Summary
Cell-type-specific domains are the anatomical domains in spatially resolved transcriptome (SRT) tissues where particular cell types are enriched coincidentally. It is challenging to use existing computational methods to detect specific domains with low-proportion cell types, which are partly overlapped with or even inside other cell-type-specific domains. Here, we propose De-spot, which synthesizes segmentation and deconvolution as an ensemble to generate cell-type patterns, detect low-proportion cell-type-specific domains, and display these domains intuitively. Experimental evaluation showed that De-spot enabled us to discover the co-localizations between cancer-associated fibroblasts and immune-related cells that indicate potential tumor microenvironment (TME) domains in given slices, which were obscured by previous computational methods. We further elucidated the identified domains and found that Srgn may be a critical TME marker in SRT slices. By deciphering T cell-specific domains in breast cancer tissues, De-spot also revealed that the proportions of exhausted T cells were significantly increased in invasive vs. ductal carcinoma.
Keywords: spatial transcriptomics, cell-type-specific domains, cell co-localizations, single cell, ensemble learning, tumor microenvironments, 3D Landscape
Graphical abstract

Highlights
-
•
Defines cell-type-specific domains and proposes De-spot to detect them precisely
-
•
Develops SMD format and extendible interfaces for diverse SRT platforms
-
•
Develops 3D Landscape for intuitively displaying cell-type-specific domains
-
•
Finds Srgn and Vim as potential tumor microenvironment markers in SRT slices
Motivation
This study was motivated by the problem of precisely deciphering the spatial heterogeneity of cell types in spatial transcriptomics, which is challenging to detect using conventional spatial domains. We formally defined “cell-type-specific domains,” which are expected to be spatially heterogeneous domains where the proportions of given cell types are significantly higher than those of others. We sought to develop an approach for precisely and effectively detecting cell-type-specific domains in both spot-level and single-cell resolution, especially for specific domains of low-proportion cell types.
Ruan et al. propose “cell-type-specific domains” and develop De-spot for detecting and displaying them precisely in spatial transcriptomics. This approach bridges the gap between cell-type distributions and anatomical spatial domains. Using De-spot, they decipher the spatial heterogeneity of low-proportion cell types and provide biological insights into tumor microenvironments.
Introduction
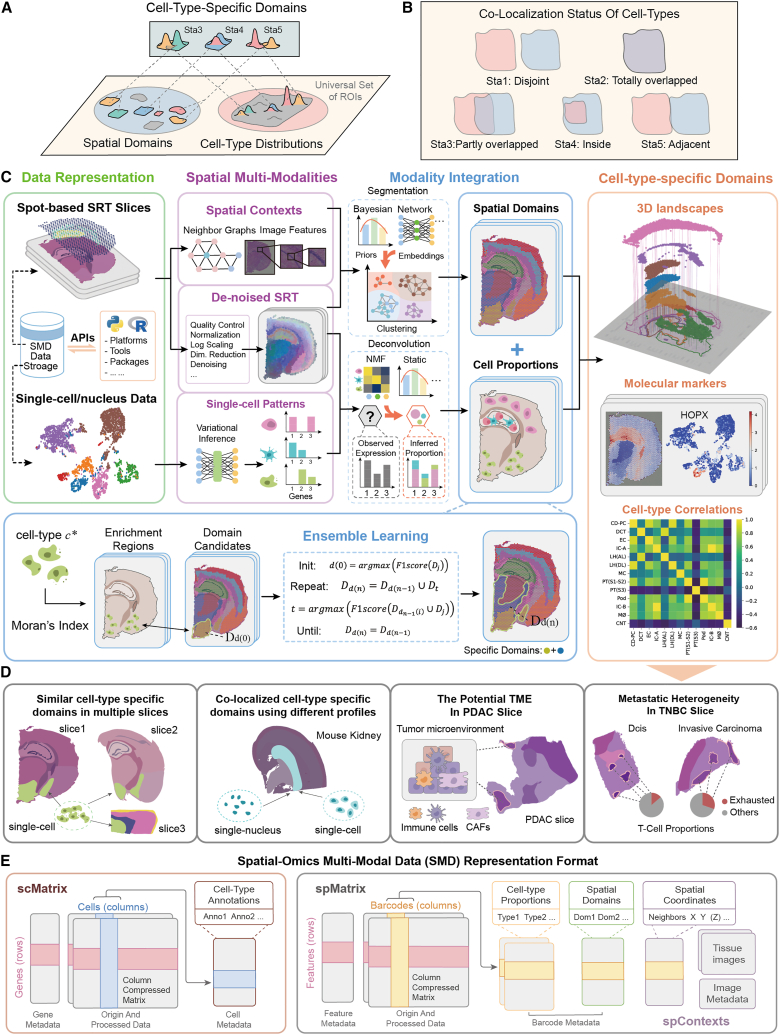
The functions of cells are closely related to the positional contexts in microenvironments.1 Recent spatially resolved transcriptomics (SRT) technologies2,3,4 can preserve invaluable spatial contexts when capturing full transcriptome information.5,6 Using the transcriptome generated by SRT technologies to decipher anatomical structures and locations of different cells is critical for arising novel insights into the biological understanding of cells, tissue structures, and their microenvironments.5 Because the information in SRT datasets is more than can be deciphered in a single analysis, choosing regions of interest (ROIs) is appropriate for further biological discoveries.7 In the universal set of ROIs, spatial domains represent the ROIs associated with anatomical structures (e.g., a specific layer in the brain), and a few of these domains are further identified with specific cell types. Likewise, cell-type distributions represent the ROIs associated with their previously known markers, and some cell types exhibit spatial heterogeneity across the tissue. In practice, precisely detecting the ROIs of anatomical structures where specific cells aggregate coincidentally is critical to promoting SRT downstream analysis,7 but such ROIs still have not been formally defined in previous research. In this paper, we denote these ROIs as “cell-type-specific domains” (Figure 1A). Compared with conventional domains, cell-type-specific domains are expected to be spatially heterogeneous domains where the proportions of certain cell types are significantly higher than other domains (see STAR Methods and Figures S1A and S1B). Within a cell-type-specific domain, we can focus on the subtypes of this cell type and evaluate their meticulous compositions, which are previously explored across the tissue and without prior enrichment domains. In addition, there generally exist cell-type co-localizations in SRT slices since dozens of cells are captured in an observation spot, which is 2–100 μm in diameter.2,3 These co-localizations are merely quantified by Pearson’s related coefficient without considering their spatial characteristics. By defining cell-type-specific domains, the spatial characteristics of cell-type co-localizations are well curved, including five statuses: disjoint, totally overlapped, partly overlapped, inside, and adjacent (Figure 1B). In particular, the partly overlapped status indicates a critical niche at their intersection regions, the inside status means a cell type may play a crucial role in the specific domains of another one, and the adjacent status depicts probable cell-cell interactions across their boundaries. Obviously, precise detection of cell-type-specific domains in SRT slices is essential for promoting an understanding of biological mechanisms, especially in cancer tissues whose anatomical structures are irregular.
Figure 1.
Overview of De-spot
(A) Cell-type-specific domains where certain cell types aggregate in some specific anatomical structures in coincidence.
(B) The co-localization status of cell types.
(C) Four modules included in De-spot: data representation, spatial multi-modalities, modality integration, and ensemble learning. The cell-type-specific domains are visualized by 3D Landscapes and are associated with the corresponding upregulated markers. Spatial correlations among cell types are also evaluated.
(D) The experimental evaluations of De-spot.
(E) The spatial-omics multi-modal data representation format.
Current mainstream SRT computational methods typically focus on segmentation or deconvolution,7,8,9,10 but they face difficulties in detecting cell-type-specific domains, which requires well-matched spatial domains and cell-type proportions. Existing segmentation and deconvolution methods require extra interventions to detect cell-type-specific domains, which are absent for most publicly accessible SRT datasets. Segmentation methods11,12,13,14 combine the SRT spatial contexts to detect spatial domains, but these detected domains lack cell-type proportion information and require manual annotation using gene expression11 or label transformation.14,15 Deconvolution methods16,17,18,19,20,21 combine single-cell profiles to infer cell-type proportions in each spot. Still, these inferred proportions lack spatial domain information and require tissue stratifications to be manually performed based on pathologist annotations.16,19,21 Even if extra interventions are introduced, segmentation or deconvolution may still fail to detect cell-type-specific domains since they cause deviations.22,23 In segmentation, spatial patterns usually vary across different resolutions and segmented approaches. Therefore, relying on a single segmentation method may overlook potential spatial patterns, leading to the deviational detection of cell-type-specific domains. Some single-cell SRT segmentation methods like BASS24 and MENDER25 detect spatial domains at multi-scale levels, but they ignore detecting spatial heterogeneity of cell-type proportions, making it hard to decipher cell co-localizations in SRT data. Likewise, a single deconvolution method may be insensitive to particular cell types, resulting in the ignoration of potential cell-type-specific domains. These deviations will unexpectedly spell gaps in which the information reflected by spatial domains and cell-type proportions is inconsistent, hindering the detection of cell-type-specific domains. Several pipelines26,27,28,29 have been equipped with segmentation and deconvolution, but they are independently utilized to decipher spatial domains and cell-type proportions without considering the gaps. SOView22 provides a web server for manually selecting ROIs associated with their markers and image morphology features. Still, it ignores cell-type correlations, which may cause the loss of some potential discoveries about cell-type proportions and their spatial correlations.
Recently, some attempts have considered integrating cell-type proportion and anatomical domains to detect cell-type-specific domains without extra interventions. An attempt called MIA30 evaluates the hypergeometric distribution of overlaps between spatially variable genes and cell-type-specific genes in single-cell data. MIA mainly relies on initial given annotations and overlaps between SRT and cell-type-specific genes. These cause a nonnegligible deviation, such that when two cell types are in partly overlapped status, MIA may mistakenly return their intersection regions as cell-type-specific domains. Another attempt called CARD31 borrows the spatial contexts to deconvolution via the conditional autoregressive-based model and then annotates the spatial domains using the dominant cell types. CARD requires no initial annotations and performs well for the spatial domains of high-proportion cell types. However, the boundaries between two adjacent cell-type-specific domains may become blurred if dominant cell types are used to annotate spatial domains without considering anatomical structures. Moreover, both MIA and CARD struggle to detect the spatial domains of low-proportion cell types. These cell-type-specific domains indicate that cells may (1) exist in tissues but have limited overlaps between SRT variable genes and cell-type markers, (2) aggregate as a small portion of subdomains inside the annotated domains, or (3) not exist in tissues. Using the hypergeometric distribution in MIA, it is hard to detect specific domains of cell types associated with limited overlaps between SRT variable genes and cell-type markers. When using CARD, extracting low-proportion cell-type-specific domains inside the domains dominated by high-proportion cell types is also challenging. Thus, MIA and CARD fail to tell whether low-proportion cell types exist in the SRT tissues.
In summary, previous methods still have severe deviations in defining the spatial locations of low-proportion cell types, which impedes the identification of potential markers and biological insights in tissues. To handle these deviations, detecting cell-type-specific domains requires the spatial patterns to be thoroughly characterized and the cell-type proportions to be precisely inferred using numeric segmentation and deconvolution methods, respectively. Therefore, it is promising to bridge the gap between cell-type distributions and anatomical domains and to use prior biological knowledge and multi-modalities simultaneously. A prerequisite for achieving this goal is to design data representations compatible with different SRT analysis tools and to develop computational methods that integrate these tools to detect cell-type-specific domains. Another prerequisite is to define the expression patterns of cell types accurately. Additionally, an intuitive visualization approach is imperative since cell-type-specific domains may overlap when co-localized cell types exist.
To address the challenges above, we proposed De-spot, which synthesizes segmentation and deconvolution to detect cell-type-specific domains automatically via ensemble learning. De-spot first generates numerous candidates of spatial domains and cell-type proportions using segmentation and deconvolution methods, respectively. Then, De-spot infers enrichment regions of these cell-type proportion candidates based on Moran’s index.32 Finally, using a greedy strategy, it detects cell-type-specific domains by mapping those spatial domain candidates to the inferred enrichment regions. In this scenario, De-spot requires no extra interventions, and it could effectively detect low-proportion cell-type-specific domains according to the spatial heterogeneity of cell-type proportions instead of evaluating overlaps between SRT and cell-type markers. De-spot introduces a compatible data representation format for spatial multi-modalities, which has extendible interfaces for diverse SRT platforms, tools, and packages in Python/R programming ecosystems. A variational inference module is also designed to derive potential cell-type patterns. With this module, the single-cell patterns are characterized clearly, facilitating the detection of accurate cell-type-specific domains. In addition, we developed a 3D Landscape visualization module for intuitively displaying cell-type-specific domains and their co-localizations. Compared with existing methods, our experimental evaluation demonstrated De-spot’s advantages in deriving single-cell patterns and detecting cell-type-specific domains running on the Visium, ST, MERFISH, and Stereo-seq SRT slices. In the pancreatic ductal adenocarcinomas (PDAC) slice, De-spot uniquely discovered the co-localizations between fibroblasts and immune-related cells (e.g., T cells, macrophages, and dendritic cells) that indicate potential tumor microenvironment (TME) domains obscured by previous computational approaches. We replicated these co-localizations in the Visium human triple-negative breast cancer (TNBC) slice. Moreover, we discovered the heterogeneous compositions of T cell subtypes between invasive cancer and ductal carcinoma in situ (DCIS) regions within the T cell-specific domains.
Results
Overview of De-spot
De-spot detects cell-type-specific domains automatically by integrating SRT data, single-cell transcriptome data, and spatial contexts (Figure 1C). De-spot begins with three inputs: (1) a spatial expression matrix of unique molecular identifier (UMI) counts, (2) the accompanying spatial coordinates and images, and (3) a single-cell (single-nucleus) gene expression profile with annotations. Specifically, single-cell and SRT profiles are either from the same sample (paired) or similar samples (unpaired). For the data curation, we developed a data representation format for spatial multi-modality data (SMD) as the core data structure of De-spot, containing scMatrix, spMatrix, and spContexts (Figure 1E). This format stores compressed intermediate results and has extendible interfaces for diverse SRT infrastructures, platforms, tools, and packages in Python/R programming ecosystems. De-spot establishes a comprehensive analysis process for spatial multi-modalities. For spatial contexts, De-spot uses the given coordinates and images to establish spatial graphs, evaluate spatial neighborhoods, and extract image morphology features. For SRT data, De-spot provides quality control, normalization, scaling, dimensional reduction, and de-noising functions to correct SRT technical covariates.33,34,35,36 For single-cell profiles, De-spot designs a variational inference module to derive potential cell-type patterns in single-cell profiles. De-spot makes inferences on each cell type’s multivariate Gaussian latent distribution.37 After that, typical molecular markers of each cell type are emphasized, and noise signals are filtered (Figures S2A– S2C).
In modality integration, De-spot integrates a series of candidates of cell-type proportions and spatial domains as an ensemble. Based on the global Moran’s index, it finds the cell types with spatial heterogeneity within the cell-type proportion candidates. Then, it infers the enrichment regions of cell types using the local Moran’s index. Next, it detects specific domains for each cell type by evaluating the F1 score among cell-type enrichment regions and spatial domain candidates. Since a cell-type-specific domain may contain different parts of tissues, De-spot repeatedly appends other domain candidates until the F1 score does not increase. Finally, De-spot examines the upregulated genes of cell-type-specific domains and generates the 3D Landscapes of cell-type-specific domains in SRT slices, especially for the overlapped domains. The generated landscapes curve the outlines of specific tissue domains, display the overlapped domains hierarchically, and draw the waterfall lines to link them together (see STAR Methods and Figure S1C).
Our experiments first evaluated whether De-spot can detect similar cell-type-specific domains in multiple SRT slices using a single-cell pattern. Then, we evaluated whether it can detect co-localized cell-type-specific domains in an SRT slice using single-cell and single-nucleus patterns from different profiles. We also used De-spot on a potential TME in the PDAC slice and deciphered the metastatic heterogeneity in the TNBC slice. Finally, we evaluated whether De-spot can deal with single-cell resolution SRT slices using multiple MERFISH and Stereo-seq mouse brain slices (Figure 1D).
De-spot identified similar cell-type-specific domains in multiple mouse brain slices
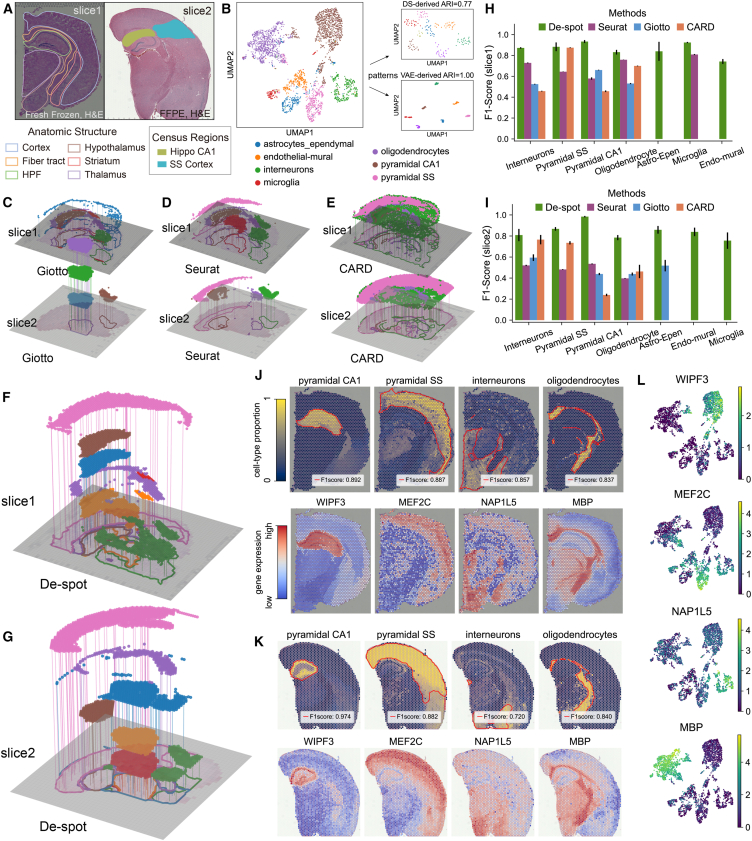
We first evaluated the ability of De-spot to detect cell-type-specific domains in multiple Visium mouse brain telencephalon samples38 (Figure 2A) associated with a single-cell profile39 (Figure 2B). The Visium samples include slice 1—a free frozen tissue with hematoxylin and eosin (H&E) stain histology—and slice 2—a formalin-fixed paraffin-embedding (FFPE) tissue with H&E stain histology. The primer brain regions, such as the cortex, fiber tracts, hippocampal formation (HPF), hypothalamus, striatum, and thalamus, were indicated by the Allen Brain Atlas.40 The cells in the single-cell profile were mainly obtained from the somatosensory cortex and hippocampal CA1 region, comprising seven major cell types in the cortex (Figures 2B and S2C).
Figure 2.
De-spot identified similar cell-type-specific domains in multiple mouse brain slices
(A) Free frozen tissue (slice 1) and FFPE tissue (slice 2). Annotations of anatomic mouse brain structure are from the Allen Brain Atlas.
(B) Previously published single-cell profiles, including seven annotated cell types: astrocytes-ependymal, endothelial-mural, interneurons, microglia, oligodendrocytes, pyramidal CA1, and pyramidal SS.
(C) 3D Landscapes of slice 1 and slice 2 generated by Giotto. The color of each domain corresponds to the cell types with the same color in (B).
(D) 3D Landscapes of slice 1 and slice 2 generated by Seurat.
(E) 3D Landscapes of slice 1 and slice 2 generated by CARD.
(F) 3D Landscapes of slice 1 generated by De-spot.
(G) 3D Landscapes of slice 2 generated by De-spot.
(H) F1 score comparison of cell-type-specific domains in slice 1 (bars indicate mean ± SEM). If a method failed to find the cell-type-specific domains of a certain cell, the F1 score bar of this method for the certain cell would not display (e.g., Giotto failed to detect pyramidal SS-specific domain in slice 1, so the F1 score bar of Giotto for pyramidal SS did not display).
(I) F1 score comparison of cell-type-specific domains in slice 2 (bars indicate mean ± SEM).
(J and K) Cell-type-specific domains of pyramidal CA1, pyramidal SS, interneurons, oligodendrocytes, and the expression of their corresponding upregulated genes (Wipf3, Mef2c, Nap1l5, and Mbp) in slice 1 (J) and slice 2 (K). The detected cell-type-specific domains are outlined in red contours.
(L) Expressions of Wipf3, Mef2c, Nap1l5, and Mbp in single-cell profiles.
We derived patterns in single-cell profiles and clustered the derived patterns using Leiden.41 The corresponding UMAP42 visualization in Figure 2B shows that De-spot’s adjusted Rand index (ARI) was 1.00, and the corresponding ARI of down-sampling approaches was 0.73. We observed that De-spot derived the patterns with more separated clustroids than previous down-sampling approaches; that is, De-spot could clearly describe the different patterns among cell types in single-cell profiles.
Next, we generated the 3D Landscapes of cell-type-specific domains detected by Giotto, Seurat, CARD, and De-spot (Figures 2C–2G) in slices 1 and 2, respectively. We found Giotto, Seurat, and CARD detected specific domains for parts of the seven major cell types in slices 1 and 2. De-spot detected all the cell-type-specific domains for the given major cell types in slices 1 and 2. Then, we evaluated the corresponding harmonic means (F1 score) by calculating the specificity and sensitivity between the segmented domains and cell-type proportions. The F1 scores of De-spot outperformed those of Giotto, Seurat, and CARD in slices 1 and 2 for the provided cell types (Figures 2H and 2I).
Further, we investigated the specific domains of pyramidal cells, interneurons, and oligodendrocytes with their corresponding upregulated genes. As illustrated previouly,39 pyramidal cells were sampled from the hippocampal CA1 region and somatosensory cortex, respectively (depicted as pyramidal CA1 and pyramidal SS). When detecting pyramidal CA1-specific domains, the detected domain should be associated with the HPF besides the hippocampal CA1 region. For slice 1, all approaches successfully detected the pyramidal CA1-specific domains in HPF (see brown spots in Figures 2D–2F). For slice 2, Giotto mistakenly detected the corresponding specific domains in the cerebral cortex (brown spots in Figure 2D). In particular, for slices 1 and 2, De-spot detected the corresponding specific domains covering not only the CA1 region but also the whole HPF (see red dashes in pyramidal CA1 in Figures 2J and 2K). By contrast, Giotto, Seurat, and CARD lost parts of spots (see blanks in the brown spot layer of Figures 2D and 2E). The pyramidal CA1-specific domains detected by De-spot were associated with Wipf3 and Hpca, which were highly expressed in HPF of slices 1 and 2, and pyramidal CA1 of single-cell profiles (Figures 2J and 2K). Likewise, the pyramidal SS-specific domains should be associated with the SS cortex and other cortex regions. Giotto failed to detect the pyramidal SS-specific domains in slice 1 and slice 2 (Figure 2D). Seurat and CARD failed to detect their overlaps with the whole SS cortex (see pink dashes of slice 1 and slice 2 in Figure 2E). De-spot successfully detected the pyramidal SS-specific domains in the cortical cortex, which includes the whole SS cortex, both in slice 1 and slice 2 (red dashed in pyramidal SS of Figures 2J and 2K). The pyramidal SS-specific domains detected by De-spot are combined with Mef2c (Figures 2J–2L), which is highly expressed in the broad cortical cortex and the pyramidal SS of single-cell profiles.43
In the following, we focused on the enrichment of interneurons, which were previously illustrated to be regionally varied in cortical contexts.44 For slice 1, interneurons were successfully detected in the hypothalamus using Giotto, Seurat, CARD, and De-spot (see green spots in Figures 2D–2F). In addition, using De-spot and Seurat, we found that interneurons were also enriched in the striatum, which conforms to previous work16 (see red dashed in interneurons, Figure 2J). For slice 2, using Giotto, Seurat, and CARD, the interneuron’s specific domains were inconsistent with those in slice 1. In particular, Giotto mistakenly associated the detected domains with the thalamus, Seurat detected them in the striatum only, and CARD detected them in the thalamus, hypothalamus, and striatum (see green spots in Figures 2C–2E). In contrast, De-spot’s interneuron-specific domains remained consistent with slice 1, with the upregulation of Nap1l5 (Figures 2J–2L).
Finally, we focused on the results of oligodendrocyte-specific domains. As discussed in previous work,3 the oligodendrocytes are preferentially localized in the fiber tracts. For slice 1, we observed that Giotto, Seurat, CARD, and De-spot can detect the oligodendrocyte (purple spots) in the fiber tracts (see purple spots in Figures 2D–2F). For slice 2, only the oligodendrocyte-specific domains detected by De-spot remained consistent with those in slice 1 (see red dashed outlines of oligodendrocytes in Figure 2K), along with a reported molecular marker of oligodendrocytes Mbp45 (Figures 2J–2L).
The results above reveal that, compared with previous approaches, De-spot could effectively detect the cell-type-specific domains of pyramidal cells, interneurons, and oligodendrocytes in multiple Visium mouse brain slices associated with their corresponding upregulated genes.
De-spot detected co-localized cell-type-specific domains using multiple profiles
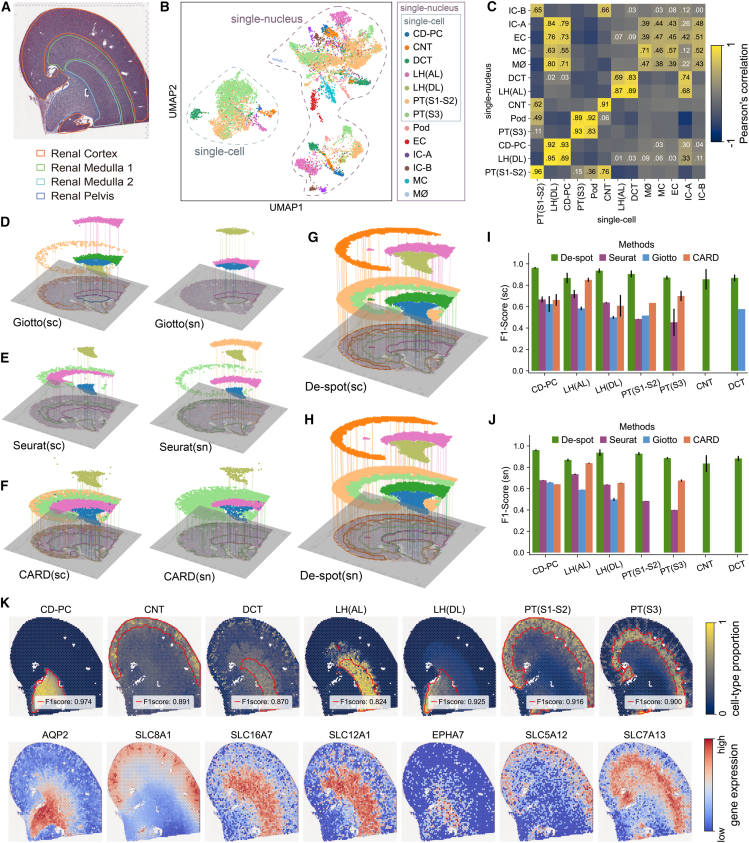
We evaluated De-spot’s capability of detecting cell-type-specific domains in a Visium mouse kidney slice46 associated with multiple single-cell and single-nucleus profiles.47 The mouse kidney slice has 19,465 unique genes and 3,124 valid spots, composed of the renal cortex, the medulla, and the pelvis (Figure 3A). The associated profiles are generated using a single-cell technology (DropSeq) and three single-nucleus technologies (sNuc-DropSeq, DroNc-seq, and 10X Chromium). Thirteen cell types were identified in these four profiles, including seven common cell types in single-cell and single-nucleus profiles and six cell types mainly in the single-nucleus profiles47 (Figures 3B, S2D, and S2E). Theoretically, the specific domains of these seven common cell types obtained from single-cell profiles should conform to those from single-nucleus profiles. We first analyzed spatial correlations among the cell-type-specific domains detected by De-spot using single-cell profiles and single-nucleus profiles, respectively (Figure 3C). We observed that the single-cell-specific domains of the seven common cell types and their corresponding single-nucleus-specific domains were well co-localized (Pearson’s correlation equals 0.83–0.96). Further, several different cell-type-specific domains detected by De-spot were also spatially co-localized (e.g., podocytes co-localized with proximal tubule S3 [PT(S3)] and connecting tubules [CNTs] co-localized with PT(S1–S2)). From the F1 score comparisons, we observed that when using the single-cell profiles, Giotto, Seurat, and CARD detected five cell-type-specific domains (Figure 3I). When using the single-nucleus profiles, the numbers of cell-type-specific domains detected by Giotto, Seurat, and CARD were 3, 5, and 4, respectively (Figure 3J). De-spot detected all the specific domains of the seven common cell types and outperformed Seurat, Giotto, and CARD (Figures 3I and 3J).
Figure 3.
De-spot detected co-localized cell-type-specific domains using multiple profiles
(A) FFPE tissue of the mouse kidney. The slice is histologically annotated to the renal cortex, renal medulla 1, renal medulla 2, and pelvis.
(B) Previously published mouse kidney profiles generated by scRNA-seq and small nuclear RNA sequencing, containing 13 cell types annotated by Wu et al.47 CD-PC, collecting duct principal cells; CNT, connecting tubule; DCT, distal convoluted tubules; EC, endothelial cells; IC, intercalated cells; LHAL, the loop of Henle ascending loop; LHDL, the loop of Henle descending loop; MC, mesangial cells; MØ, macrophages; PT, proximal tubules; Pod, podocytes.
(C) Correlation heatmap of pairwise cell type proportion between single-nucleus profiles (rows) and single-cell profiles (columns). Non-significant correlations (p < 0.05) are shown in gray; the color bar indicates Pearson’s r values; the Pearson’s r values among cell types greater than zero are marked in elements of the heatmap.
(D) 3D Landscapes of cell-type-specific domains generated by Giotto according to single-cell and single-nucleus profiles. The color of each domain corresponds to the color of cell types in (B).
(E) 3D Landscapes of cell-type-specific domains generated by Seurat.
(F) 3D Landscapes of cell-type-specific domains generated by CARD.
(G) 3D Landscapes of cell-type-specific domains generated by De-spot using single-cell profiles.
(H) 3D Landscapes of cell-type-specific domains generated by De-spot using single-nucleus profiles.
(I) F1 score comparison of the cell-type-specific domains when using single-cell profiles (bars indicate mean ± SEM).
(J) F1 score comparison of the cell-type-specific domains when using single-nucleus profiles (bars indicate mean ± SEM).
(K) Proportions of De-spot’s seven common cell types: CD-PC, CNT, DCT, LHAL, LHDL, PT(S1–S2), and PT(S3) and their corresponding upregulated genes.
Further, we generated the 3D Landscapes to validate whether they are detected in proper domains (Figures 3D–3H). For the loop of Henle (LH), we found that by using Giotto, Seurat, CARD, and De-spot, the ascending loops (LHAL; Slc12a1) and descending loops (LHDL; Epha7) specific domains were detected in the medulla and the pelvis along with their expected markers47 (Figure 3H), respectively, remaining consistent between single-cell and single-nucleus profiles. In particular, collecting duct principal cell (CD-PC)-specific domains are totally overlapped with LH(DL) along with Aqp2. For the PTs, Giotto detected the PT(S1–S2)-specific domains in the renal cortex using the single-cell profiles but failed to detect them using single-nucleus profiles. In addition, it failed to detect the PT(S3)-specific domains when using single-cell or single-nucleus profiles (Figure 3D). Seurat mistakenly detected the PT(S1–S2)-specific domains in the medulla instead of the renal cortex. The PT(S3)-specific domains detected by Seurat were expected to be located in the renal cortex using single-cell profiles. Still, their spatial locations were inconsistent with those using single-nucleus profiles (Figure 3E). CARD successfully detected the PT(S1–S2) and PT(S3)-specific domains in the renal cortex using the single-cell profiles but failed to detect the PT(S1–S2)-specific domains when using single-nucleus profiles (Figure 3F). Compared with these previous methods, De-spot correctly detected the PT(S1–S2) and PT(S3)-specific domains in the renal cortex, verified by their upregulated genes Slc5a12 and Slc7a1347 (Figures 3G, 3H, and 3K). Moreover, Giotto, Seurat, and CARD failed to detect the CNT- and distal convoluted tubule (DCT)-specific domains (Figure 3D–3F). By contrast, De-spot could successfully detect them in single-cell and single-nucleus profiles, whose corresponding upregulated genes are Slc8a1 and Slc16a7, respectively (Figures 3G, 3H, and 3K). Overall, paired single-cell and single-nucleus cell-type-specific domains detected by De-spot showed high overall agreement and are correctly co-localized in proper spatial locations.48 These results demonstrated that De-spot was robust and unbiased compared with previous methods.
De-spot discovered a potential TME in pancreatic ductal adenocarcinomas
To demonstrate the applicability of detecting precise cell-type-specific domains, we applied De-spot to the ST pancreatic ductal adenocarcinoma (PDAC) slice30 (Figure 4A). The PDAC slice was pathologically stratified into carcinoma regions, pancreatic tissues, ductal epithelium, and stroma regions. The paired single-cell RNA sequencing (scRNA-seq) profiles from this PDAC patient contain cell types including acinar cells, cancer cells, ductal cells, endocrine cells, endothelial cells, fibroblasts, infiltrating immune cells, and tuft cells (Figures 4B and S3A).
Figure 4.
De-spot discovered a potential TME in pancreatic ductal adenocarcinomas
(A) The spatial transcriptomics (ST) slide of PDAC tumor cryosection, which is stratified into cancer cells and desmoplasia, normal pancreatic tissue, and duct epithelium.
(B) UMAP visualization of paired PDAC single-cell profiles containing 20 cell types annotated by Yanai et al.30
(C) 3D Landscapes of high-proportion cell types, including cancer clone A (orange), cancer clone B (green), acinar cells (blue), centroacinar ductal cells (purple), and fibroblasts (cyan).
(D) 3D Landscapes of high-proportion cell types detected by Seurat, Giotto, and CARD.
(E) F1 score comparison of high-proportion cell types in multiple runs (bars indicate mean ± SEM).
(F) Cell-type-specific domains of four ductal cell types and the corresponding upregulated genes.
(G) 3D Landscapes of immune-related cells, including T and natural killer (NK) cells, monocytes, mast cells, macrophages, and DC cells.
(H) Cell-type proportions of fibroblasts, T and NK cells, monocytes, mast cells, macrophages, DC cells, and fibroblasts.
(I) Upregulated genes of acinar cells, ductal cells, immune-related cells, and fibroblasts.
The specific domains of high-proportion cell types, including cancer clone cells, acinar cells, ductal cells, and fibroblasts, were detected by Giotto, Seurat, CARD, and De-spot, respectively (Figures 4C and 4D). De-spot successfully detected these high-proportion cell types in their proper spatial locations, associated with their corresponding upregulated genes (Figures 4C and 4I). In particular, De-spot detected the intermix of cancer clone A- and B-specific domains (green and orange spots in Figure 4C) in the cancer region (red outlined regions in Figure 4A), associated with the tumor promoters Tm4sf1 and S100A4.49 De-spot also detected the fibroblast-specific domains (cyan spots in Figure 4C) located in the large cancer region (Col1a1), indicating these fibroblasts are associated with cancer cells. It detected the acinar-specific domains (blue spots in Figure 4C) in the normal pancreatic tissue (Ctrb1) and the ductal-specific domains (purple spots in Figure 4C) in the duct epithelium (Crisp3). By contrast, Seurat failed to detect the cell-type-specific domains of fibroblasts, Giotto failed to detect those of cancer clone B, and CARD failed to detect those of fibroblasts and acinar cells (Figure 4D). From the results of the F1 scores in Figure 4E, we also observed that De-spot outperformed Giotto, Seurat, and CARD.
As illustrated in previous work,30 ductal cells could be further classified into four subtypes: centroacinar ductal cells (ductal-centroacinar), antigen-presenting ductal cells (ductal-MHC class II), terminal ductal cells (ductal-terminal), and ductal cells with hypoxic-response gene expression (ductal-hypoxic). We further analyzed the spatial distribution of these ductal subtypes obtained using De-spot. Similar to MIA, the main cell-type-specific domains of these four ductal subtypes detected by De-spot were located in the duct epithelium associated with their corresponding markers (Figure 4F). However, MIA failed to delineate the local heterogeneity of ductal subtypes within the duct epithelium. Using De-spot, we further found that the ductal-hypoxic-specific domains aggregate in the upper-left and lower-left domains of the duct epithelium (Figure 4F). In addition, we found the ductal-MHC class II cells were also located in the upper-left and lower-left domains of the duct epithelium, indicating that professional antigen-presenting cells (e.g., monocytes, macrophages, and dendritic cells) appear in these domains.50 We further found the specific domains of five immune-related cells partly overlapped with the ductal-hypoxic (Figures 4G and 4H), while other previous methods failed to detect the five immune-related cell-type-specific domains (Figure 4D). Specifically, De-spot detected the specific domains of macrophages (Cd68), mast cells (Tpsb2 and Tpsab1), and dendritic cells (DCs; C1qa)51 (Figures 4G, 4H, and S3B). De-spot also detected the T cell- and natural-killer-cell-specific domains with their molecular markers (Cd3d and Il32) and detected the monocyte specific domains associated with Srgn and S100a9, in which S100a9 was reported to be a biomarker for predicting the benefits of immunotherapy52 (Figures 4H and 4I). We found Srgn was upregulated in the upper-left and lower-left domains of the duct epithelium, co-localizing with those five immune-related cell types (Figure 4I). As illustrated in previous reports,53,54 Srgn is highly expressed in infiltrating immune cells and promotes the epithelial-to-mesenchymal transition. Thus, these domains may be associated with the interactions between invasive carcinoma and infiltrating immune cells.
We further analyzed the spatial locations of cancer-associated fibroblasts (CAFs), which constitute a favorable environment for tumor development55 and reshape the immune landscape to facilitate cancer metastasis.56 We observed that De-spot detected the specific domains of these fibroblasts in the immunoreaction domains (Figures 4G and 4H), indicating that the infiltrating immune cells were partly overlapped with fibroblast-specific domains. We found Vimentin (Vim), a mesenchymal marker of tumor cells that reveals a high possibility of invasion,57 was highly expressed in the upper-left and lower-left domains of the duct epithelium and co-localizes with the fibroblasts and infiltrating immune cells (Figure 4G). Observing several spots with high expressions of Tm4sf1 in the upper-left and lower-left domains of the duct epithelium (Figure 4I), we anticipated a potential TME located in these domains (green outlines, Figure 4A). These results further demonstrated the nice sensitivity of De-spot for detecting cell-type-specific domains.
De-spot characterized the metastatic heterogeneity in breast cancer slices
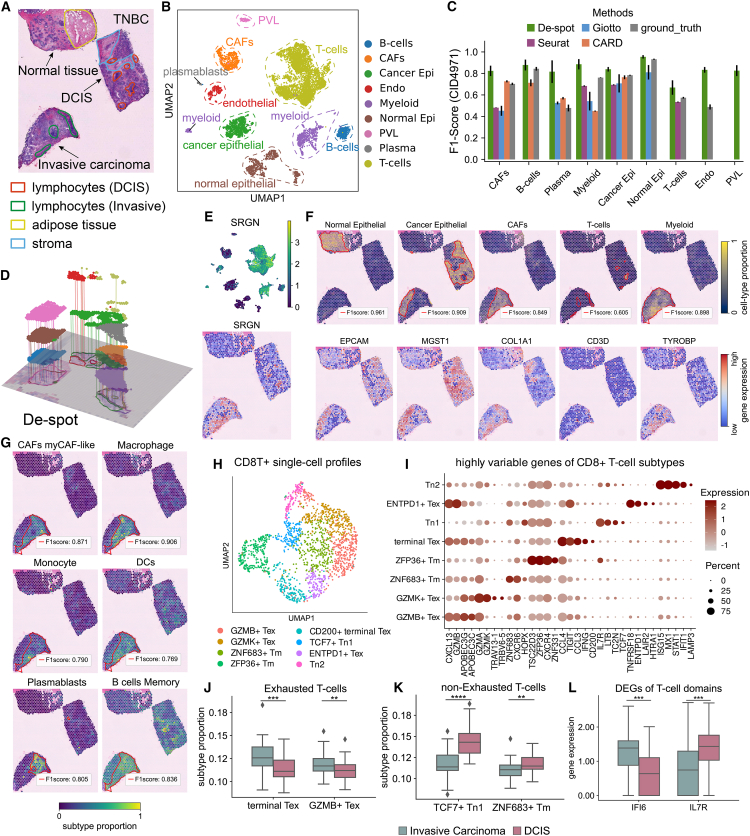
To evaluate De-spot’s capability in characterizing cancer metastatic heterogeneity, we performed experiments on the previously published and annotated Visium TNBC slice CID4971.58 The slice contains tissues of the normal breast region, DCIS, and invasive carcinoma (Figure 5A). The corresponding single-cell profile GSM5354531 was annotated into nine major cell types58 (Figures 5B and S4G). Figure 5C shows that Giotto, Seurat, and CARD detect 5, 4, and 5 specific domains of the primary cell types, respectively (Figure S4B). Through mapping the cell proportion to the ground truth (depicted as ground-truth mapping), the specific domains of eight cell types were detected, except perivascular-like (PVL) cells (Figure 5C). Compared with them, De-spot successfully detected all the specific domains of the nine cell types, especially including endothelial cells and PVL cells (Figures 5D and S4C). In comparing detection accuracy, De-spot outperformed Seurat, Giotto, CARD, and the ground-truth mapping (Figure 5C).
Figure 5.
De-spot characterized the metastatic heterogeneity in breast cancer slices
(A) H&E images of the TNBC slice CID4971 with anatomical domains outlined. The histology image contains tissues of the normal breast region, DCIS, and invasive carcinoma.
(B) UMAP visualization of the TNBC single-cell profiles (GSM5354531).
(C) F1 score comparison of the cell-type-specific domains generated by Giotto, Seurat, CARD, ground-truth mapping, and De-spot (bars indicate mean ± SEM).
(D) 3D Landscapes of 9 major cell-type-specific domains generated by De-spot. The color of each cell-type-specific domain corresponds to the cell types with the same color in (C).
(E) The expression of Srgn in SRT and single-cell profiles.
(F) Specific domains of normal epithelial, cancer epithelial, CAFs, T cells, and myeloid cells detected by De-spot with their corresponding markers.
(G) De-spot’s cell-type-specific domains and cell-type proportions of myCAFs and the subtypes co-localized with myCAFs.
(H) UMAP visualization of the CD8+ T cells selected from the single-cell profiles.
(I) Markers of CD8+ T cell subtypes.
(J) Differential analysis for the subtype proportion of exhausted T cells with statistical significance (∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
(K) Differential analysis for the subtype proportion of non-exhausted T cells (∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
(L) Differential expression genes of exhausted and non-exhausted T cells in invasive carcinoma and DCIS (∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
In line with the pathological annotation, De-spot clearly detected the normal epithelial cells in the normal tissue (Epcam), the cancer epithelial cells mainly in the DCIS (Cd2459 and Mgst160), and the CAFs in the invasive carcinoma tissue (Col1a161) (Figure 5F). We further found that some cancer epithelial cells were detected in part of the invasive cancer region containing minimal CAFs, indicating that an antagonistic relationship existed between the cancer epithelial cells and the CAFs in the TNBC slice (Figure 5F). In addition, the detected endothelial cells located in the stroma of DCIS tissue associated with Pecam1, and the detected PVL cells located in the normal tissue associated with Acta2 (Figures S4A and S4C). Consistent with the co-localizations of CAFs and immune-related cells above in the PDAC slice, De-spot detected the myeloid-specific domains (Tyrobp and Cst3) preferentially co-localizing with CAFs in the invasive carcinoma (Figures 5F and S4A). De-spot detected the T cell-specific domains in the annotated lymphocytes of the DCIS and the invasive carcinoma tissue associated with the T cell molecular markers Cd3d and Cd3e (Figures 5F and S4F). In addition, the spatial distribution of Srgn co-localized with the myeloid and T cell-specific domains, consisting of the distribution of Srgn in the PDAC slice (Figure 5E). In the following, we further investigated the immunoreaction in the TME based on spatial locations of CAFs, myeloid cells, and T cells.
To uncover the spatial associations between CAFs and myeloid cells, we further analyzed the detected specific domains of the single-cell subtypes. From the single-cell subtypes of CAFs, the resembled myofibroblast-like CAFs (myCAFs), whose pathways are associated with extracellular matrix (ECM),58 were obtained at first. We then evaluated the Pearson correlation between pairwise subtype proportions, and the results revealed that myCAFs co-localized with several myeloid subtypes, such as macrophages, monocytes, and DCs in the invasive carcinoma domains (Figure S4D). In particular, macrophages were fulfilled in invasive carcinoma. Monocytes were preferentially detected around the lymphocytes with lower proportions. DCs were detected in the invasive carcinoma domains and partly overlapped with T cells in the DCIS (Figure 5G). We also found other immune-related cells, including plasmablast (Jchain), lymphatic epithelial cells, and memory B cells (Cd79a) co-localize with myCAFs (Figures 5G, S4D, and S4F). In addition to the collagen genes (Col1a1), we observed that Decorin (Dcn) and Lumican (Lum) were highly expressed in the mesenchymal of the three tissues, but they had a high centralized distribution in the myCAFs of invasive carcinoma (Figures 5G and S4E). Decorin is reported to be highly expressed in ECM and to suppress tumorigenic growth when tumor invasion or metastasis occurs.62 Lumican is a key regulator of epithelial-mesenchymal transition.63 From the gene expression and cell co-localization aspect, the above results further demonstrated that myCAFs have biological relevance to the invadopodia with infiltrating immune cells in human breast cancer.
For infiltrating T cells, the states of T cells in invasive carcinoma and DCIS were difficult to classify in single-cell profiles. To characterize the heterogeneity of infiltrating T cells in different metastatic states, we dichotomized the spatial location of T cells into the T cell infiltrating lymphocytes (TILs) inside the myCAFs-specific domains in invasive carcinoma, and the TILs partly overlapped with DCIS. Meanwhile, we re-clustered and annotated the CD8+ T cells from the single-cell profiles (Figures 5H and 5I). For the CD8+ T cells, subtypes including terminal exhausted T cells (terminal Tex; Cd200 and Tigit), Gzmk+ Tex, Gzmb+ Tex, memory T cells (Tm; Znf683, Cxcr6, and Zfp36), naive CD8+ T cells (Tn; Tcf7 and Il7r), and Tex-expressing regulatory T cell markers (Entpd1 and Tnfrsf18) were identified according to the known markers64 (Figure 5I). Next, we used De-spot to infer the proportions of these subtypes in the dichotomized spatial location of T cells. We observed that terminal Tex cells and Gzmb+ Tex cells were highly enriched in the invasive carcinoma with statistical significance (Figure 5J), and memory T cells and naive T cells were highly enriched in the DCIS region with statistical significance (Figure 5K). We further examined the differential expressions of T cell exhaustion factors64,65,66,67 in the invasive carcinoma tissue versus the DCIS tissue. We found that Ifi6 and Ccl3 (factors with exhausting response) expressed higher in the invasive carcinoma TILs with statistical significance, meaning the spatial enrichment of exhausted T cells. In addition, Il7r (another factor that lacks exhausting response) was upregulated in the DCIS TILs with statistical significance, meaning the spatial enrichment of non-exhausted T cells, including naive T cells and memory T cells (Figure 5L). As a consequence of the results above, De-spot revealed that exhausting T cells have higher proportions in invasive carcinoma tissue than those in DCIS tissue.
De-spot showed consistent cell-type-specific domains in single-cell SRT slices
To demonstrate the capability of De-spot in detecting cell-type-specific domains on image-based single-cell SRT data, we applied De-spot to twelve slices from a MERFISH atlas68 of the mouse brain (Figure 6A). These twelve slices contain 156,775 cells and 13 cell types, which are divided into neurons and non-neurons. (Figures 6B and S5A). Consistent with the application of De-spot in 10X Visium mouse brain slices, we still used the single-cell mouse brain profiles from Zeisel et al.39 (Figure S5B).
Figure 6.
De-spot showed similar cell-type-specific domains in single-cell SRT slices
(A) The mouse brain sections in MERFISH SRT slices are stratified into 4 layers: striatum, fiber tracts, cortex, and pia.
(B) UMAP visualization of the 156,775 co-embedded cells from 12 MERFISH slices annotated with neurons and non-neurons. Neurons include excitatory neurons (ExN), inhibitory neurons (InN), and medium spiny neurons (MSN). Non-neurons include oligodendrocytes (Oligo), oligodendrocyte precursor cells (OPC), astrocytes (Astro), ependymal cells (Epen), pericytes (Peri), vascular leptomeningeal cells (VLMC), endothelial cells (Endo), microglia (Micro), macrophages (Macro), and T cells.
(C) F1 score comparison of the cell-type-specific domains in 12 MERFISH slices generated by Giotto, Seurat, CARD, and De-spot (bars indicate mean ± SEM).
(D) 3D Landscapes of 12 MERFISH slices generated by De-spot.
(E) Cell-type-specific domains marked in UMAP embeddings.
(F) UMAP visualization of cell markers corresponding to cell-type-specific domains in (E).
(G) 3D Landscapes of the Stereo-seq slice generated by De-spot.
(H) Spatial markers of cell-type-specific domains detected by De-spot.
(I) F1 score comparison of the cell-type-specific domains in the Stereo-seq slice generated by Giotto, Seurat, CARD, and De-spot.
We generated the 3D Landscapes of seven cell-type-specific domains detected by De-spot, Seurat, Giotto, and CARD in these twelve slices (Figures 6D and S5D–S5F). We found that Seurat recovered the structure of striatum, fiber tracts, and cortex, but it failed to find astrocyte- and microglia-specific domains. Giotto found astrocyte-specific domains in several slices but failed to decipher the specific domains of the other 6 cell types. CARD deciphered neuron-specific domains and non-neuron domains, but it ignored the specific domains of astrocytes and failed to distinguish interneurons, pyramidal CA1, and pyramidal SS. Only De-spot detected all the cell-type-specific domains for the given (seven) major cell types in twelve slices. The F1 scores of De-spot outperformed those of Giotto, Seurat, and CARD in the twelve slices for the provided seven cell types (Figure 6C).
Then we investigated whether De-spot reconstructed the structure of given mouse brain slices. De-spot detected interneuron-specific domains adjacent to the specific domains of oligodendrocytes (co-localization status 5). According to the structure of the mouse brain in Figure 6A, their specific domains are in striatum and fiber tracts, respectively (Figure 6D). De-spot detected pyramidal SS-specific domains also adjacent to oligodendrocytes in twelve slices, indicating that pyramidal SS cells are located in the cortex, consistent with the pyramidal SS-specific domains in 10X mouse brain slices. De-spot detected pyramidal CA1 proximal to pyramidal SS in the front cortex associated with their shared marker genes. Astrocytes and ependymal cells were previously reported to tend to aggregate in pia regions and are distributed uniformly throughout the cortex.68 De-spot uniquely detected astrocytes enriched in pia regions, co-localized with endothelial and mural cells. De-spot also detected astrocytes and ependymal distributed uniformly in the cortex of slices 1, 2, and 10. Microglia were randomly distributed uniformly in the whole tissues for most slices. De-spot still found a higher density of microglia in the pia regions of slices 6, 9, and 11.
Next, according to the UMAP reduction of twelve MERFISH slices after batch effect correction (Figures 6E and 6F), we explored whether cell-type-specific domains detected by De-spot stay consistent among slices. The oligodendrocyte-specific domains were well-matched to the oligodendrocyte and oligodendrocyte precursor cell (OPC) clusters associated with Plp1. The astrocyte-ependymal-specific domains were similar to the endothelial-mural cell domains, indicating that their co-localization patterns are generalizable across different slices. Astrocyte-ependymal- and endothelial-mural-specific domains mainly matched to Astro, Epen, Endo, and Peri clusters associated with their markers Aqp4 and Acta2. Notably, pericyte was a subcluster in endothelial-mural from single-cell profiles (Figure S5C). All the pyramidal CA1 and pyramidal SS-specific domains from twelve slices matched to ExN clusters associated with Mef2c and Tbr1. The interneuron-specific domains typically matched to medium spiny neuron cluster associated with corresponding markers Gad1 and Gad2.
To demonstrate the capability of microarray-based single-cell SRT data, we applied De-spot to another single-cell mouse brain SRT slice generated by Stereo-seq69 from STOmicsDB.70 We still used single-cell profiles from Zeisel to generate 3D Landscapes of cell-type-specific domains detected by De-spot, Seurat, Giotto, and CARD (Figures 6G and S5G–S5I). We found that Seurat failed to detect astrocyte- and microglia-specific domains, Giotto failed to decipher the cortex, and CARD failed to distinguish interneurons, oligodendrocytes, and microglia. Only De-spot detected all the specific domains of the given cell types, and these domains are matched with their cell markers (Figure 6I). In particular, astrocyte-, microglia-, and endothelial-specific domains are partly overlapped in the boundary of the tissue associated with their respective markers AQP4, C1QA, and ACTA2. Interneurons and oligodendrocytes are partly overlapped and associated with GAD2 and MBP, respectively. Pyramidal-specific domains are in the cortex associated with MEF2C (Figure 6H).
In brief, these results further demonstrated that De-spot can precisely decipher the anatomical structures of both image-based and microarray-based SRT mouse brain slices and stay sustainable among different batches.
Discussion
Conventional spatial domains are expected to have high intra-cluster similarity within each domain and low inter-cluster similarity between different domains. They are usually determined by the distributions of high-proportion cell types. In addition to these high-proportion cell types, low-proportion cell types also play important roles in the microenvironment. Using conventional spatial domains, deciphering the spatial heterogeneity of low-proportion cell types is challenging because they have a lower frequency of occurrence, and they are usually intermixed with high-proportion cell types. To unbiasedly decipher the spatial heterogeneity of cell types with different proportions, we defined “cell-type-specific domains,” which are further expected to be spatially heterogeneous domains where the proportions of given cell types are significantly higher than other domains. Further, we presented De-spot to enable precise detection of cell-type-specific domains in both spot-level and single-cell SRT slices. De-spot bridges the gap between cell-type proportions and spatial domains by integrating potential candidates, inferring enrichment regions of cell-type proportions, and mapping enrichment regions to spatial domains. De-spot takes advantage of segmentation and deconvolution simultaneously, making it more effective in selecting cell-type-specific domains as ROIs without manual fine-tuning, including dealing with low-proportion cell types. De-spot requires no extra interventions and avoids the dependency on overlapped highly variable genes. Using De-spot, researchers could effectively explore cell-type-specific domains and their co-localization information through the 3D Landscapes.
Our experimental evaluation showed that De-spot could detect cell-type-specific domains in proper spatial locations and outperformed existing methods in accuracy and robustness. Additionally, it could sensitively detect the specific domains of low-proportion cell types obscured in previous methods. It overcame the challenges in detecting low-proportion cell-type-specific domains from the complex microenvironment, especially in detecting meticulous cells within a potential TME. In the experiments conducted on the PDAC slice, De-spot uniquely identified a TME by observing that several specific domains of low-proportion immune cells were inside the CAF-specific domains combined with the expression of tumor-specific markers Tm4sf1. In the experiments conducted on the TNBC slice, De-spot effectively revealed a high possibility of exhausting T cell proportions by observing that specific domains of T cells and invasive carcinoma were in partly overlapped status.
Further, De-spot elucidated the cell-type-specific domains associated with molecular markers. Previous studies examined single-cell data and found that Srgn plays an important role in epithelial-mesenchymal transition and is upregulated in highly metastatic cells.71 We found that Srgn was upregulated in the TME domains where the immune-related cells and CAFs were co-localized, indicating that Srgn may be a significant marker of TME. Additionally, Vim has been demonstrated to be upregulated in tumor cells with a high possibility of invasion.57 In accordance with this, we deciphered its spatial locations and found that it was also upregulated in TME domains, with co-localization of fibroblasts and infiltrating immune cells. Moreover, cell states of T cell infiltrates in human tumors are subdivided using scRNA-seq.65 The detailed T cell proportions and spatial distributions in TME are difficult to identify,58 which are critical to understanding the functions of different T cell states in tumorigenesis and metastasis. We identified T cell proportions and spatial distributions using De-spot and discovered that exhausted T cells with the upregulation of Ccl3 and Ifi6 were enriched in the TILs of invasive carcinoma. In contrast, non-exhausted T cells with the upregulation of Il7r were enriched in the TILs of DCIS in breast cancer slices.
Limitations of the study
The processing speed of De-spot is promising to be improved because it integrates numerous decontamination, segmentation, and deconvolution methods. We intend to optimize De-spot though parallel programming for large-scale cohort data and to design a real-time analysis module to involve newly developed methods. In addition, displaying cell-type-specific domains still has limitations because 3D Landscapes can only be shown from limited perspectives in the publications. A web server for dynamically displaying 3D Landscapes is required. By conducting intensive studies on more spatial omics including spatial proteomics and epigenomics, De-spot is promising to detect more spatial regions with biological meanings in tissue development, tumor environment, and diseases. Leveraging our discoveries, we intend to excavate their underlying mechanisms and further promote clinical research, pathologic diagnosis, and therapeutic intervention.
STAR★Methods
Key resources table
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the lead contact, Jian Liu (jianliu@nankai.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The data were acquired from the following websites or accession numbers. The mouse brainscRNA-seq data from Zeisel et al.39 have been deposited at GEO: GSE60361, and its annotated data have been deposited athttp://linnarssonlab.org/cortex; Visium SRT data are available at 10x Visium official websites (https://www.10xgenomics.com/resources/datasets/mouse-brain-section-coronal-1-standard-1-0-0; https://www.10xgenomics.com/resources/datasets/adult-mouse-brain-ffpe-1-standard-1-3-0). The scRNA-seq data of the mouse kidney and the annotations from Wu et al. are available at GEO: GSE119531; the 10x Visium SRT data are available from 10x Visium official websites(https://www.10xgenomics.com/resources/datasets/adult-mouse-kidney-ffpe-1-standard-1-3-0). The ST PDAC data and paired scRNA-seq data are available at GEO: GSE111672.The scRNA-seq datasets of human breast cancer from Wu et al.58 are available at GEO: GSE176078; the 10x Visium SRT data are available from the Zenodo data repository (https://doi.org/10.5281/zenodo.4739739). The MERFISH data are available at the CELL x GENE repository (https://cellxgene.cziscience.com/collections/31937775-06024e52-a799-b6acdd2bac2e). The Stereo-seq data of mouse brain are available at the STOmicsDB repository (https://db.cngb.org/stomics/datasets/STDS0000234).
-
•
The original code of De-spot is available at GitHub: https://github.com/lyotvincent/Despot.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Method details
De-spot aims to precisely detect cell-type-specific domains in both spot-level and single-cell resolution SRT data via ensemble learning. We developed a data representation called smd as the core data structure of De-spot. In addition to spatial modalities analysis, De-spot provides a variational inference module to derive single-cell patterns. De-spot integrates several segmentation and deconvolution methods as an ensemble to detect cell-type-specific domains in the modality integration module. After detecting cell-type-specific domains, De-spot examines the upregulated genes of these domains, deciphers the cell-type co-localizations, and finally visualizes them using 3D Landscapes.
Cell-type-specific domains
Cell-type-specific domains are expected to be spatial heterogeneous domains where the proportions of given cell types are significantly higher than other domains. Formally, suppose the SRT slice contains spots and single-cell profiles provide potential cell types . Each cell type is combined with its detected specific domain as a cell-type-specific domain tuple , and the set of all these tuples is denoted as .
| (Equation 1) |
Cell-type-specific domains in have two properties: (1) they allow existing such that and (2) . It means that a spot may belong to one or multiple cell-type-specific domains, meanwhile some spots are not encompassed by any cell-type-specific domains. For the optimization of cell-type-specific domain tuple , let denote the difference in the proportion of between and other domains.
| (Equation 2) |
Here, denotes the cell proportions of within spot in spot-level slices. In single-cell level SRT slices, denotes the probability that the ground truth cell type of spot is . . The cell-type-specific domain is optimized to be spatially heterogeneous and have the maximization of .
We can see that the definition of cell-type-specific domains is suitable for SRT slices in both spot-level and single-cell resolution. Further, cell-type-specific domains mainly focus on the location of potential cell types and relax restrictions on regional segmentation, making the cell-type co-localizations well-curved. Assuming two different cell-type-specific domain tuples and , The co-localization of and is deciphered into five potential statuses:
-
•
sta1 disjoint:
-
•
sta2 totally overlapped:
-
•
sta3 partly overlapped:
-
•
sta4 inside:
-
•
sta5 adjacent: .
In particular, the partly overlapped status (sta3) indicates a critical niche at their intersection regions, the inside status (sta4) means a cell type may play a crucial role in the specific domains of another one, and the adjacent status (sta5) depicts probable cell-cell interactions across their boundaries. A diagram is provided for illustration in Figures S1A and S1B. For real-world examples, in the application of the Visium mouse kidney slices (Figures 3G and 3H), De-spot detected CD-PC totally overlapped with LH(DL) (sta2). In the application of the PDAC slice (Figure 4C), De-spot detected fibroblasts partly overlapped with immune-related cells (sta3). In the application of the TNBC slices (Figure 5D), De-spot detected exhausted T cells inside the CAFs (sta4). In the application of MERFISH slices (Figure 6D), De-spot detected interneurons adjacent to oligodendrocytes (sta5).
Data representation
De-spot begins with three inputs: (1) a spatial expression matrix of unique molecular identifier (UMI) counts, (2) the accompanying two or three-dimensional spatial coordinates, and (3) a single-cell UMI matrix with annotations. Specifically, paired single-cell and SRT profiles are recommended. De-spot designs a spatial multi-omics data representation format called smd based on HDF5 binary data (Figures 1E and S1D). The smd files offer features to (1) provide compressed data format for SRT data, spatial contexts, and single-cell profiles, (2) store compatible profiles generated from diverse SRT and single-cell sequencing platforms, (3) store intermediate data profiles generated during downstream analysis and (4) provide compatible interfaces for popular infrastructures in Python (stData,14 Squidpy,28 and Scanpy29) and R ecosystems (Seurat,26 Giotto,27 SpatialExperiment,72 SingleCellExpreiment,73 and STUtility74).
The smd files contain three sections: spMatrix, spContexts, and scMatrix (Figure S1). The spMatrix manages the original input of spatial expression matrices, the generated matrices from the de-noising phase, and their submatrices. These matrices have their own set of spatial domains and cell-type proportions candidates. Each generated matrix retains the same barcodes and features as the origin matrix. Each submatrix stores the selected barcode and feature indexes of the original matrix. Column compression is used to store sparse matrices in spMatrix. The spContexts store spatial coordinates, size of sampling units, histology images at various resolutions, and scale factors among high-resolution and low-resolution images. The scMatrix manages the original input single-cell profiles and the VAE-derived single-cell patterns. Annotations for cell types in scMatrix are required. The smd files enable De-spot to store multi-modality SRT data in a compressed file and facilitate the subsequent analysis and visualization.
SMD file is initialized with function SMD_Init. They accept the input from 10X Visium folder, Slide-seq folder, h5ad for single-cell SRT data, and txt for other matrix-based format data. In the Python environment, spatial and single-cell data in SMD files can be converted to AnnData with functions Load_smd_to_Anndata and Load_smdsc_to_Anndata, respectively. In the R environment, SMD files can be converted to R objects with the function Load_smd_to_X, and X can be set to Seurat, SpatialExperiment, SingleCellExperiment, and Giotto. For other formats, it’s easy to obtain them through the conversion of these intermediate infrastructures. In addition, SMD also provides Load_h5ad_to_sce for loading single-cell h5ad data into the R environment. As a result, it’s easy to transfer data between Python and R ecosystems using the functions above. To store intermediate data generated from downstream analysis, De-spot provides Save_idents_to_smd for saving segmentation data, Save_deconv_to_smd for saving deconvolution data, and Save_reduction_to_smd for storing embedding data with the same length of barcodes. De-spot also provides Save_mat_to_smd for storing denoised or imputed data, and Save_subset_to_smd for subset data.
Spatial multi-modalities analysis
Spatial graph
The spatial neighborhood relationship is represented using a spatial graph in which each node denotes a spot (cell or bead), and each edge connects a pair of adjacent spots. For the SRT sequencing platforms using gridded spots (ST, Visium), De-spot defines the number of neighbors as 4 for square spots and 6 for hex spots, respectively. De-spot creates a Delaunay triangulation network for other SRT sequencing platforms to identify each spot’s corresponding neighbors. Alternatively, De-spot provides k-nearest neighborhood or Euclidean distance to evaluate the spatial proximity by setting k or radius values, respectively. The neighbors of each spot are stored in spContexts.
SRT analysis
For each spMatrix, De-spot first removes the genes of low expression to reduce computational cost. Then, gene expression values in each spot are normalized and divided by the total UMI count across all genes in a given spot. After normalization, the expression values are multiplied by 10,000 and converted to a natural log scale. Considering SRT sequencing platforms usually suffer from contaminations such as sparsity, dropout events, platform-specific noise, and mRNA exchange,33 De-spot integrates previous de-noising methods33,34,35,36 to handle the sparsity and particular noise in spMatrix under the premise that adjacent units are more likely to express similarly.
Single-cell pattern derivation
De-spot develops a deep-learning model based on the Bayesian variational inference37 to generate high-quality single-cell patterns and accelerate modality integration. The developed model uses an “Encoder-Latent Distribution-Decoder” neural network structure, and it trains the embedding patterns of each cell type in scMatrix. After the training, all cell-type patterns are inferred, down-sampled, and aggregated to form a complete single-cell pattern profile for the deconvolution.
The variational inference module contains an input layer, a hidden layer, and an out layer. The hidden layer is obtained by sampling from a hidden variable distribution satisfying a multivariate Gaussian distribution, whose parameters are generated by forward propagation. The formulation of our variational inference module is given below:
| (Equation 3) |
| (Equation 4) |
| (Equation 5) |
Where E denotes an encoder composed of fully connected layers, (·) denotes a nonlinear activation function such as ReLU. W denotes the weight matrix of the corresponding layers. and are the outputs of the Encoder, denoting the mean and variance of the hidden variable Z, respectively. denotes the preprocessed matrix for a cell-type subset with log and min-max scaler, and it is defined as below:
| (Equation 6) |
The acquisition of hidden variable Z is sampled from the distribution N (, 2). Considering the sampling operation is not derivable in backpropagation, as reported by previous research,37 we do reparameterization as below:
| (Equation 7) |
After that, the gradient can be back-propagated directly through and . Finally, a decoder D composed of fully connected layers is used to generate , the final expression patterns of cell subsets are defined as follows:
| (Equation 8) |
| (Equation 9) |
By adjusting the weights of the neural network, the variational inference module in an unsupervised manner to efficiently compress and subsequently reconstruct the data using the combination of typically mean square error (MSE) loss function and K-L divergence:
| (Equation 10) |
Modality integration
Integration analysis of spatial transcriptome and spatial contexts is to take full advantage of image features and spatial neighbor graphs as prior or embeddings for clustering. De-spot provides some Bayesian-based methods,11 which use spatial contexts as priors and encourage adjacent spots to belong to the same cluster, and neural-network-based methods,12,13 which encode the spatial neighbor graphs and image features as additional information to the embedding layers of SRT gene expression. In addition, De-spot provides image-based methods,14,28 which homogenize gene expression by H&E stain images and neighborhood information of each spot at first and then conduct unsupervised clustering. De-spot also integrates previous standard clustering algorithms,41 and single-cell SRT segmentation methods BASS and MENDER.
De-spot provides two strategies to integrate spatial transcriptome and single-cell profiles: (1) mapping cells into space positions and (2) inferring the cell-type proportion at each spot using deconvolution. The mapping methods15,75 transform SRT and single-cell data into a shared space in which a unique cell-type label classifies each cell or spot. The correspondence scores between spots and cell types are further evaluated, and spots classified to certain cell types with high confidence will receive high correspondence scores. Finally, De-spot converts the correspondence scores to cell-type proportions by normalization. The deconvolution methods infer cell-type proportions based on probability distributions in the Bayesian framework or non-negative matrix factorization (NMF) regression. The Bayesian-based methods fit the SRT data to some prior distributions, such as negative binomial distribution16,18 and Poisson distribution,17 then use maximum likelihood estimation or Expectation Maximization (E-M) Algorithm to determine the parameters of the SRT data distribution model with the scRNA-seq data as a priori. Next, the gene expressions of spots are used as input, producing output as the maximum posterior estimate of the distribution of cell subsets. The regression-based methods use NMF to create cell-type-specific topic profiles, then use nonnegative least squares19 or damped weighted least squares20 to determine cell-type proportions at each spot. The deconvolution (mapping) result is a matrix where the rows denote the barcode of spots, the columns denote the specified cell types, and the elements denote the cell-type proportions. De-spot uses raw single-cell datasets for the deconvolution tools, which have strict prior distribution assumptions for single-cell expression patterns (Cell2Location16). For others, De-spot uses the variational inference module to generate single-cell patterns as input. For each spMatrix, De-spot integrates all the methods above to detect spatial domain candidates and cell-type proportion candidates, then stores them into barcode metadata (Figure 1E).
In addition, De-spot can deal with batch effects when comparing slices between conditions by Harmony.75 De-spot could load different batches by setting SMD files as a list through the function Despot_Embedding to correct batch effects and generate integrated reductions.
Detection of cell-type-specific domains
De-spot uses ensemble learning to select the best pairs between the region and cell-type proportion candidates to find the accurate cell-type-specific domains. Suppose a given cell type have cell-type proportion candidates . According to the global Moran’s Index, De-spot removes cell types without spatial heterogeneity for each cell-type proportion candidate. Suppose the proportion of detected by deconvolution (mapping) method in each spot is , and its average proportion is ; the global Moran’s Index is evaluated as below:
| (Equation 11) |
where N denotes the number of total spots; wij denotes the distance between spot and spot . When two spots are adjacent to each other, wij = 1; otherwise, wij = 0. W denotes the sum of wij. De-spot selects the cell types with Moran’s Index larger than 0.5 (larger than 0.05 in single-cell resolution SRT data), which are generally considered to be spatially heterogeneous.
Then, De-spot evaluates the enrichment regions of cell types with spatial heterogeneity according to the local Moran’s Index. For each spot and its certain cell-type proportion , the local Moran’s Index is evaluated as below:
| (Equation 12) |
Here, , denotes the spatial distance between spot and spot , and N denotes the number of spots in tissue. Next, De-spot evaluates the significance of the local Moran’s Index using Z-test:
| (Equation 13) |
Focusing on the aggregation of cells in tissues, spot satisfied with three conditions below are included in spatial heterogeneity spots set for cell-type :
| (Equation 14) |
The first condition denotes that the cell-type proportion of spot is larger than the average proportion. The second condition denotes that the average cell-type proportion of surrounding spots is also larger than the average proportion. The third condition denotes that the spots with adjusted p values in the Z-test less than 0.05 are considered to have spatial heterogeneity.
Next, De-spot associates generated spatial domains with the enrichment regions of cell types with spatial heterogeneity. For all spatial domain candidates, assuming that the slice is totally segmented into k spatial domains , the F1-score is evaluated as below:
| (Equation 15) |
| (Equation 16) |
| (Equation 17) |
where denotes the weight between precision and recall , the default is 1. De-spot finds the most suitable domain for this cell type :
| (Equation 18) |
However, a cell type may be abundant in different parts of tissues, meaning that more than one spatial domain should be associated with a cell type. Here, we propose a greedy strategy to extend the best spatial domain. De-spot repeatedly appends other regions to and recalculates the F1 score to decide the inclusion of :
| (Equation 19) |
If new domains are added to make the F1 score larger, De-spot repeatedly appends these domains to until . Through the above steps, De-spot finds the spatial-heterogeneous specific domain of using the cell-type proportions generated by method . De-spot detects cell-type-specific domains using cell proportion candidates , and finds to generate the cell-type-specific domain tuple .
| (Equation 20) |
Finally, with the given spots and cell types , De-spot successfully detects cell-type-specific domains .
Upregulated genes detection
After finding the most suitable domains corresponding to cell type in tissue, we examine the upregulated genes and detect the abundance of cell types. We investigate the genes that are differentially expressed in region and the cell type in single-cell (single-nucleus) profiles (adjusted p value <10-5, Wilcoxon rank-sum test; effect size >0.5, Cohen’s d). Then, we find the intersection of differentially expressed genes between them and query the significance of the overlap using the hypergeometric cumulative distribution:
| (Equation 21) |
N denotes the number of all background genes; m and n denote the differentially expressed genes in region and the cell type , and denotes the length of the intersection part.
Cell-type co-localizations
De-spot evaluates Pearson correlations between pairwise cell type proportions in the usage of all spots in tissue to estimate the co-occurrence of spatial cell types. Due to the spatial hysteresis effect, we append a spatial lag item to the cell type composition score. For each spot and each cell-type , let be the origin cell type composition score. We have the adjusted cell-type proportion of spot i and cell-type below:
| (Equation 22) |
where denotes the weight of the spatial lag item, the default is 1; when spot is adjacent to spot , wij = 1; otherwise wij = 0. The Pearson correlation r between pairwise cell type k and l is calculated as follows:
| (Equation 23) |
3D Landscapes
De-spot designs 3D Landscapes to visualize the detected cell-type-specific domains. The generated landscapes curve the outlines of cell-type-specific domains in tissues and display the overlapped domains in layered structures. De-spot uses the Alpha-shape algorithm to curve the outlines in tissues and uses dots with colors to represent the layers of cell-type-specific domains. To map the layered structures onto outlines in tissues, De-spot randomly selects dots of 10 percent for each layer and creates waterfall lines to link the dots and outlined regions together. De-spot arranges the domains in stack mode or stairway mode. The stack mode arranges domains in the bottom layer unless other overlapped domains exist. The stairway mode arranges domains according to their centroids. The domains with centroids closer to the coordinate axis are arranged in the lower layer. With 3D Landscapes, researchers can intuitively observe the spatial location of cell-type-specific domains and the co-localizations among cell types. For SRT data with no images (e.g., MERFISH data), 3D Landscape sets the largest domain to the bottom layer and hides the contour lines. 3D Landscapes can be plotted using the function Show_3DLandscape by receiving an output SMD file from De-spot. An example of 3D Landscape is shown in Figure S1C.
Quantification and statistical analysis
Statistical analyses were performed in Python with Scanpy and Scipy package. The differential analysis of gene expression was performed by empirical Bayes moderated t tests with a Benjamini Hochberg correction for multiple testing. Pairwise comparisons of cell-type proportions were performed using Wilcoxon rank-sum test. The significance of the local and global Moran’s Index was evaluated using Z-test. The spatial co-localizations of paired cell types were quantified by Pearson’s R with t tests. For all comparisons, p values less than 0.05 were considered statistically significant.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFA0908700 and 2020YFA0908702), the National Natural Science Foundation of China (62272246), and the CAAI-MindSpore Open Fund.
Author contributions
J.L. conceived and designed the project. Z.R. and J.L. developed the approach and conducted bioinformatics analysis. Z.R., W.Z., H.L., J.W., and S.C. conducted the case studies. W.Z. and H.L. assisted with interpreting the data. X.W. provided help with landscape visualization. Z.R., Y.P., Chaoyang Yan, W.X., and Chengwei Yan performed the preprocessing and data integration for the scRNA-seq and SRT data. Z.R. and J.L. wrote the manuscript with input from all authors. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: August 9, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2024.100841.
Supplemental information
References
- 1.van den Brink S.C., Sage F., Vértesy Á., Spanjaard B., Peterson-Maduro J., Baron C.S., Robin C., van Oudenaarden A. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat. Methods. 2017;14:935–936. doi: 10.1038/nmeth.4437. [DOI] [PubMed] [Google Scholar]
- 2.Ståhl P.L., Salmén F., Vickovic S., Lundmark A., Navarro J.F., Magnusson J., Giacomello S., Asp M., Westholm J.O., Huss M., et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 3.Lewis S.M., Asselin-Labat M.L., Nguyen Q., Berthelet J., Tan X., Wimmer V.C., Merino D., Rogers K.L., Naik S.H. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods. 2021;18:997–1012. doi: 10.1038/s41592-021-01203-6. [DOI] [PubMed] [Google Scholar]
- 4.Rodriques S.G., Stickels R.R., Goeva A., Martin C.A., Murray E., Vanderburg C.R., Welch J., Chen L.M., Chen F., Macosko E.Z. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363:1463–1467. doi: 10.1126/science.aaw1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marx V. Method of the Year: spatially resolved transcriptomics. Nat. Methods. 2021;18:9–14. doi: 10.1038/s41592-020-01033-y. [DOI] [PubMed] [Google Scholar]
- 6.Moses L., Pachter L. Museum of spatial transcriptomics. Nat. Methods. 2022;19:534–546. doi: 10.1038/s41592-022-01409-2. [DOI] [PubMed] [Google Scholar]
- 7.Rao A., Barkley D., França G.S., Yanai I. Exploring tissue architecture using spatial transcriptomics. Nature. 2021;596:211–220. doi: 10.1038/s41586-021-03634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker B.L., Cang Z., Ren H., Bourgain-Chang E., Nie Q. Deciphering tissue structure and function using spatial transcriptomics. Commun. Biol. 2022;5:220. doi: 10.1038/s42003-022-03175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palla G., Fischer D.S., Regev A., Theis F.J. Spatial components of molecular tissue biology. Nat. Biotechnol. 2022;40:308–318. doi: 10.1038/s41587-021-01182-1. [DOI] [PubMed] [Google Scholar]
- 10.Longo S.K., Guo M.G., Ji A.L., Khavari P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 2021;22:627–644. doi: 10.1038/s41576-021-00370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao E., Stone M.R., Ren X., Guenthoer J., Smythe K.S., Pulliam T., Williams S.R., Uytingco C.R., Taylor S.E.B., Nghiem P., et al. Spatial transcriptomics at subspot resolution with BayesSpace. Nat. Biotechnol. 2021;39:1375–1384. doi: 10.1038/s41587-021-00935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J., Li X., Coleman K., Schroeder A., Ma N., Irwin D.J., Lee E.B., Shinohara R.T., Li M. SpaGCN: Integrating gene expression, spatial location and histology to identify spatial domains and spatially variable genes by graph convolutional network. Nat. Methods. 2021;18:1342–1351. doi: 10.1038/s41592-021-01255-8. [DOI] [PubMed] [Google Scholar]
- 13.Fu H., Xu H., Chong K., Li M., Ang K.S., Lee H.K., Ling J., Chen A., Shao L., Liu L., Chen J. Unsupervised Spatially Embedded Deep Representation of Spatial Transcriptomics. bioRxiv. 2021 doi: 10.1101/2021.06.15.448542. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duy P., Xiao T., Jun X., Laura F.G., Pui Y.L., Arti R., Jana V., Marc J.R., Quan N. stLearn: integrating spatial location, tissue morphology and gene expression to find cell types, cell-cell interactions and spatial trajectories within undissociated tissues. bioRxiv. 2020 doi: 10.1101/2020.05.31.125658. Preprint at. [DOI] [Google Scholar]
- 15.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleshchevnikov V., Shmatko A., Dann E., Aivazidis A., King H.W., Li T., Elmentaite R., Lomakin A., Kedlian V., Gayoso A., et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 2022;40:661–671. doi: 10.1038/s41587-021-01139-4. [DOI] [PubMed] [Google Scholar]
- 17.Cable D.M., Murray E., Zou L.S., Goeva A., Macosko E.Z., Chen F., Irizarry R.A. Robust decomposition of cell type mixtures in spatial transcriptomics. Nat. Biotechnol. 2022;40:517–526. doi: 10.1038/s41587-021-00830-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson A., Bergenstråhle J., Asp M., Bergenstråhle L., Jurek A., Fernández Navarro J., Lundeberg J. Single-cell and spatial transcriptomics enables probabilistic inference of cell type topography. Commun. Biol. 2020;3:565. doi: 10.1038/s42003-020-01247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elosua-Bayes M., Nieto P., Mereu E., Gut I., Heyn H. SPOTlight: seeded NMF regression to deconvolute spatial transcriptomics spots with single-cell transcriptomes. Nucleic Acids Res. 2021;49 doi: 10.1093/nar/gkab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong R., Yuan G.C. SpatialDWLS: accurate deconvolution of spatial transcriptomic data. Genome Biol. 2021;22:145. doi: 10.1186/s13059-021-02362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J., Luo T., Jiang M., Liu J., Gupta G.P., Li Y. Cell composition inference and identification of layer-specific spatial transcriptional profiles with POLARIS. Sci. Adv. 2023;9 doi: 10.1126/sciadv.add9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Z., Pan W., Zhao X., Zhao F., Xu Z., Li X., Zhao Y., Zhang M.Q., Yao J. SODB facilitates comprehensive exploration of spatial omics data. Nat. Methods. 2023;20:387–399. doi: 10.1038/s41592-023-01773-7. [DOI] [PubMed] [Google Scholar]
- 23.Li B., Zhang W., Guo C., Xu H., Li L., Fang M., Hu Y., Zhang X., Yao X., Tang M., et al. Benchmarking spatial and single-cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat. Methods. 2022;19:662–670. doi: 10.1038/s41592-022-01480-9. [DOI] [PubMed] [Google Scholar]
- 24.Li Z., Zhou X. BASS: multi-scale and multi-sample analysis enables accurate cell type clustering and spatial domain detection in spatial transcriptomic studies. Genome Biol. 2022;23:168. doi: 10.1186/s13059-022-02734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Z. MENDER: fast and scalable tissue structure identification in spatial omics data. Nat. Commun. 2024;15:207. doi: 10.1038/s41467-023-44367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dries R., Zhu Q., Dong R., Eng C.H.L., Li H., Liu K., Fu Y., Zhao T., Sarkar A., Bao F., et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 2021;22:78. doi: 10.1186/s13059-021-02286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palla G., Spitzer H., Klein M., Fischer D., Schaar A.C., Kuemmerle L.B., Rybakov S., Ibarra I.L., Holmberg O., Virshup I., et al. Squidpy: a scalable framework for spatial omics analysis. Nat. Methods. 2022;19:171–178. doi: 10.1038/s41592-021-01358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf F.A., Angerer P., Theis F.J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moncada R., Barkley D., Wagner F., Chiodin M., Devlin J.C., Baron M., Hajdu C.H., Simeone D.M., Yanai I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020;38:333–342. doi: 10.1038/s41587-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y., Zhou X. Spatially informed cell-type deconvolution for spatial transcriptomics. Nat. Biotechnol. 2022;40:1349–1359. doi: 10.1038/s41587-022-01273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmal C., Myung J., Herzel H., Bordyugov G. Moran’s I quantifies spatio-temporal pattern formation in neural imaging data. Bioinformatics. 2017;33:3072–3079. doi: 10.1093/bioinformatics/btx351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni Z., Prasad A., Chen S., Halberg R.B., Arkin L.M., Drolet B.A., Newton M.A., Kendziorski C. SpotClean adjusts for spot swapping in spatial transcriptomics data. Nat. Commun. 2022;13:2971. doi: 10.1038/s41467-022-30587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Song B., Wang S., Chen M., Xie Y., Xiao G., Wang L., Wang T. Sprod for de-noising spatially resolved transcriptomics data based on position and image information. Nat. Methods. 2022;19:950–958. doi: 10.1038/s41592-022-01560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., Pan Y., Ruan Z., Guo J. SCDD: a novel single-cell RNA-seq imputation method with diffusion and denoising. Briefings Bioinf. 2022;23 doi: 10.1093/bib/bbac398. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Wang T., Duggan B., Sharpnack M., Huang K., Zhang J., Ye X., Johnson T.S. SPCS: a spatial and pattern combined smoothing method for spatial transcriptomic expression. Briefings Bioinf. 2022;23 doi: 10.1093/bib/bbac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingma D.P., Welling M. Auto-Encoding Variational Bayes. arXiv. 2014 doi: 10.48550/arXiv.1312.6114. Preprint at. [DOI] [Google Scholar]
- 38.10x Genomics Mouse brain cortex data. 2019. https://www.10xgenomics.com/resources/datasets/mouse-brain-section-coronal-1-standard-1-0-0 10x Genomics.
- 39.Zeisel A., Muñoz-Manchado A.B., Codeluppi S., Lönnerberg P., La Manno G., Juréus A., Marques S., Munguba H., He L., Betsholtz C., et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz C., Navarro J.F., Jurek A., Märtin A., Lundeberg J., Meletis K. Molecular atlas of the adult mouse brain. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traag V.A., Waltman L., van Eck N.J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 2019;9:5233. doi: 10.1038/s41598-019-41695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becht E., McInnes L., Healy J., Dutertre C.A., Kwok I.W.H., Ng L.G., Ginhoux F., Newell E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2019;37:38–44. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z., Zhao Y. Progress on the roles of MEF2C in neuropsychiatric diseases. Mol. Brain. 2022;15:8. doi: 10.1186/s13041-021-00892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krienen F.M., Goldman M., Zhang Q., Del Rosario R.C.H., Florio M., Machold R., Saunders A., Levandowski K., Zaniewski H., Schuman B., et al. Innovations present in the primate interneuron repertoire. Nature. 2020;586:262–269. doi: 10.1038/s41586-020-2781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meffre D., Massaad C., Grenier J. Lithium chloride stimulates PLP and MBP expression in oligodendrocytes via Wnt/β-catenin and Akt/CREB pathways. Neuroscience. 2015;284:962–971. doi: 10.1016/j.neuroscience.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 46.10x Genomics . 2021. Mouse kidney data.https://www.10xgenomics.com/resources/datasets/adult-mouse-kidney-ffpe-1-standard-1-3-0 10x Genomics. [Google Scholar]
- 47.Wu H., Kirita Y., Donnelly E.L., Humphreys B.D. Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J. Am. Soc. Nephrol. 2019;30:23–32. doi: 10.1681/ASN.2018090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao X., Li C., Yang H., Lu X., Liao J., Qian J., Wang K., Cheng J., Yang P., Chen H., et al. Knowledge-graph-based cell-cell communication inference for spatially resolved transcriptomic data with SpaTalk. Nat. Commun. 2022;13:4429. doi: 10.1038/s41467-022-32111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu F., Yang X., Zheng M., Zhao Q., Zhang K., Li Z., Zhang H., Zhang S. Role of Transmembrane 4 L Six Family 1 in the Development and Progression of Cancer. Front. Mol. Biosci. 2020;7:202–889X. doi: 10.3389/fmolb.2020.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wosen J.E., Mukhopadhyay D., Macaubas C., Mellins E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018;9:2144–3224. doi: 10.3389/fimmu.2018.02144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., Gong P., Chen T., Gao S., Wu Z., Wang X., Li J., Marjani S.L., Costa J., Weissman S.M., et al. Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single-Cell Analysis of Transcriptome and Telomeres. Adv. Sci. 2021;8:2004320. doi: 10.1002/advs.202004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rad Pour S., Pico de Coaña Y., Demorentin X.M., Melief J., Thimma M., Wolodarski M., Gomez-Cabrero D., Hansson J., Kiessling R., Tegner J. Predicting anti-PD-1 responders in malignant melanoma from the frequency of S100A9+ monocytes in the blood. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young M.D., Mitchell T.J., Vieira Braga F.A., Tran M.G.B., Stewart B.J., Ferdinand J.R., Collord G., Botting R.A., Popescu D.M., Loudon K.W., et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science. 2018;361:594–599. doi: 10.1126/science.aat1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z., Deng Y., Zheng G., Jia X., Xiong Y., Luo K., Qiu Q., Qiu N., Yin J., Lu M., et al. SRGN-TGFβ2 regulatory loop confers invasion and metastasis in triple-negative breast cancer. Oncogenesis. 2017;6:e360–e9024. doi: 10.1038/oncsis.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng B., Wu J., Shen B., Jiang F., Feng J. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int. 2022;22:166–2867. doi: 10.1186/s12935-022-02599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong Z., Li Q., Shi J., Wei J., Li P., Chang C.H., Shultz L.D., Ren G. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity. 2022;55:1483–1500.e9. doi: 10.1016/j.immuni.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohebi M., Ghafouri-Fard S., Modarressi M.H., Dashti S., Zekri A., Kholghi-Oskooei V., Taheri M. Expression analysis of vimentin and the related lncRNA network in breast cancer. Exp. Mol. Pathol. 2020;115:104439–104800. doi: 10.1016/j.yexmp.2020.104439. [DOI] [PubMed] [Google Scholar]
- 58.Wu S.Z., Al-Eryani G., Roden D.L., Junankar S., Harvey K., Andersson A., Thennavan A., Wang C., Torpy J.R., Bartonicek N., et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 2021;53:1334–1347. doi: 10.1038/s41588-021-00911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barkal A.A., Brewer R.E., Markovic M., Kowarsky M., Barkal S.A., Zaro B.W., Krishnan V., Hatakeyama J., Dorigo O., Barkal L.J., Weissman I.L. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuang F., Liu J., Xie Y., Tang D., Kang R. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem. Biol. 2021;28:765–775.e5. doi: 10.1016/j.chembiol.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Comba A., Faisal S.M., Dunn P.J., Argento A.E., Hollon T.C., Al-Holou W.N., Varela M.L., Zamler D.B., Quass G.L., Apostolides P.F., et al. Spatiotemporal analysis of glioma heterogeneity reveals COL1A1 as an actionable target to disrupt tumor progression. Nat. Commun. 2022;13:3606. doi: 10.1038/s41467-022-31340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neill T., Schaefer L., Iozzo R.V. Decorin as a multivalent therapeutic agent against cancer. Adv. Drug Deliv. Rev. 2016;97:174–185. doi: 10.1016/j.addr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karamanou K., Franchi M., Vynios D., Brézillon S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin. Cancer Biol. 2020;62:125–133. doi: 10.1016/j.semcancer.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Zheng L., Qin S., Si W., Wang A., Xing B., Gao R., Ren X., Wang L., Wu X., Zhang J., et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. 2021;374 doi: 10.1126/science.abe6474. [DOI] [PubMed] [Google Scholar]
- 65.Van der Leun A.M., Thommen D.S., Schumacher T.N. CD8+ T cell states in human cancer: insights from single-cell analysis. Nat. Rev. Cancer. 2020;20:218–232. doi: 10.1038/s41568-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolina J.S., Van Braeckel-Budimir N., Thomas G.D., Salek-Ardakani S. CD8+ T Cell Exhaustion in Cancer. Front. Immunol. 2021;12:1664–3224. doi: 10.3389/fimmu.2021.715234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sade-Feldman M., Yizhak K., Bjorgaard S.L., Ray J.P., de Boer C.G., Jenkins R.W., Lieb D.J., Chen J.H., Frederick D.T., Barzily-Rokni M., et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen W.E., Blosser T.R., Sullivan Z.A., Dulac C., Zhuang X. Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell. 2023;186:194–208.e18. doi: 10.1016/j.cell.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei X., Fu S., Li H., Liu Y., Wang S., Feng W., Yang Y., Liu X., Zeng Y.Y., Cheng M., et al. Single-cell Stereo-seq reveals induced progenitor cells involved in axolotl brain regeneration. Science. 2022;377 doi: 10.1126/science.abp9444. [DOI] [PubMed] [Google Scholar]
- 70.Xu Z., Wang W., Yang T., Li L., Ma X., Chen J., Wang J., Huang Y., Gould J., Lu H., et al. STOmicsDB: a comprehensive database for spatial transcriptomics data sharing, analysis and visualization. Nucleic Acids Res. 2024;52:D1053–D1061. doi: 10.1093/nar/gkad933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tellez-Gabriel M., Tekpli X., Reine T.M., Hegge B., Nielsen S.R., Chen M., Moi L., Normann L.S., Busund L.T.R., Calin G.A., et al. Serglycin Is Involved in TGF-β Induced Epithelial-Mesenchymal Transition and Is Highly Expressed by Immune Cells in Breast Cancer Tissue. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.868868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Righelli D., Weber L.M., Crowell H.L., Pardo B., Collado-Torres L., Ghazanfar S., Lun A.T.L., Hicks S.C., Risso D. SpatialExperiment: infrastructure for spatially-resolved transcriptomics data in R using Bioconductor. Bioinformatics. 2022;38:3128–3131. doi: 10.1093/bioinformatics/btac299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amezquita R.A., Lun A.T.L., Becht E., Carey V.J., Carpp L.N., Geistlinger L., Marini F., Rue-Albrecht K., Risso D., Soneson C., et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods. 2020;17:137–145. doi: 10.1038/s41592-019-0654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergenstråhle J., Larsson L., Lundeberg J. Seamless integration of image and molecular analysis for spatial transcriptomics workflows. BMC Genom. 2020;21:482. doi: 10.1186/s12864-020-06832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., Baglaenko Y., Brenner M., Loh P.R., Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The data were acquired from the following websites or accession numbers. The mouse brainscRNA-seq data from Zeisel et al.39 have been deposited at GEO: GSE60361, and its annotated data have been deposited athttp://linnarssonlab.org/cortex; Visium SRT data are available at 10x Visium official websites (https://www.10xgenomics.com/resources/datasets/mouse-brain-section-coronal-1-standard-1-0-0; https://www.10xgenomics.com/resources/datasets/adult-mouse-brain-ffpe-1-standard-1-3-0). The scRNA-seq data of the mouse kidney and the annotations from Wu et al. are available at GEO: GSE119531; the 10x Visium SRT data are available from 10x Visium official websites(https://www.10xgenomics.com/resources/datasets/adult-mouse-kidney-ffpe-1-standard-1-3-0). The ST PDAC data and paired scRNA-seq data are available at GEO: GSE111672.The scRNA-seq datasets of human breast cancer from Wu et al.58 are available at GEO: GSE176078; the 10x Visium SRT data are available from the Zenodo data repository (https://doi.org/10.5281/zenodo.4739739). The MERFISH data are available at the CELL x GENE repository (https://cellxgene.cziscience.com/collections/31937775-06024e52-a799-b6acdd2bac2e). The Stereo-seq data of mouse brain are available at the STOmicsDB repository (https://db.cngb.org/stomics/datasets/STDS0000234).
-
•
The original code of De-spot is available at GitHub: https://github.com/lyotvincent/Despot.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.