 29.Jain V., Ravindran E., Dhingra N.K. Differential expression of Brn3 transcription factors in intrinsically photosensitive retinal ganglion cells in mouse. J. Comp. Neurol. 2012;520:742–755. doi: 10.1002/cne.22765. [DOI] [PubMed] [Google Scholar]
- 30.Valiente-Soriano F.J., García-Ayuso D., Ortín-Martínez A., Jiménez-López M., Galindo-Romero C., Villegas-Pérez M.P., Agudo-Barriuso M., Vugler A.A., Vidal-Sanz M. Distribution of melanopsin positive neurons in pigmented and albino mice: evidence for melanopsin interneurons in the mouse retina. Front. Neuroanat. 2014;8:131. doi: 10.3389/fnana.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provencio I., Rodriguez I.R., Jiang G., Hayes W.P., Moreira E.F., Rollag M.D. A novel human opsin in the inner retina. J. Neurosci. 2000;20:600–605. doi: 10.1523/jneurosci.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez A.R., de Sevilla Müller L.P., Brecha N.C. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014;522:1411–1443. doi: 10.1002/cne.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran N.M., Shekhar K., Whitney I.E., Jacobi A., Benhar I., Hong G., Yan W., Adiconis X., Arnold M.E., Lee J.M., et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron. 2019;104:1039–1055.e12. doi: 10.1016/j.neuron.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C.K., Kiyama T., Weber N., Whitaker C.M., Pan P., Badea T.C., Massey S.C., Mao C.A. Characterization of Tbr2-expressing retinal ganglion cells. J. Comp. Neurol. 2021;529:3513–3532. doi: 10.1002/cne.25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pottackal J., Walsh H.L., Rahmani P., Zhang K., Justice N.J., Demb J.B. Photoreceptive Ganglion Cells Drive Circuits for Local Inhibition in the Mouse Retina. J. Neurosci. 2021;41:1489–1504. doi: 10.1523/jneurosci.0674-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E.S., Lee J.Y., Kim G.H., Jeon C.J. Identification of calretinin-expressing retinal ganglion cells projecting to the mouse superior colliculus. Cell Tissue Res. 2019;376:153–163. doi: 10.1007/s00441-018-2964-1. [DOI] [PubMed] [Google Scholar]
- 37.Do M.T.H., Yau K.-W. Intrinsically Photosensitive Retinal Ganglion Cells. Physiol. Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt T.M., Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J. Comp. Neurol. 2011;519:1492–1504. doi: 10.1002/cne.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estevez M.E., Fogerson P.M., Ilardi M.C., Borghuis B.G., Chan E., Weng S., Auferkorte O.N., Demb J.B., Berson D.M. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J. Neurosci. 2012;32:13608–13620. doi: 10.1523/jneurosci.1422-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stabio M.E., Sabbah S., Quattrochi L.E., Ilardi M.C., Fogerson P.M., Leyrer M.L., Kim M.T., Kim I., Schiel M., Renna J.M., et al. The M5 Cell: A Color-Opponent Intrinsically Photosensitive Retinal Ganglion Cell. Neuron. 2018;97:150–163.e4. doi: 10.1016/j.neuron.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quattrochi L.E., Stabio M.E., Kim I., Ilardi M.C., Michelle Fogerson P., Leyrer M.L., Berson D.M. The M6 cell: A small-field bistratified photosensitive retinal ganglion cell. J. Comp. Neurol. 2019;527:297–311. doi: 10.1002/cne.24556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veldman M.B., Park C.S., Eyermann C.M., Zhang J.Y., Zuniga-Sanchez E., Hirano A.A., Daigle T.L., Foster N.N., Zhu M., Langfelder P., et al. Brainwide Genetic Sparse Cell Labeling to Illuminate the Morphology of Neurons and Glia with Cre-Dependent MORF Mice. Neuron. 2020;108:111–127.e6. doi: 10.1016/j.neuron.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Souza S.P., Upton B.A., Eldred K.C., Glass I., Grover K., Ahmed A., Ngyuen M.T., Gamlin P., Lang R.A. Developmental adaptation of rod photoreceptor number via photoreception in melanopsin (OPN4) retinal ganglion cells. bioRxiv. 2023 doi: 10.1101/2023.08.24.554675. Preprint at. [DOI] [Google Scholar]
- 44.Baden T., Euler T., Berens P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 2020;21:5–20. doi: 10.1038/s41583-019-0242-1. [DOI] [PubMed] [Google Scholar]
- 45.Wässle H., Boycott B.B. Functional architecture of the mammalian retina. Physiol. Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 46.Berry M.H., Moldavan M., Garrett T., Meadows M., Cravetchi O., White E., Leffler J., von Gersdorff H., Wright K.M., Allen C.N., Sivyer B. A melanopsin ganglion cell subtype forms a dorsal retinal mosaic projecting to the supraoptic nucleus. Nat. Commun. 2023;14:1492. doi: 10.1038/s41467-023-36955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osterhout J.A., Josten N., Yamada J., Pan F., Wu S.W., Nguyen P.L., Panagiotakos G., Inoue Y.U., Egusa S.F., Volgyi B., et al. Cadherin-6 mediates axon-target matching in a non-image-forming visual circuit. Neuron. 2011;71:632–639. doi: 10.1016/j.neuron.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nadal-Nicolás F.M., Kunze V.P., Ball J.M., Peng B.T., Krishnan A., Zhou G., Dong L., Li W. True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field. Elife. 2020;9 doi: 10.7554/eLife.56840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hattar S., Kumar M., Park A., Tong P., Tung J., Yau K.W., Berson D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beier C., Zhang Z., Yurgel M., Hattar S. Projections of ipRGCs and conventional RGCs to retinorecipient brain nuclei. J. Comp. Neurol. 2021;529:1863–1875. doi: 10.1002/cne.25061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faust T.E., Feinberg P.A., O'Connor C., Kawaguchi R., Chan A., Strasburger H., Frosch M., Boyle M.A., Masuda T., Amann L., et al. A comparative analysis of microglial inducible Cre lines. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bedolla A.M., McKinsey G.L., Ware K., Santander N., Arnold T.D., Luo Y. A comparative evaluation of the strengths and potential caveats of the microglial inducible CreER mouse models. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2023.113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novak A., Guo C., Yang W., Nagy A., Lobe C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 54.Lobe C.G., Koop K.E., Kreppner W., Lomeli H., Gertsenstein M., Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev. Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- 55.Sexton T.J., Bleckert A., Turner M.H., Van Gelder R.N. Type I intrinsically photosensitive retinal ganglion cells of early post-natal development correspond to the M4 subtype. Neural Dev. 2015;10:17. doi: 10.1186/s13064-015-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallego-Ortega A., Norte-Muñoz M., Di Pierdomenico J., Avilés-Trigueros M., de la Villa P., Valiente-Soriano F.J., Vidal-Sanz M. Alpha retinal ganglion cells in pigmented mice retina: number and distribution. Front. Neuroanat. 2022;16 doi: 10.3389/fnana.2022.1054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behrens C., Schubert T., Haverkamp S., Euler T., Berens P. Connectivity map of bipolar cells and photoreceptors in the mouse retina. Elife. 2016;5 doi: 10.7554/eLife.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine J.N., Schwartz G.W. The Olivary Pretectal Nucleus Receives Visual Input of High Spatial Resolution. bioRxiv. 2020 doi: 10.1101/2020.06.23.168054. Preprint at. [DOI] [Google Scholar]
- 59.Sabbah S., Papendorp C., Behrendt I., Rasras H., Cann J., Leyrer M.L., Koplas E., Beltoja M., Etebari C., Gunesch A.N., et al. Intrinsically photosensitive retinal ganglion cells evade temporal filtering to encode environmental light intensity. bioRxiv. 2022 doi: 10.1101/2022.04.09.487733. Preprint at. [DOI] [Google Scholar]
- 60.Andrabi M., Upton B.A., Lang R.A., Vemaraju S. An Expanding Role for Nonvisual Opsins in Extraocular Light Sensing Physiology. Annu. Rev. Vis. Sci. 2023;9:245–267. doi: 10.1146/annurev-vision-100820-094018. [DOI] [PubMed] [Google Scholar]
- 61.Zhang K.X., D'Souza S., Upton B.A., Kernodle S., Vemaraju S., Nayak G., Gaitonde K.D., Holt A.L., Linne C.D., Smith A.N., et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature. 2020;585:420–425. doi: 10.1038/s41586-020-2683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bárez-López S., Bishop P., Searby D., Murphy D., Greenwood M.P. Male rat hypothalamic extraretinal photoreceptor Opsin3 is sensitive to osmotic stimuli and light. J. Neuroendocrinol. 2024;36 doi: 10.1111/jne.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Upton B.A., Nayak G., Schweinzger I., D'Souza S.P., Vorhees C.V., Williams M.T., Earl B.R., Lang R.A. Comprehensive Behavioral Analysis of Opsin 3 (Encephalopsin)-Deficient Mice Identifies Role in Modulation of Acoustic Startle Reflex. eNeuro. 2022;9 doi: 10.1523/eneuro.0202-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matynia A., Nguyen E., Sun X., Blixt F.W., Parikh S., Kessler J., Pérez de Sevilla Müller L., Habib S., Kim P., Wang Z.Z., et al. Peripheral Sensory Neurons Expressing Melanopsin Respond to Light. Front. Neural Circ. 2016;10:60. doi: 10.3389/fncir.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q., Yue W.W.S., Jiang Z., Xue T., Kang S.H., Bergles D.E., Mikoshiba K., Offermanns S., Yau K.W. Synergistic Signaling by Light and Acetylcholine in Mouse Iris Sphincter Muscle. Curr. Biol. 2017;27:1791–1800.e5. doi: 10.1016/j.cub.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sikka G., Hussmann G.P., Pandey D., Cao S., Hori D., Park J.T., Steppan J., Kim J.H., Barodka V., Myers A.C., et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc. Natl. Acad. Sci. USA. 2014;111:17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ondrusova K., Fatehi M., Barr A., Czarnecka Z., Long W., Suzuki K., Campbell S., Philippaert K., Hubert M., Tredget E., et al. Subcutaneous white adipocytes express a light sensitive signaling pathway mediated via a melanopsin/TRPC channel axis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonoda T., Li J.Y., Hayes N.W., Chan J.C., Okabe Y., Belin S., Nawabi H., Schmidt T.M. A noncanonical inhibitory circuit dampens behavioral sensitivity to light. Science. 2020;368:527–531. doi: 10.1126/science.aay3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins E.C., Pannell R., Simpson E.M., Forster A., Rabbitts T.H. Inter-chromosomal recombination of Mll and Af9 genes mediated by cre-loxP in mouse development. EMBO Rep. 2000;1:127–132. doi: 10.1093/embo-reports/kvd021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zong H., Espinosa J.S., Su H.H., Muzumdar M.D., Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Liu J., Willet S.G., Bankaitis E.D., Xu Y., Wright C.V.E., Gu G. Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis. 2013;51:436–442. doi: 10.1002/dvg.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tong Q., Ye C., McCrimmon R.J., Dhillon H., Choi B., Kramer M.D., Yu J., Yang Z., Christiansen L.M., Lee C.E., et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metabol. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng F., Han Y., Srisai D., Belakhov V., Farias M., Xu Y., Palmiter R.D., Baasov T., Wu Q. New inducible genetic method reveals critical roles of GABA in the control of feeding and metabolism. Proc. Natl. Acad. Sci. USA. 2016;113:3645–3650. doi: 10.1073/pnas.1602049113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stringer C., Wang T., Michaelos M., Pachitariu M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods. 2021;18:100–106. doi: 10.1038/s41592-020-01018-x. [DOI] [PubMed] [Google Scholar]
- 75.Warren E.J., Allen C.N., Brown R.L., Robinson D.W. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur. J. Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker M.T., Brown R.L., Cronin T.W., Robinson P.R. Photochemistry of retinal chromophore in mouse melanopsin. Proc. Natl. Acad. Sci. USA. 2008;105:8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lauridsen K., Ly A., Prévost E.D., McNulty C., McGovern D.J., Tay J.W., Dragavon J., Root D.H. A Semi-Automated Workflow for Brain Slice Histology Alignment, Registration, and Cell Quantification (SHARCQ) eneuro. 2022;9 doi: 10.1523/eneuro.0483-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Reanalysis of the ipRGC and ipRGC-proximal clusters from Tran et al., 2019 have been deposited and are available through Mendeley (https://doi.org/10.17632/36tpcd3ykb.1).
-
•
This manuscript did not generate any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.