Summary
The availability of data from profiling of cancer patients with multiomics is rapidly increasing. However, integrative analysis of such data for personalized target identification is not trivial. Multiomics2Targets is a platform that enables users to upload transcriptomics, proteomics, and phosphoproteomics data matrices collected from the same cohort of cancer patients. After uploading the data, Multiomics2Targets produces a report that resembles a research publication. The uploaded matrices are processed, analyzed, and visualized using the tools Enrichr, KEA3, ChEA3, Expression2Kinases, and TargetRanger to identify and prioritize proteins, genes, and transcripts as potential targets. Figures and tables, as well as descriptions of the methods and results, are automatically generated. Reports include an abstract, introduction, methods, results, discussion, conclusions, and references and are exportable as citable PDFs and Jupyter Notebooks. Multiomics2Targets is applied to analyze version 3 of the Clinical Proteomic Tumor Analysis Consortium (CPTAC3) pan-cancer cohort, identifying potential targets for each CPTAC3 cancer subtype. Multiomics2Targets is available from https://multiomics2targets.maayanlab.cloud/.
Keywords: multiomics, GPT, LLM, transcriptomics, KEA3, ChEA3, X2K, Expression2Kinases
Graphical abstract

Highlights
-
•
A bioinformatics platform for multiomics analysis of cancer patient cohorts
-
•
Identifies ranked targets based on transcriptomics, proteomics, and phosphoproteomics
-
•
Generates reports exportable as citable PDFs and Jupyter Notebooks
-
•
Application to the CPTAC3 pan-cancer cohort identifies targets for individual patients
Motivation
Finding targets and driver cell signaling pathways that are uniquely activated in tumors while absent from normal tissues and cell types remains a significant challenge. In addition, identifying targets by analyzing transcriptomics does not ensure that the targets are also expressed and are active at the proteome level. Furthermore, heterogeneity within the tumor, across patients of the same cancer type, and across cancers underscores the need for subtype-specific personalized target identification. The Multiomics2Targets workflow addresses some of these challenges by combining phosphoproteomics, proteomics, and transcriptomics with comprehensive background knowledge databases and algorithms to identify safer targets for cancer subtypes and individual patients.
Deng et al. present Multiomics2Targets, a bioinformatics workflow that can be used to identify drug targets for cohorts of cancer patients profiled with transcriptomic, proteomic, and phosphoproteomic approaches. After uploading the results from such multiomics profiling, the platform automatically produces a report with ranked targets for individual patients.
Introduction
A prominent challenge in cancer immunotherapy and targeted therapy is identifying targets and drugs that can selectively kill, or stop the proliferation of, tumor cells without negatively impacting normal cells and tissues. Furthermore, personalized cancer therapy requires that target and drug candidates are tailored to individuals or subgroups of patients. Specific cell signaling pathways can be characterized as the drivers of molecular alterations in regulatory mechanisms across different tumor types and individuals.1 Because effective cancer drugs often target these driver pathways, identifying these pathways from a patient’s tumor sample can lead to effective personalized treatments. Previously, we have developed the eXpression2Kinases (X2K) pipeline.2,3 The X2K pipeline takes a set of differentially expressed genes (DEGs) and infers the upstream regulatory cell signaling pathway likely responsible for the observed changes in gene expression. Other software tools that have been developed for pathway analysis from RNA sequencing (RNA-seq) often use gene expression changes as a proxy for protein activity. Often such methods are limited to identifying canonical literature-curated cell signaling pathways.4,5 By contrast, X2K chains several enrichment and network analysis steps to reconstruct an original cell signaling pathway from information encoded in transcription factor (TF), protein-protein interaction (PPI), and kinase-substrate databases. Once such upstream cell signaling pathways have been identified and constructed, these pathways can be targeted, for example, by inhibiting the top ranked kinases from the last step of the workflow.6
At the same time, computational approaches have been developed to identify cell-surface targets for a specific patient, or a group of cancer patients, with experimental demonstrations that these approaches can work. For example, to identify immunotherapeutic targets for a neuroblastoma patient,7 gene expression data from the patient’s tumor were compared to RNA-seq data from GTEx.8 Targets were prioritized if they were highly expressed in the tumor compared with their expression across 54 normal GTEx tissues. Genes that met this criterion were further filtered for those that encode cell-surface proteins, because these can be targeted by immunotherapies such as antibody-drug conjugates (ADCs)9 or chimeric antigen receptor (CAR) T cells.10,11 The final candidate, GPC2, was experimentally validated by demonstrating that a GPC2-targeted ADC safely and effectively delivered cytotoxic drugs to eliminate neuroblastoma cells in vitro and in vivo. Similarly, another study12 compared tissue-level gene expression from 12 pediatric cancers to normal tissue samples. Through individual identification of highly expressed membrane proteins in the tumors, targetable antigens were identified. Novel candidates as well as targets that are currently being tested in clinical trials were identified, including GPC2. Other similar studies integrated transcriptomics and proteomics profiling to identify and rank differentially expressed cell-surface proteins in prostate cancer13 and multiple myeloma14 cell lines.
As immunotherapies to remove tumor cells gain more research interest, several computational methods have emerged to harness omics datasets for identifying novel targets. For example, Dannenfelser et al.15 developed a gene expression-based computational workflow applied to pan-cancer data to identify pairs of antigens that can discriminate tumor cells from normal cells. The predictions made by the workflow are provided for browsing from a dedicated website. However, the site does not provide mechanisms to upload expression data collected by a user to obtain targets. A similar application called QSurface identified 519 candidate cell-surface proteins, and then, by examining gene expression levels in 14 cancer types, it ranked these 519 candidates by their relevance to each cancer type.16 Another tool called Open Cancer TherApeutic Discovery (OCTAD)17 enables users to upload their data, but instead of screening for targets, the application prioritizes small molecules from the LINCS L1000 collection.18 We developed TargetRanger,19 a web-server application that can be used to identify and visualize candidate immunotherapeutic targets given gene expression data from a single patient or a cluster of similar patients. TargetRanger identifies highly expressed genes or transcripts in a patient sample compared to normal tissues and cell types gathered from processing data from several normal tissue and cell-type transcriptomics and proteomics atlases. Importantly, TargetRanger also offers analysis of tumors profiled with both transcriptomics and proteomics. Since it is well known that the transcriptome does not always correlate with the proteome,20,21 incorporating proteomics data can help to further distill targets with greater potential. While most pan-cancer omics projects do not include proteomics data, the National Cancer Institute (NCI) Clinical Proteomic Tumor Analysis Consortium has profiled 1,020 tumors with transcriptomics, proteomics, and phosphoproteomics.22 Since the Clinical Proteomic Tumor Analysis Consortium proteomics data are mostly profiled with multiplex tandem mass tag (TMT), which only provides relative expression levels for proteins, Wang et al.23 preprocessed the raw Clinical Proteomic Tumor Analysis Consortium data and converted them to a format that provides an estimation of absolute expression. This step is critical for surface-antigen discovery.
While there is an increasing trend of collecting multiomics data from cohorts of patients,22 single cells,24 cell lines,25 and animal models,26 analysis of multiomics data is not trivial, and there are not many bioinformatics tools available for biologists with no computational background. Here we present Multiomics2Targets, a bioinformatics workflow that enables users to upload three data matrices collected from the same cohort of patients. After uploading transcriptomics, proteomics, and phosphoproteomics data matrices, as well as accompanying metadata, Multiomics2Targets produces a report that resembles a research publication. The uploaded data matrices are processed, analyzed, and visualized using the tools Enrichr,27 KEA3,28 ChEA3,29 X2K,3 and TargetRanger19 to produce ∼80 figures and ∼30 tables. To perform various multiomics analyses for the identification of patient-specific driver pathways and targets, the Multiomics2Targets workflow implements three separate primary workflows with a combination of these tools (Figure 1).
Figure 1.
Workflow for X2K pathway recovery and the target identification process
(A) In the transcriptomics component of the X2K workflow, sets of 500 up and down genes are submitted to ChEA3 for TF enrichment analysis, followed by gene set augmentation using one of six methods. The expanded gene set is then submitted to KEA3 to rank the most likely kinases upstream of the observed transcriptional changes.
(B) In the phosphoproteomics component of the workflow, sets of 500 up and down phosphosites are submitted to KEA3 for kinase enrichment analysis to rank the most likely kinases responsible for the observed phosphoproteomic changes. The top-ranked kinases from the transcriptomic and phosphoproteomic pipelines are then compared for agreement to recover likely signaling pathways.
(C) For target identification, CPTAC3 pan-cancer transcriptomics data are first analyzed by the Leiden algorithm to identify clusters for each cancer type. At the same time, gene expression from normal tissues and cell types is compiled from GTEx and ARCHS4. Each cluster of samples from each cancer subtype is compared to the normal tissues and cell types from the two background atlases to identify genes that are highly expressed in the tumors. The results are visualized in boxplots and volcano plots. Top-ranked genes are then filtered to include only genes that give rise to transmembrane proteins using proteomics resources: COMPARTMENTS, CPTAC3 proteomics, the Human Protein Atlas (HPA), and the Human Proteome Map (HPM). The results across clusters are visualized as heatmaps.
Results
X2K parameter search and pathway discovery
The Clinical Proteomic Tumor Analysis Consortium profiled 1,020 tumor samples from invasive breast carcinoma (BR), clear cell renal cell carcinoma (CCRCC), colon adenocarcinoma (CO), glioblastoma multiforme (GBM), head-and-neck squamous cell carcinoma (HNSCC), lung squamous cell carcinoma (LSCC), lung adenocarcinoma (LUAD), ovarian serous cystadenocarcinoma (OV), pancreatic ductal adenocarcinoma (PDA), and uterine corpus endometrial carcinoma (UCEC) with bulk RNA-seq as well as mass spectrometry proteomics and phosphoproteomics.30 To infer upstream cell signaling pathways associated with each cluster of Clinical Proteomic Tumor Analysis Consortium patients, we employed the X2K pipeline to infer kinase activity from sets of DEGs.2,3 For each tumor sample, the top 500 DEGs with the highest and lowest expression relative to the other samples of the same tumor type were submitted to X2K. Since we also have the sets of differentially phosphorylated proteins from each tumor, we used these as input to KEA328 to obtain additional ranked lists of kinases for each tumor. The kinase predictions obtained from the phosphoproteomics were then used to calibrate the parameters of the X2K pipeline. For each tumor sample, the 500 phosphosites with the highest and lowest phosphorylation activity relative to other samples of the same tumor type were first converted into a protein set and then submitted to KEA3 for kinase enrichment analysis. To normalize the ranks of the inferred kinases, the expected rank distribution of each kinase was estimated by submitting random gene sets to KEA3, and the raw KEA3 ranks were then Z scored against this distribution.
For each tumor sample, the top 500 DEGs with the highest and lowest expression relative to other samples of the same tumor type were submitted to ChEA329 for TF enrichment analysis. Similar to the KEA328 phosphoproteomic workflow, the raw ChEA3 ranks were normalized by Z scoring against the expected rank distribution of each TF, which was estimated by submitting random gene sets to ChEA3. After obtaining ranked lists of the most likely TFs responsible for the observed changes in the transcriptomics, each set of TFs was augmented using known PPIs, gene-gene co-expression similarity from ARCHS4,31 or protein-protein co-expression from the cancer cell line encyclopedia (CCLE) proteomics.32 The expanded gene sets were then submitted to KEA3 for kinase enrichment analysis, followed by the same rank normalization used in the phosphoproteomics pipeline. Since phosphoproteomics captures kinase activity more directly, we considered the KEA3 phosphoproteomics pipeline to be the “ground truth” for kinase enrichment analysis. Thus, we calibrated the parameters of the X2K pipeline to achieve maximum agreement with the kinase ranks obtained from submitting the phosphoproteomics gene sets to KEA3. Three parameters were optimized: the number of TFs to pass on for gene set augmentation, the database of gene set augmentation, and the number of genes to add to the pathway.
Gene set augmentation using the CCLE protein-protein co-expression matrix outperformed other methods for all tumor types other than LSCC and CCRCC, where expansion with ARCHS4 co-expression surpassed protein co-expression (Figure 2A). However, due to technical limitations of protein quantification with mass spectrometry, the protein-protein co-expression matrix includes 8,892 proteins representing less than half the human coding genes, whereas the ARCHS4 co-expression matrix captures all known human coding genes. Co-expression values from the CCLE proteomics matrix are weakly correlated with ARCHS4 gene co-expression (mean r = 0.13, standard deviation = 0.11). To limit the potential bias introduced by proteomics, we focus this study on the predictions obtained from using ARCHS4 gene-gene co-expression for gene set augmentation, and we report the X2K enrichment results using CCLE protein-protein co-expression in the supplemental information. Using ARCHS4 gene co-expression, the optimal gene set size for kinase enrichment analysis varied between tumor types; for instance, the optimal number of TFs was as low as 13 for UCEC and up to 29 for BR, GBM, CO, and PDA. The optimal number of augmented genes varied between 83 for CO and 147 for LSCC (Figure 2B). We define a kinase to be recovered if it was ranked among the top 30 in both the X2K and the KEA3 phosphoproteomics pipelines. Across all tumor types, on average, more kinases were recovered than expected by random draws from the gene space, although recovery for samples from the same patient was not significantly higher than the recovery expected from two different patients of the same tumor type. Kinases enriched at up-regulated phosphosites were primarily recovered in up-regulated gene sets, and kinases enriched at down-regulated phosphosites were primarily recovered in down-regulated protein sets (Figure 2C). Spearman’s rank correlation was used to assess the alignment between the X2K enrichment scores and the KEA3 phosphoproteomics enrichment scores of recovered kinases. X2K enrichment scores from up-gene sets and down-gene sets were positively correlated (mean r = 0.18) with KEA3 enrichment scores from phosphosite activity in the same direction (i.e., up-phosphosites and down-phosphosites, respectively). However, X2K enrichment scores did not show strong correlation (mean r = −0.05) with KEA3 enrichment from phosphosite activity in the opposite direction (Figure 2D).
Figure 2.
X2K parameter optimization
(A) Jaccard similarity between the phosphoproteomic and the X2K kinase ranks, obtained from sets of 20 TFs augmented with 100 genes using six different gene set expansion methods. Error bars indicate 1 standard deviation, and the dashed line indicates the Jaccard similarity expected by random chance.
(B) For each tumor type, the numbers of TFs (x axis) and augmented genes (y axis) that maximize the Jaccard similarity of the top 30 kinases ranked by X2K and KEA phosphoproteomics.
(C) Mean overlap of the top 30 kinases inferred from the KEA phosphoproteomic pipeline and recovered in the X2K pipeline. Each bar comprises up-kinases (phosphoproteomics) recovered in up-regulated gene sets (transcriptomics), up-kinases recovered in down-regulated gene sets, down-kinases recovered in down-regulated gene sets, and down-kinases recovered in up-regulated gene sets. The dashed line indicates the kinase overlap expected by random sampling from the input gene space. The slashed bar indicates the mean kinase overlap expected by random sampling from other patients of the same tumor type.
(D) Spearman rank correlation between the X2K and the KEA phosphoproteomics enrichment scores of the top 50 most commonly recovered kinases within each tumor type. For each kinase, up-gene set enrichment scores are correlated with up-kinase (top left) and down-kinase enrichment scores (top right) and down-gene set enrichment scores are correlated with up-kinase (bottom left) and down-kinase enrichment scores (bottom right). Kinases are ordered by their average X2K rank in up- and down-gene sets across all samples of a given tumor type.
Visualizing pan-cancer signaling pathway activity
After applying the optimized workflow to all samples in each cancer type, pan-cancer results were visualized as heatmaps with hierarchical clustering of samples based on enrichment scores of the 30 most enriched TFs and kinases. Tumor type groupings (e.g., LSCC and LUAD; BR and CO) in the gene expression space (Figure 3A) are generally maintained in the transformation to TF enrichment vectors (Figure 3B) and kinase enrichment vectors (Figure 3C). In the up-regulated gene sets, the most enriched kinases across all cancer types are involved in cell-cycle regulation (Table S1). Specifically, CDK2, PLK1, and AURKB were enriched in over 67.6% of all HNSCC, LSCC, and LUAD samples, as well as in smaller proportions of BR, CCRCC, CO, GBM, OV, PDA, and UCEC samples. CDK2 was also predicted from up-regulated gene sets in over 72.5% of pan-cancer samples using CCLE protein-protein co-expression. The up-regulated gene sets showed common TF enrichment across multiple tumor types: the oncogenic TFs ETV4, HMGA1, and HMGA2 were the most enriched TFs in the up-regulated gene sets of HNSCC, LSCC, and LUAD samples, while the developmental TFs SOX15 and PITX1 were enriched in high proportions of PDA and UCEC samples.
Figure 3.
Pan-cancer X2K enrichment results
Results from each step of the X2K pipeline are visualized as hierarchically clustered heatmaps. Columns are patient samples, and rows are genes, TFs, or kinases.
(A) RNA-seq Z scores of the 500 most common up- and down-genes across all tumor types.
(B) ChEA3 normalized enrichment scores of the 50 most commonly enriched TFs in up- and down-genes across all tumor types.
(C) X2K normalized enrichment scores of the 30 most commonly enriched kinases from up- and down-genes across all tumor types.
(D) KEA3 normalized enrichment scores of the 30 most commonly enriched kinases from up- and down-phosphosites across all tumor types.
For all plots, column tracks show clinical metadata associated with each sample, including tumor type, tumor stage, patient age group, and categorized survival duration.
In the down-regulated gene sets, the most enriched kinase across all cancer types was SRC, which was inferred in 46.3% of all samples and in 78.5% of the HNSCC, LSCC, LUAD, PDA, and UCEC samples. MAPK3, AKT1, and ERBB3 were also enriched in high proportions of LSCC, LUAD, and PDA samples. Using CCLE protein-protein co-expression for the X2K predictions, only MAPK3 showed similar pan-cancer enrichment in 50.0% of samples. The DNA repair kinase PRKDC was inferred in the down-regulated gene sets of 62.1% of BR, CO, and GBM samples. The down-regulated gene sets were also most enriched for the sex-determining TF DMRT2, which was inferred in 73.2% of CCRCC, HNSCC, LSCC, LUAD, PDA, and UCEC samples. The diabetes-linked TFs PAX4 and HNF1A were enriched in 91.4% of PDA and CCRCC samples, and the developmental TF TBX4 was enriched in 98.2% of LSCC and LUAD samples.
Kinase predictions were also produced by applying KEA3 to up- and down-regulated phosphosites (Figure 3D). As seen in the up-regulated gene sets, when using the up-regulated phosphosites as the input to KEA3, the most enriched kinases across all cancer types are also involved in the cell cycle. CLK2, CDK12, SRPK3, SRPK1, and CLK1 were enriched in 73.6% of all CO, HNSCC, LSCC, and LUAD samples compared to 22.3% of BR, CCRCC, GBM, OV, PDA, and UCEC samples. Similarly, when the down-regulated phosphosites were submitted to KEA3, the kinases PTK2B, PAK2, PRKACA, and TTN were enriched in 60.9% of CO, HNSCC, LSCC, and LUAD samples compared to only 10.5% of BR, CCRCC, GBM, OV, PDA, and UCEC samples, distinguishing these two groups of tumor types. WNK1 was the most enriched kinase in the down-regulated phosphosites, inferred in 50.6% of all tumor samples.
In both up- and down-regulated gene sets from the transcriptomics, the 30 most enriched pathways across all cancers were tested for association with patient survival using Cox regression models, adjusting for tumor type, tumor stage, patient age, and patient race (Table S2). In the down-regulated genes, enrichment of PTK2, with FAXDC2 and EMCN as downstream intermediates and DMRT2, GATA1, TBX4, and TCF21 as downstream TFs, was significantly associated with poor survival (p < 0.05). PTK2, which is also called focal adhesion kinase 1, has been previously linked to cancer growth and poor outcomes.33 Inhibition of PTK2 was experimentally shown to improve treatment response in EGFR-TKI-resistant non-small cell lung cancers.34 Low expression of TBX4 has previously been found to predict poor survival in PDA patients,35 and TCF21 has been identified as a crucial TF in coordinating normal cell growth and maturation.36 These TFs, along with DMRT2 and GATA1, play important roles in healthy cell development and differentiation, and their dysregulation is associated with cancer development.37,38 Similarly, low expression of the predicted pathway intermediates FAXDC2 and EMCN has also been identified as strongly correlated with tumor recurrence and metastasis.39 These findings suggest that in multiple cancer types, PTK2 signaling in cancer cells may have an inhibitory effect on downstream intermediates and TFs crucial for healthy cell maturation, thus promoting cancer cell growth and survival. In the up-regulated genes, enrichment of AURKB, CDC25A, and ETV4 was most strongly associated with worse overall survival, although not statistically significant. AURKB is a well-studied cell-cycle kinase implicated in many cancer types,40 and ETV4 is an oncogenic TF whose overexpression is often associated with tumorigenesis and poor patient prognosis.41 Although a direct signaling pathway between AURKB and ETV4 has yet to be identified in the literature, our analysis hypothesizes that CDC25A is a possible intermediate to connect AURKB to ETV4. The overexpression of CDC25A is also implicated in tumorigenesis.42
Tumor-specific signaling pathways
Utilizing version 3 of the Clinical Proteomic Tumor Analysis Consortium (CPTAC3) transcriptomics data matrix, the expression profiles from each of the 10 cancer types were also divided into subtypes based on X2K enrichment vectors. The uniform manifold approximation and projection (UMAP) of Leiden clusters per cancer subtype (Figure S1) identifies distinct groups of patients that share similar kinase enrichment vectors inferred from the transcriptomics data matrix (Figure S2). For each cancer type, between two and seven clusters were identified, each comprising at least six samples (Table S3). Compared to clustering applied to the raw RNA-seq data matrix, clustering applied to the X2K enrichment vectors achieved more distinct separation in the survival outcomes of each cluster. For some cancer types, such as GBM and HNSCC, clusters with distinct kinase enrichment profiles were also characterized by significant differences in overall survival. However, for tumor types with lower variability across gene set signatures, such as LSCC and UCEC, we observed similar X2K enrichment vectors across clusters with no separation in survival outcomes (Figure S2).
For both GBM and HNSCC, at least one subtype showed different survival outcomes compared with the rest of the subtypes (Figure S2). In GBM, cluster 0 was associated with better survival compared to cluster 1 (log rank p = 0.0033). CHEK1 down-regulation of the ZFP62 TF through various intermediates was commonly enriched in the poor prognosis cluster and associated with worse overall survival (hazard ratio [HR] = 3.96, p = 0.01; Figure S3A). Although ZFP62 is not a well-studied TF in the context of GBM, inhibition of CHEK1 is an effective treatment for multiple cancer types, including GBM.43 CHEK1 signaling may lead to worse patient outcomes in GBM by suppressing ZFP62. Up-regulation of the RUNX3 TF by various kinases through the S1PR4 intermediate was also associated with worse survival (HR = 3.19, p = 0.02). The RUNX family of TFs is believed to be involved in GBM malignancy, and RUNX signaling pathways have been proposed as targets for GBM therapy in previous work.44,45 Although the specific relationship between RUNX3 and S1PR4 is not yet well studied, sphingosine-1-phosphate (S1P) signaling is involved in GBM progression.46 In HNSCC, cluster 2 was associated with better survival compared to cluster 0 or 1 (log rank p = 0.011). As seen in the global survival analysis, down-regulation of DMRT2 by PTK6 and the ERBB family of kinases was associated with worse survival (HR = 2.36, p = 0.02) (Figure S3B). The remaining tumor types did not show significant separation of survival curves between subtypes.
Comparison and validation of kinase prediction methods
Because driver kinases do not necessarily show alterations in protein or transcript abundance, the X2K method leverages external knowledge from libraries of TF targets, PPIs, proteomics, and kinase substrates, enabling the identification of kinases that may not be detected by more traditional tumor vs. normal differential expression analyses. To illustrate this unique advantage of X2K, we examined the agreement between X2K enrichment scores derived from transcriptomics, KEA enrichment scores derived from phosphoproteomics, and differential protein abundance scores derived from proteomics (Figure S4). For each tumor type, we also used DepMap’s gene-knockout-effect scores47 as an independent validation of whether the driver kinases predicted by each method are also essential to the viability of cancer cell lines. Across all CPTAC3 samples, we observed that 32.1% of the X2K-predicted kinases, 32.7% of the KEA-predicted kinases, and 36.1% of the kinases recovered by both X2K and KEA also show differential protein abundance in the same patient. In BR, HNSCC, LSCC, LUAD, and UCEC, we identified groups of kinases predicted by X2K, KEA, and differential proteomics that have strong DepMap gene-knockout effects, i.e., < −1 (Figure S4). The kinases in these clusters also display known PPIs with one another, suggesting that these key drivers may operate in interconnected subnetworks rather than in isolation. Although kinases predicted by all three methods have the strongest predicted effect on cancer cell viability, 18.5% of all non-differentially expressed kinases predicted by X2K or KEA also show robust gene-knockout effects (<−0.5) in the corresponding DepMap cell lines. This population of possible driver kinases cannot be detected by differential protein expression but can be inferred by the X2K and/or KEA3 methods. For example, in GBM, clusters of kinases with large DepMap gene-knockout effects are predicted by X2K but not KEA3, nor are they differentially expressed in the proteomics data (Figure S4D).
Target identification with TargetRanger
Global gene and transcript targets were first identified by submitting the RNA-seq transcriptomics data from all 10 tumor types to TargetRanger19 (Figure 4). The RNA-seq data were also submitted by tumor type to TargetRanger to process subtype-specific targets at the gene (Figures S5A–S5J) and transcript levels (Figures S6A–S6J) for each of the 10 cancers.
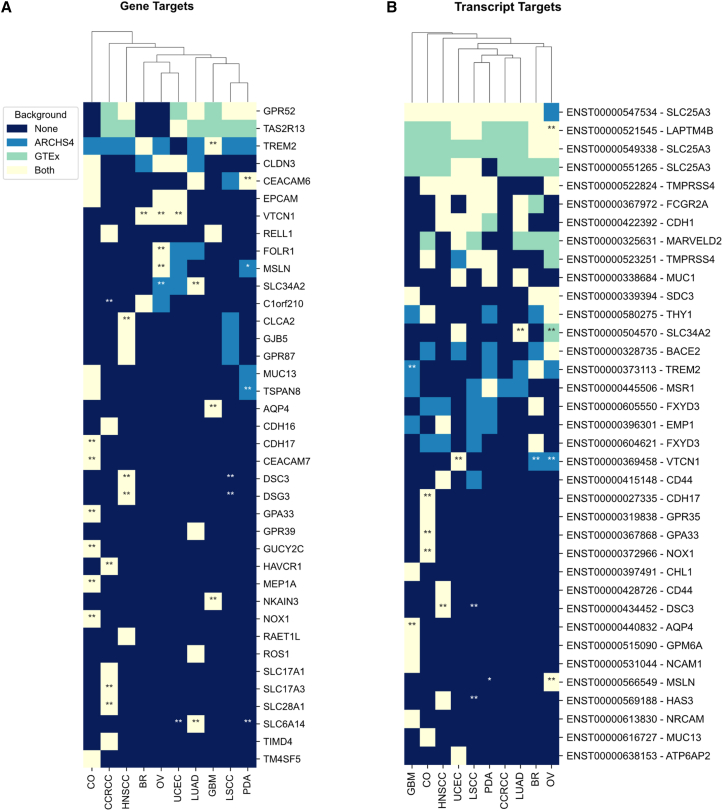
Figure 4.
Global gene and transcript target identification
Top cell-surface (A) gene and (B) transcript targets in each tumor type. Columns are tumor types, hierarchically clustered using Euclidean distance. Rows are the top 30 target genes (A) and transcripts (B), sorted by frequency across subtypes and filtered for genes and transcripts identified against both ARCHS4 and GTEx backgrounds. Star assignments indicate proteomic confirmation of the targets: single stars are assigned to genes and transcripts highly expressed in at least 10% of the samples of a given tumor type. A second star is assigned if the gene or transcript is also expressed at least twice as much in the indicated cancer compared to the average across all other cancer types.
Pan-cancer targets
Although most targets were cancer-type specific, several targets were identified across multiple cancers (Figure 4). At the gene level, GPR52, TAS2R13, triggering receptor expressed on myeloid cells 2 (TREM2), CLDN3, carcinoembryonic antigen cell adhesion molecule 6 (CEACAM6), and EPCAM were significantly differentially expressed across multiple cancer types. However, proteomics confirmation supports TREM2 only as a GBM target and CEACAM6 only as a PDA target. TREM2 is a transmembrane receptor highly expressed in myeloid cells, and it has been shown to be up-regulated in gliomas. Its expression is closely related to the overall survival of patients.48 In addition, in the glioma cell lines U87 and U373, TREM2 reduction inhibited cell proliferation and increased apoptosis and immune invasion.49 The identification of TREM2 as a candidate for GBM demonstrates the ability of TargetRanger to identify targets that were previously investigated in the literature. CEACAM6 was identified as a potential target at the gene level for all PDA clusters and at the proteomics level for three of the four clusters (Figure S5I). CEACAM6 is involved in cell adhesion, apoptosis, angiogenesis, and other biological processes.50,51 CEACAM6 is already known to be highly expressed in PDA52 and was already selected as a therapeutic target for ADC development.53 Thus, CEACAM6 provides another example of the ability of TargetRanger to recover targets for PDA.
The top pan-cancer target supported by gene, transcript, and protein level analysis is the V-set domain-containing T cell activation inhibitor 1 (VTCN1). VTCN1 is a T cell regulator, which was identified as a potential target for BR, OV, and UCEC. VTCN1 is known to block cell-cycle progression and interleukin-2 (IL-2) production in CD4+ and CD8+ cells, and it is up-regulated in many cancers, including breast cancer.54 VTCN1 was reported in the literature to be a potential target for ovarian cancer due to its consistent overexpression in serous, endometrioid, and clear cell ovarian cancers, compared to normal tissues,55 and its potential role in reducing immune response.56 In UCEC, overexpression of VCTN1 can lead to inhibition of T cell infiltration and interferon-γ (IFN-γ) concentration.57 In our analysis of BR samples, VTCN1 was highly expressed at the proteomics level for 6 of the 10 BR clusters. Specifically, the isoform ENST00000369458 of VTCN1 was identified as a top target at the transcript level for BR (Figure S6A). In OV, VTCN1 was identified as a target for all 8 clusters and was also highly expressed at the protein level in all OV clusters (Figure S5H). In addition, the ENST00000369458 isoform of VTCN1 was identified as a transcript-level target for OV (Figure S6H). Notably, VTCN1 was expressed most highly at the protein level in OV cluster 7, suggesting a VTCN1-targeted therapy may be most effective for this subgroup of patients (Figure S5H). In UCEC samples, VTCN1 was identified as a target for all 10 clusters and highly expressed in the proteomics of 9 of 10 clusters (Figure S5J). Like OV, the ENST00000369458 isoform of VTCN1 was also identified as a transcript-level target for UCEC (Figure S6J). Altogether, evidence from this analysis and prior literature suggest VTCN1 may serve as a common therapeutic target for BR, OV, and UCEC due to the potential benefit in the interference with immune responses and should be investigated more thoroughly.
Tumor-specific targets
Through TargetRanger, both known and unknown targets were identified for tumors and their Leiden subtypes (Figures S5A–S5J and S6A–S6J). We observed that most targets identified by the pipeline are highly specific to each cancer type (Figure 4). For example, in CCRCC, the targets HAVCR1, SLC17A3, and SLC28A1 were highly expressed at the gene level and are also supported by protein-level evidence. HAVCR1 is involved in immune activation and kidney regeneration and has additionally been linked to cancer progression.58 HAVCR1 is expressed in immune cells that have a key role in promoting immune cell activation and proliferation, the secretion of cytokines, and the regulation of the immune response.59,60 HAVCR1 is also known to directly bind to TIMD461 and was previously identified as a potential therapeutic target for CCRCC.62 Thus, HAVCR1 likely plays an important role in mediating immune responses in CCRCC. SLC17A3 and SLC28A1 are less studied in the context of CCRCC, but evidence from both transcriptomics and proteomics supports their potential as highly specific targets for CCRCC.
Many targets were also specific for CO at the gene, transcript, and protein levels, including NOX1 (ENST00000372966), MEP1A, GUCY2C, GPA33 (ENST00000367868), CEACAM7, and cadherin-17 (CDH17) (ENST00000027335). All six of these targets are highly expressed in the proteomics for all three CO clusters (Figure S5C). GPA33 is a type I transmembrane protein and a member of the immunoglobulin superfamily (IgSF).63 GPA33 is also known to be associated with inhibitory effects on T cells through its relation to CD27664 and VSIG4,65 as well as other immune dysregulations.66,67 GPA33 expression in CD4+ T cells was an indicator of undifferentiated T cells and their inability to produce pro-inflammatory cytokines such as IL-2, IFN-γ, and IL-17.68,69 GPA33 was also identified as a potential target in intestinal intraductal papillary mucinous neoplasm.70 Overall, GPA33 association with immune dysfunction and T cells makes it a promising candidate to target CO. Another target identified for CO is CDH17 (Figure S5C). CDH17 is highly expressed in all three clusters at the proteome level. CDH17 is a calcium-dependent transmembrane protein involved in cell adhesion and it is known to facilitate cell-cell interactions.71 In both mice and humans, under normal physiological conditions, CDH17 is expressed solely in the small intestine and colon epithelial cells.72 In addition, CDH17 has been identified as a biomarker in gastric adenocarcinoma.73 It was targeted and knocked down in vivo, causing limited tumor progression.74 More recently, CDH17 was also identified as a biomarker in colorectal adenocarcinoma along with CDX2.75 Given CDH17’s knockdown effect on gastric adenocarcinoma progression, and its recognition as a biomarker in colorectal adenocarcinoma, CDH17’s potential as a therapeutic target in CO should be further investigated.
Tumor-specific targets identified for GBM include NKAIN3 and aquaporin-4 (AQP4). NKAIN3 encodes a subunit of a sodium/potassium-transporting ATPase expressed in neuronal tissue. NKAIN3 is not well studied in the context of GBM, but gene- and protein-level evidence in GBM cluster 0 supports its potential as a therapeutic target. AQP4 was identified as a target appearing for both GBM clusters at the gene and proteome levels (Figure S5D). An isoform of AQP4 (ENST00000440832) was also identified as highly expressed at the transcript level (Figure S6D). AQP4 is a member of the aquaporin protein family. It is most highly expressed in astrocytes, where it mediates the homeostasis of water.76 Following both traumatic brain and non-traumatic brain injury, AQP4 expression is elevated in rodents.77,78 In addition, its elevated expression is linked with blood-brain barrier deterioration and edema.79,80 AQP4 was found to be generally up-regulated in brain tumors, where its pattern of expression varied with tumor type. It was hypothesized that AQP4 facilitates the movement of water in brain tumors,81 given that brain tumors usually exhibit vascular permeability and edema surrounding the tumor.82 More specifically, AQP4 was shown to play a vital role in the progression of malignant glioma, where its inhibition led to reduced proliferation83 and improved response to therapy.84 AQP4 is currently investigated as a potential target for targeted therapy, specifically for GBM. The independent rediscovery of AQP4 by the Multiomics2Targets pipeline supports the overall relevance of the method for tumor target identification.
The top HNSCC-specific targets include desmocollin-3 (DSC3) (ENST00000434452), desmoglein 3 (DSG3), and chloride channel accessory 2 (CLCA2). Both DSC3 and DSG3 were identified as top targets at the gene level for HNSCC for all three clusters, while also being highly expressed at the proteome level for each cluster (Figure S5E). DSC3 plays a critical role in maintaining epithelial integrity and cell adhesion, and it is believed to have tumor suppressive activity in some cancer types. DSG3, a transmembrane glycoprotein, is mainly found in the squamous epithelium and keratinocyte desmosomes, and it is involved in cell adhesion.85,86 DSG3 plays a role in cell signaling pathways related to cell migration and proliferation of keratinocytes.87 High DSG3 expression has been identified in a variety of cancers, including squamous cell carcinomas.88 However, the roles of both DSC3 and DSG3 in cancer remain unclear. There are studies suggesting their down-regulation in cancer enables squamous cell tumor progression,89,90,91 while, on the other hand, DSG3 was shown to have an oncogenic effect in multiple cancers.92,93 DSG3 has also been specifically identified as a target in head-and-neck cancers, where it was observed to be overexpressed. DSG3 inhibition in vitro and in vivo led to protective effects, reducing malignancy.94 Although the role of DSG3 in cancer has not been fully elucidated, it has been confirmed in the literature as a potential target for treating HNSCC, making it another worthy target for further investigation. CLCA2 was also identified as a target at the gene level and showed expression at the protein level for all three HNSCC clusters (Figure S5E). CLCA2 is a chloride channel that is involved in cell proliferation and DNA damage response.95 CLCA2 is also involved in basal cell adhesion and detachment.96 In addition, CLCA2 was investigated in the context of tumorigenesis and development specifically in breast cancer, where it acts as a p53-inducible growth inhibitor.95 Interestingly, its down-regulation in breast cancer enables the survival of tumor cells. Its role in other cancers is less studied; however, its expression was identified to be an indicator of poor outcomes in LSCC.97,98 Although CLCA2 has not been specifically implicated in HNSCC, its overexpression and known mechanisms of action (MOAs) in other cancers qualify it as a potential candidate for targeted therapy in HNSCC.
In OV, FOLR1 and MSLN (ENST00000566549) showed high transcriptomics and proteomics specificity, although both targets were also detected in the transcriptomics samples of other tumor types against the ARCHS4 background. Both FOLR1 and MSLN were highly expressed in both the transcriptomics and the proteomics of both OV clusters (Figure S5H). Both were also identified at the transcript level: the FOLR1 isoform ENST00000393676 and three MSLN isoforms: ENST000002662101, ENST00000566549, and ENST00000563941 (Figure S6H). CAR-T cell therapy targeting both FOLR1 and MSLN in vivo resulted in increased survival time, decreased tumor volume, and an increase in T cell infiltration and persistence in OV.99 Thus, our analysis pipeline identified two OV-specific targets that have been experimentally validated.
Comparison and validation of gene target identification methods
While the TargetRanger algorithm performs differential expression analysis of tumor RNA-seq against RNA-seq from many different normal tissues and cell types, traditional differential expression analyses typically compare expression values between tumor samples and their matched normal tissues. While this approach to target identification is expected to detect genes that are highly expressed in other vital tissues, and thus would be less ideal candidates for immunotherapy, we applied it as an alternative approach. For each tumor subtype with enough matched normal samples (N > 2), we computed DEGs with adjusted p < 0.01 using limma-voom. Next, we compared these DEGs to the targets identified by TargetRanger (Table S4). DEGs were not computed for BR, CO, GBM, or OV, because these cancers did not have matched normal adjacent tissue samples. Across all tumor types, on average, 15.1% of the predicted targets from TargetRanger were also significant DEGs, and 8.6% were validated by DepMap with a gene effect that is < −0.5. The DepMap gene effects of our predicted targets (median = −0.056) did not differ significantly from the gene effects of the DEGs (median = −0.063), although the differential gene expression analysis identified a larger portion (10.2%) of outlying genes with strong gene effect.
The Multiomics2Targets website
The Multiomics2Targets website is an enhanced Appyter100 that provides a user-friendly input form to enable users to upload their datasets and customize the parameters of the workflow (Video S1). In contrast with other Appyters, Multiomics2Targets has customized input and results pages, providing additional features such as exporting to a PDF report, sharing the URL of the analysis on LinkedIn and X, and exporting a citation of the results page. While the Multiomics2Targets website was initially designed to only process transcriptomics, proteomics, and phosphoproteomics together, it is expected that such datasets are not widely available. Hence, to broaden the usefulness of the resource, users are only required to upload a minimum of one data file. The analysis report is customized to only provide analyses based on the combination of input datasets. The output report includes many aspects of a published research paper, including abstract, introduction, methods, results, discussion, conclusions, and references. The code producing the report can be viewed directly on the results report page by pressing the toggle code button. The code is hidden by default. As an integral part of the report, a large language model (LLM) (GPT-4o) is used to compose a description of the results in several sections of the report. These sections are highlighted in yellow boxes with a warning to readers that the text was generated by an LLM. In addition, users may load example files for each of the 10 CPTAC3 cancer types processed in this study. These analyses are canned and load immediately to prevent the reexecution of the same pipelines when there is no need to do so.
Discussion
Identification of literature-supported pathways and targets
The X2K workflow identified enriched kinases, intermediates, and TFs for key tumor types and subtypes. Many of these automatically identified pathways already have known roles in tumorigenesis and are associated with poor cancer prognosis. For instance, substantial literature evidence supports the roles of PTK2 and AURKB in cancer cell signaling33,34 in multiple tumor types, as well as the roles of the downstream intermediates and TFs that were identified. Furthermore, many of the top targets across the cancer subtypes are also supported by prior evidence documented in the literature. For example, TREM2 was identified as a target for GBM at the gene level with high expression in a majority of the GBM clusters at the proteome level. TREM2 has been previously reported as a target for GBM due to its overexpression and relation to patient survival.48 DSG3 was identified as a target at the gene level for both HNSCC and LSCC. DSG3 was previously identified to be highly expressed in squamous cell carcinomas and specifically has been identified as a biomarker and a target for both HNSCC and LSCC.101,102,103 In OV, VTCN1 was identified as overexpressed in all clusters at both the gene and the transcript levels and was also up-regulated at the proteome level in all clusters. VTCN1 has been previously identified as a gene overexpressed in ovarian serous cancer, and it is used as a biomarker.55,104,105 Overall, the Multiomics2Targets pipeline ranks highly many already known targets across cancer subtypes at the gene, transcript, and protein levels. Thus, since the Multiomics2Targets approach is unbiased and does not consider prior literature, we expect that many novel targets can become effective biomarkers and immunotherapeutic targets.
Identification of novel pathways and targets
While the X2K pipeline predicted many kinases and TFs with well-studied individual roles in cancer, the signaling pathways and connecting regulatory networks are less well known. By inferring co-expressed intermediates and identifying kinases and TFs enriched in the same tumor sample, the X2K analysis enables discovery of regulatory pathways linking upstream kinases to their downstream targets. For instance, while the kinase AURKB and the TF ETV4 have been studied independently for their roles in cancer, the X2K analysis predicted a possible pathway linking these two proteins through CDC25A, a known key driver of tumorigenesis.42 The TargetRanger component of the pipeline also facilitates hypothesis generation regarding cancer targets. Many predicted targets were not directly linked to a specific cancer subtype, but rather connected to other cancers, immune processes, or mechanisms related to cell proliferation and cancer cell survival. For instance, in HNSCC, CLCA2 was identified as a target at the gene level and expressed for all clusters at the proteomics level. CLCA2 is known to have protective effects in certain populations with breast cancer106 and is a biomarker for LSCC.97 GPA33 was identified at the gene and proteomics levels for all CO clusters. GPA33 is associated with immune dysfunction in T cells and has been identified as a potential target for intraductal papillary mucinous neoplasm.70 Although it has not been specifically implicated in CO or related cancers, its consistently high expression in CO suggests its potential as a therapeutic target for CO. In addition, in CO, CDH17 was identified as a target at the gene and protein levels for each cluster. CDH17 has been implicated in gastric adenocarcinoma as a biomarker and an immunotherapeutic target.107 These are a few examples of many more; hence, the Mulitomics2Targets approach produced hypotheses about targets known to be associated with other related cancers. Given the evidence surrounding many of these targets, their potential to be translated into therapies in the subtypes identified by our analysis pipeline warrants further investment.
Future applications—Single-cell omics
Considering the increasing availability and amounts of single-cell omics data, we are currently considering how the algorithms implemented for Multiomics2Targets could be applied to multiomics single-cell data. Patient cohort data matrices and single-cell data matrices are of a similar shape: the genes and proteins are the rows, and the cells or patients are the columns. Hence, the heatmaps, the X2K pipeline, and the TargetRanger algorithm can be applied to single-cell multiomics data in a similar way. Furthermore, if such multiomics data are collected from single cells of cohorts of cancer patients, we could identify the pathways and targets at the cell-type population level for each patient. This would produce a more precise targeted precision-medicine approach, because the most critical cell types within a tumor will be those that we would aim to target. Hence, for each tumor, we may identify combinations of targets and pathways that represent various single-cell subpopulations that could be targeted in parallel.
Future applications—Personalized medicine
Cancer is a highly personalized disease characterized by genetic alterations unique to each patient, and thus therapies that are customized to the individual are of growing interest for realizing precision personalized medicine. In this study, we summarized our predictions at the tumor subtype level. However, the X2K pipeline can infer kinase activity given the transcriptomics signature of a single patient, and the TargetRanger pipeline can also predict patient-specific targets if provided with multiple samples collected from the same patient. Both pipelines can also be adapted to work for single samples using simulated replicates or single-sample differential expression analysis methods.108 In future work, as data acquisition becomes more patient specific, we aim to apply the Multiomics2Targets workflow to individual patient data, enabling personalized predictions of kinases and cell-surface targets that can be targeted for individualized treatments.
Limitations of the study
One of the limitations of the pathway analysis with X2K is that we used a hard-coded rank cutoff. For example, only the top 30 kinases are involved in each pathway. Adjustments to the cutoff may yield slightly different results. For instance, a less stringent cutoff may enable the identification of pathways that are less studied. Another important limitation is the lack of direct wet-lab experimental validation of the pathways and targets predicted by the analysis. Furthermore, the tumor samples were clustered using a transformed version of the transcriptomics data through enrichment analysis with X2K. Clustering for subtypes could be done with genomics, proteomics, imaging, and clinical classifications or a combination of these. Thus, although our results highlight subtype-specific pathways and targets, the subtypes themselves should be interpreted with caution. It should be noted, too, that the approach can be applied without the clustering step by examining each tumor individually or determining the subtype by a different method.
Proteomics data were incorporated in the Multiomics2Targets workflow at two steps: first, as an option for gene set expansion in the X2K pipeline, and second, as a source of target validation for the identified X2K protein kinases and TargetRanger’s cell-surface proteins. However, protein quantification with mass spectrometry is prone to abundance biases, as low-abundance proteins tend to be underdetected, and mass spectrometry proteomics is also limited in the number of measured molecules compared to RNA-seq. To reduce the presence of such proteomics bias, the X2K pipeline has the option of substituting protein-protein co-expression with gene-gene co-expression to augment the set of predicted TFs at the second step of the X2K pipeline. The tumor proteomics data offer information about the protein expression in each sample, or cluster of samples, which provides valuable insights regarding the presence and specificity of each target for a certain tumor type or subtype. However, this approach may fail to confirm viable targets that exhibit lower abundance at the protein level.
Another limitation of the current version of the Multiomics2Targets workflow is the background gene expression data created from ARCHS4 and used by TargetRanger. This normal tissue background was created with tokenized term labeling of RNA-seq samples describing tissues and cell types. While the statistics computed for each cell type and tissue should be relatively robust to outliers, it is not exact or direct and contains diseased tissues and cell types. In addition, TargetRanger’s differential expression analysis prioritizes candidates that are highly expressed in the tumor samples, but there is no guarantee that the target is poorly expressed across all tissues and all cell types. It can be poorly expressed across most tissues and cell types but highly expressed in one or a few tissues and cell types. The consideration of bulk RNA-seq from tumors and tissues masks the expression of the targets in single cells. This limitation can be mitigated by considering data from scRNA-seq as the input to the workflow and comparing to scRNA-seq data from normal tissue backgrounds. While current knowledge about single-cell protein expression in normal cells and tissues is limited, several atlases have emerged to provide mRNA expression in single cells across tissues. Finally, although many of the targets identified here have literature evidence with relevance to cancer, their safety and potential as therapeutic targets must be further tested experimentally.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| CPTAC3 Cohort Li Y et al. Clinical Proteomic Tumor Analysis Consortium. Proteogenomic data and resources for pan-cancer analysis. Cancer Cell. 2023 Aug 14; 41(8):1397–1406. |
https://doi.org/10.1016/j.ccell.2023.06.009. PMID: 37582339; Li et al.22 PMCID: PMC10506762; Li et al.22 |
https://pdc.cancer.gov/pdc/cptac-pancancer, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001287.v16.p6 |
| Software and algorithms | ||
| The source code for the Multiomics2Targets | This paper |
https://github.com/MaayanLab/Multiomics2Targets https://doi.org/10.6084/m9.figshare.26199887 |
| The source code for the eXpression2Kinases analysis | PMID: 22080467 Chen et al.,2 Clarke et al.3 29800326 | https://github.com/MaayanLab/x2k-pathway-analysis |
| The Multiomics2Targets website | This paper | https://multiomics2targets.maayanlab.cloud/ |
| ChEA3 | PMID: 31114921 Keenan et al.29 | https://maayanlab.cloud/chea3/ |
| Enrichr | PMID: 23586463 Chen et al.109 2714196133780170 Kuleshov et al.,110 Xie et al.27 | https://maayanlab.cloud/Enrichr/ |
| TargetRanger | 37166966 Marino et al.19 | https://targetranger.maayanlab.cloud/ |
| Geneshot | PMID: 31114885 Lachmann et al.111 | https://maayanlab.cloud/geneshot/ |
| KEA3 | PMID: 34019655 Kuleshov et al.28 | https://maayanlab.cloud/kea3/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Avi Ma’ayan, PhD (avi.maayan@mssm.edu).
Materials availability
The study did not generate any new unique reagents.
Data and code availability
-
•
This paper analyzes publicly accessible data. Accessions are listed in the Key Resources Table.
-
•
The Multiomics2Targets website can be accessed at: https://multiomics2targets.maayanlab.cloud/
The source code for the Multiomics2Targets site can be accessed at https://github.com/MaayanLab/Multiomics2Targets. A snapshot of the Multiomics2Targets code was deposited into Figshare at https://figshare.com/articles/software/Multiomics2Targets_source_code_snapshot_from_07082024/26199887, with https://doi.org/10.6084/m9.figshare.26199887. The source code for the eXpression2Kinases analysis can be accessed at https://github.com/MaayanLab/x2k-pathway-analysis.
-
•
Any additional information needed to re-analyze the data reported in this paper is available from the lead contact upon request.
Method details
Pan-cancer transcriptomics, proteomics, and phosphoproteomics
The Clinical Proteomic Tumor Analysis Consurtium (CPTAC) 1,020 tumor samples were organized into three data matrices where the rows are genes/proteins, and the columns are the patient/tumor samples profiled with transcriptomics, mass spectrometry proteomics, and phosphoproteomics. The processed data matrices from these samples were obtained from a recent study30 that details the data processing steps used to convert the raw data into these data matrices.
Kinase prioritization with eXpression2Kinases and KEA3
The X2K pipeline prioritizes kinases using patient transcriptomics: first, a gene set is submitted for transcription factor enrichment analysis (TFEA) with ChEA329, which ranks transcription factors (TFs) most likely responsible for the observed changes in gene expression. Next, the set of enriched TFs is augmented with known protein-protein interactions (PPIs) to form an enriched subnetwork using the Genes2Networks algorithm112, which is then submitted for kinase enrichment analysis (KEA) with KEA3 28. KEA3 uses processed data from several databases of kinase-substrate phosphorylation to rank kinases by their likelihood to regulate a set of input proteins (Figure 1A).
X2K pipeline and parameter optimization
Since CPTAC3 profiled tumors from patients with transcriptomics and phosphoproteomics we can calibrate the X2K pipeline by comparing the ranked kinases produced by X2K from the transcriptomics with the kinases produced by KEA3 from the phosphoproteomics. The parameter search space for X2K was fixed based on the combination of preset parameter values that maximized the Jaccard similarity between the top 30 ranked kinases by the two workflows. To expand the options for gene set augmentation in the X2K pipeline (Figure 1B), we computed protein-protein co-expression values from the Cancer Cell Line Encyclopedia (CCLE) proteomics profiling32. The non-normalized version of the CCLE proteomics data matrix was downloaded from the CCLE website.
Subtype clustering with X2K enrichment vectors
Since tumors with similar gene expression profiles reflect similar pathogenesis, it is expected that patients with similar tumors will respond similarly to personalized treatments. Although subtyping in the clinical setting has been mostly based on morphological and genetic markers, research suggests that cancer treatments may be better personalized based on molecular subtyping from more complex omics-based signatures113. Thus, we aimed at identifying cancer subtypes using a data-driven approach by clustering tumor samples based on their inferred cell signaling profiles from the X2K pipeline. For each tumor type, dimensionality reduction with uniform manifold approximation and projection (UMAP)114 (minimum distance = 0.01) was applied to the normalized X2K enrichment scores from the up- and down- gene sets of each sample, and the Leiden community detection algorithm115 was applied using the scanpy Python toolkit. The Leiden subtypes for each cancer were then analyzed for survival-associated pathways, and the samples within each cluster submitted to TargetRanger19 for target identification.
Survival-associated driver pathway identification
The kinases, protein intermediates that are used to connect the TFs, and the TFs that are highly ranked in the same tumor sample constitute a cell signaling pathway likely involved in driving the tumor phenotype. Such cell signaling pathways are defined as triplets of kinases, intermediates, and TFs that are highly enriched in the up- or down-regulated gene sets of the same sample. To identify potentially pathological pathways associated with poor survival, or potentially protective pathways associated with good survival, pathways highly enriched in poor-prognosis and good-prognosis clusters were tested for association with overall survival using Cox regression models, adjusting for tumor stage, patient age, and patient sex. Only datasets with more than 20 recorded death events (CCRCC, GBM, HNSCC, LSCC, LUAD, and PDA) were deemed sufficient for such survival analysis.
Target identification
Gene and transcript FPKM for each cancer subtype as determined by the Leiden algorithm115 were submitted to the TargetRanger19 for analysis. TargetRanger identifies highly expressed genes or transcripts within the input samples compared to a background dataset of non-diseased tissues and cell types processed from GTEx8 or ARCHS431 bulk RNA-seq datasets. TargetRanger performs differential expression analysis using limma-voom116. To further filter the results, highly expressed membrane-associated proteins are selected using a membrane/surface protein filter. The intersection of protein sets from COMPARTMENTS117 and Human Protein Atlas (HPA)118 was used as a master set of membrane proteins in all analyses. Proteins with "plasma membrane" and "cell surface" localization terms in COMPARTMENTS with a confidence score greater than or equal to 3 were retained. From HPA, we downloaded a set of membrane proteins where the filter "Evidence at protein level" was applied. Then, we removed proteins with "Low tissue specificity." We then ranked significantly differentially expressed candidates. Among the top 30 differentially expressed targets for each cluster, those that appeared as significant when selecting both the GTEx and the ARCHS4 backgrounds are visualized as a hierarchically clustered heatmap. The targets identified from the transcriptomics were subsequently tested for their protein expression relative to all other tumor samples. Proteomics confirmation is indicated by stars overlayed on the heatmaps: a single star indicates that the target is highly expressed in at least 10% of the samples in the cluster. A second star is assigned if the target is also expressed at least twice as much in the samples from the cluster compared to the other clusters in the same cancer, as well as compared with the average across the other cancer types. Due to the success of ADCs and CAR-T cell therapy in cancer treatment, here we focus on gene targets genes localized to the cell surface. However, secreted molecules also present an important class of genes with potential to be easily accessible therapeutic targets. To enable users to identify these candidates, the Multiomics2Targets algorithm also includes a secretome filter of 1891 genes predicted to have at least one secreted product by the HPA118.
Validation of predicted kinases and targets
To compare the X2K predictions with the kinases predicted by differential protein expression, we computed patient-specific differential protein abundance scores. For each cancer, we standardized the protein abundance data by subtracting the average abundance of each protein in the normal adjacent tissue samples from its abundance in each tumor sample. We then scaled these values by the standard deviation of the protein abundances across tumor samples. For four cancer types, namely BR, GBM, PDA, and UCEC, there are no adjacent-normal tissue samples. For these cancer types, we Z-scored the abundances of each protein across the tumor samples. To validate the predicted driver kinases, we downloaded the CRISPR knockout screen data from the DepMap portal119 to quantify the efficacy of knocking out the predicted kinases in cell lines corresponding to each of the 10 cancer types. To identify targets predicted by TargetRanger that already have known approved drugs, we queried the OpenTargets resource120, for ‘known drugs’ through the OpenTargets GraphQL API. We performed three queries per target: searching for any known drugs targeting the identified target protein; searching for drugs targeting a disease including the term cancer, carcinoma, or adenocarcinoma; and searching for known targets with the specific cancer type as the disease.
Multiomics2Targets website development
The Multiomics2Targets web-based platform is developed from the Appyter framework100. The Appyter framework creates an input form from custom components referenced in a Jupyter Notebook which are defined using Jinja2, and Svelte templates. Input components and the site itself are styled with Bootstrap, CSS, and HTML. The pipeline is implemented in Python through code cells in Jupyter notebook and analysis is performed with a variety of packages including Scanpy121, Scipy122. Plots are produced with matplotlib and seaborn. Cells are omitted or included based on the input values using Appyter ‘magics.’ Runs of the pipeline are stored in a dedicated S3 bucket along with the produced figures and titles, enabling access of any past run given the corresponding url. The site is hosted in a Kubernetes cluster, wherein each run of the pipeline is executed through a Kuberenetes job.
Additionally, we provide textual descriptions of the results throughout the pipeline utilizing GPT-4o. For determining cluster labels based upon enriched terms, for instance, the model is instructed to summarize the significant terms from the up- and down-regulated genes in five words or less. The system prompt is specified as follows: "You are an assistant to a biologist who has performed enrichment analysis on a set of up-regulated and down-regulated genes for a certain cluster of patient samples. You must determine a consensus label for a given cluster based on the significantly enriched terms which relate to biological processes and phenotypes." The user prompt on each run is then formatted as follows: "The most significantly enriched terms for the upregulated genes of cluster {cluster} are: {up-regulated genes enriched terms}. For the down genes the significantly enriched terms are: {down-regulated genes enriched terms}. Please provide a consensus label for this cluster with no other reasoning. The label should be at maximum 5 words in length." For the description of the results throughout the pipeline, the system prompt is defined to contextualize the overall pipeline: “You are an assistant to a biologist who has performed various analyses on gene, protein and phosphoprotein data related to tumor expression. A certain result or set of results will be provided with a section header that describes the analysis. Do not include the headers in your response. Write a discussion of the results that mainly describes the results without interpretation. You may specifically denote differences between clusters or specific samples/patients.” To produce the description, the result is formatted and as a string alongside an additional customized prompt which describes the specific analysis performed in that step.
Quantification and statistical analysis
To deal with missing values in the CPTAC proteomics data, we imputed the data matrix with average expression across all samples for each protein, and then z-scored the rows/proteins based on the expression value across all tumor samples. The top 250 significantly differentially expressed proteins for each sample were retained, and these proteins were regarded as highly or lowly expressed in each tumor sample. The cut-off of 30 kinases from the KEA3 analyses was determined based on an inflection point in the distribution of the KEA3 MeanRank scores across the inferred kinases, where the score values are most extreme within the top 30. The Tree Parzen Estimators algorithm was implemented within the hyperopt Python module123 was used to converge on the optimal number of TFs (between 5 and 30) and augmented genes (between 20 and 150) to submit for kinase enrichment analysis. The data matrix was imputed by substituting NaN values with the average expression of each protein after dropping proteins with > 50% NaNs. We then applied quantile and Z-score normalization to the data matrix. Co-expression correlations were computed with Pearson's correlation coefficients. The number of clusters in each CPTAC cohort were determined by the number of neighbors in the UMAP embedding (5 to 30) and the Leiden clustering resolution (0 to 1). These parameters were selected to maximize the separation of the clusters’ overall survival curves, given by the log-rank test statistic. For the TargetRanger analysis we identify significantly differentially expressed candidates as those with an adjusted p-value that is < 0.01.
Acknowledgments
This project is funded by NIH grants U24CA271114, U24CA264250, OT2OD030160, R01DK131525, and U24CA224260. The work was partially funded by the U.S. National Cancer Institute's Office of Cancer Clinical Proteomics Research (Clinical Proteomic Tumor Analysis Consortium [CPTAC]). CPTAC collaborates with international organizations/institutions to accelerate the understanding of the molecular basis of cancer through the application of proteogenomics, standards development, and publicly available datasets.
Author contributions
G.B.M. and E.Z.D. developed the Multiomics2Targets website, performed the TargetRanger and X2K analyses, generated the figures, and wrote the paper. D.J.B.C. developed the Appyter and performed some of the initial analyses. I.D. created the protein co-expression data matrix from CCLE. A.C.R. and P.W. contributed ideas and participated in discussions. W.M. assisted in preparing the inputs to the ChEA3 and KEA3 analyses. A.M. initiated the project, provided direction and ideas, managed the project, and wrote the paper.
Declaration of interests
The authors declare no competing interests.
Published: August 9, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2024.100839.
Supplemental information
References
- 1.Sanchez-Vega F., Mina M., Armenia J., Chatila W.K., Luna A., La K.C., Dimitriadoy S., Liu D.L., Kantheti H.S., Saghafinia S., et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen E.Y., Xu H., Gordonov S., Lim M.P., Perkins M.H., Ma’ayan A. Expression2Kinases: mRNA profiling linked to multiple upstream regulatory layers. Bioinformatics. 2012;28:105–111. doi: 10.1093/bioinformatics/btr625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke D.J.B., Kuleshov M.V., Schilder B.M., Torre D., Duffy M.E., Keenan A.B., Lachmann A., Feldmann A.S., Gundersen G.W., Silverstein M.C., et al. eXpression2Kinases (X2K) Web: linking expression signatures to upstream cell signaling networks. Nucleic Acids Res. 2018;46:W171–W179. doi: 10.1093/nar/gky458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge S.X., Son E.W., Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinf. 2018;19:534. doi: 10.1186/s12859-018-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., Wadi L., Meyer M., Wong J., Xu C., et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019;14:482–517. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y., Ratnam K., Chuang P.Y., Fan Y., Zhong Y., Dai Y., Mazloom A.R., Chen E.Y., D’Agati V., Xiong H., et al. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat. Med. 2012;18:580–588. doi: 10.1038/nm.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosse K.R., Raman P., Zhu Z., Lane M., Martinez D., Heitzeneder S., Rathi K.S., Kendsersky N.M., Randall M., Donovan L., et al. Identification of GPC2 as an Oncoprotein and Candidate Immunotherapeutic Target in High-Risk Neuroblastoma. Cancer Cell. 2017;32:295–309.e12. doi: 10.1016/j.ccell.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters C., Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 2015;35 doi: 10.1042/BSR20150089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadelain M., Rivière I., Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 11.Ho W.Y., Blattman J.N., Dossett M.L., Yee C., Greenberg P.D. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 12.Orentas R.J., Yang J.J., Wen X., Wei J.S., Mackall C.L., Khan J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front. Oncol. 2012;2:194. doi: 10.3389/fonc.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.K., Bangayan N.J., Chai T., Smith B.A., Pariva T.E., Yun S., Vashisht A., Zhang Q., Park J.W., Corey E., et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc. Natl. Acad. Sci. USA. 2018;115:E4473–E4482. doi: 10.1073/pnas.1802354115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson I.D., Patiño-Escobar B., Tuomivaara S.T., Lin Y.-H.T., Nix M.A., Leung K.K., Kasap C., Ramos E., Nieves Vasquez W., Talbot A., et al. The surfaceome of multiple myeloma cells suggests potential immunotherapeutic strategies and protein markers of drug resistance. Nat. Commun. 2022;13:4121. doi: 10.1038/s41467-022-31810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dannenfelser R., Allen G.M., VanderSluis B., Koegel A.K., Levinson S., Stark S.R., Yao V., Tadych A., Troyanskaya O.G., Lim W.A. Discriminatory Power of Combinatorial Antigen Recognition in Cancer T Cell Therapies. Cell Syst. 2020;11:215–228.e5. doi: 10.1016/j.cels.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Y., Park C., Kim N., Cho J., Moon S.U., Kim J., Jeong E., Yoon S. QSurface: fast identification of surface expression markers in cancers. BMC Syst. Biol. 2018;12:17. doi: 10.1186/s12918-018-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng B., Glicksberg B.S., Newbury P., Chekalin E., Xing J., Liu K., Wen A., Chow C., Chen B. OCTAD: an open workspace for virtually screening therapeutics targeting precise cancer patient groups using gene expression features. Nat. Protoc. 2021;16:728–753. doi: 10.1038/s41596-020-00430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keenan A.B., Jenkins S.L., Jagodnik K.M., Koplev S., He E., Torre D., Wang Z., Dohlman A.B., Silverstein M.C., Lachmann A., et al. The Library of Integrated Network-Based Cellular Signatures NIH Program: System-Level Cataloging of Human Cells Response to Perturbations. Cell Syst. 2018;6:13–24. doi: 10.1016/j.cels.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marino G.B., Ngai M., Clarke D.J.B., Fleishman R.H., Deng E.Z., Xie Z., Ahmed N., Ma’ayan A. GeneRanger and TargetRanger: processed gene and protein expression levels across cells and tissues for target discovery. Nucleic Acids Res. 2023;51:W213–W224. doi: 10.1093/nar/gkad399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Eraslan B., Wieland T., Hallström B., Hopf T., Zolg D.P., Zecha J., Asplund A., Li L.-H., Meng C., et al. A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol. Syst. Biol. 2019;15 doi: 10.15252/msb.20188503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghazalpour A., Bennett B., Petyuk V.A., Orozco L., Hagopian R., Mungrue I.N., Farber C.R., Sinsheimer J., Kang H.M., Furlotte N., et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Dou Y., Da Veiga Leprevost F., Geffen Y., Calinawan A.P., Aguet F., Akiyama Y., Anand S., Birger C., Cao S., et al. Proteogenomic data and resources for pan-cancer analysis. Cancer Cell. 2023;41:1397–1406. doi: 10.1016/j.ccell.2023.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Yu W., D’Anna R., Przybyla A., Wilson M., Sung M., Bullen J., Hurt E., D’Angelo G., Sidders B., et al. Pan-Cancer Proteomics Analysis to Identify Tumor-Enriched and Highly Expressed Cell Surface Antigens as Potential Targets for Cancer Therapeutics. Mol. Cell. Proteomics. 2023;22 doi: 10.1016/j.mcpro.2023.100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baysoy A., Bai Z., Satija R., Fan R. The technological landscape and applications of single-cell multi-omics. Nat. Rev. Mol. Cell Biol. 2023;24:695–713. doi: 10.1038/s41580-023-00615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross S.M., Dane M.A., Smith R.L., Devlin K.L., McLean I.C., Derrick D.S., Mills C.E., Subramanian K., London A.B., Torre D., et al. A multi-omic analysis of MCF10A cells provides a resource for integrative assessment of ligand-mediated molecular and phenotypic responses. Commun. Biol. 2022;5:1066. doi: 10.1038/s42003-022-03975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MoTrPAC Study Group. Lead Analysts. MoTrPAC Study Group Temporal dynamics of the multi-omic response to endurance exercise training. Nature. 2024;629:174–183. doi: 10.1038/s41586-023-06877-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Z., Bailey A., Kuleshov M.V., Clarke D.J.B., Evangelista J.E., Jenkins S.L., Lachmann A., Wojciechowicz M.L., Kropiwnicki E., Jagodnik K.M., et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021;1 doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuleshov M.V., Xie Z., London A.B.K., Yang J., Evangelista J.E., Lachmann A., Shu I., Torre D., Ma’ayan A. KEA3: improved kinase enrichment analysis via data integration. Nucleic Acids Res. 2021;49:W304–W316. doi: 10.1093/nar/gkab359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keenan A.B., Torre D., Lachmann A., Leong A.K., Wojciechowicz M.L., Utti V., Jagodnik K.M., Kropiwnicki E., Wang Z., Ma’ayan A. ChEA3: transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 2019;47:W212–W224. doi: 10.1093/nar/gkz446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petralia F., Ma W., Yaron T.M., Caruso F.P., Tignor N., Wang J.M., Charytonowicz D., Johnson J.L., Huntsman E.M., Marino G.B., et al. Pan-cancer proteogenomics characterization of tumor immunity. Cell. 2024;187:1255–1277.e27. doi: 10.1016/j.cell.2024.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachmann A., Torre D., Keenan A.B., Jagodnik K.M., Lee H.J., Wang L., Silverstein M.C., Ma’ayan A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018;9:1366. doi: 10.1038/s41467-018-03751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nusinow D.P., Szpyt J., Ghandi M., Rose C.M., McDonald E.R., 3rd, Kalocsay M., Jané-Valbuena J., Gelfand E., Schweppe D.K., Jedrychowski M., et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell. 2020;180:387–402.e16. doi: 10.1016/j.cell.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J., Guan J.-L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 34.Tong X., Tanino R., Sun R., Tsubata Y., Okimoto T., Takechi M., Isobe T. Protein tyrosine kinase 2: a novel therapeutic target to overcome acquired EGFR-TKI resistance in non-small cell lung cancer. Respir. Res. 2019;20:270. doi: 10.1186/s12931-019-1244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zong M., Meng M., Li L. Low expression of TBX4 predicts poor prognosis in patients with stage II pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2011;12:4953–4963. doi: 10.3390/ijms12084953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ao X., Ding W., Zhang Y., Ding D., Liu Y. TCF21: a critical transcription factor in health and cancer. J. Mol. Med. 2020;98:1055–1068. doi: 10.1007/s00109-020-01934-7. [DOI] [PubMed] [Google Scholar]
- 37.Yu J., Liu M., Liu H., Zhou L. GATA1 promotes colorectal cancer cell proliferation, migration and invasion via activating AKT signaling pathway. Mol. Cell. Biochem. 2019;457:191–199. doi: 10.1007/s11010-019-03523-w. [DOI] [PubMed] [Google Scholar]
- 38.Daino K., Nishimura M., Imaoka T., Takabatake M., Morioka T., Nishimura Y., Shimada Y., Kakinuma S. Epigenetic dysregulation of key developmental genes in radiation-induced rat mammary carcinomas. Int. J. Cancer. 2018;143:343–354. doi: 10.1002/ijc.31309. [DOI] [PubMed] [Google Scholar]
- 39.Zhang G., Li M., Zhou D., Yang X., Zhang W., Gao R. Loss of endothelial EMCN drives tumor lung metastasis through the premetastatic niche. J. Transl. Med. 2022;20:446. doi: 10.1186/s12967-022-03649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang A., Gao K., Chu L., Zhang R., Yang J., Zheng J. Aurora kinases: novel therapy targets in cancers. Oncotarget. 2017;8:23937–23954. doi: 10.18632/oncotarget.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang W., Xu Y., Chen X., Pan S., Zhu X. E26 transformation-specific variant 4 as a tumor promotor in human cancers through specific molecular mechanisms. Mol. Ther. Oncolytics. 2021;22:518–527. doi: 10.1016/j.omto.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen T., Huang S. The role of Cdc25A in the regulation of cell proliferation and apoptosis. Anti Cancer Agents Med. Chem. 2012;12:631–639. doi: 10.2174/187152012800617678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y., Dai Y., Grant S., Dent P. Enhancing CHK1 inhibitor lethality in glioblastoma. Cancer Biol. Ther. 2012;13:379–388. doi: 10.4161/cbt.19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J., Li B., Jia Z., Zhang A., Wang G., Chen Z., Shang Z., Zhang C., Cui J., Yang W. RUNX3 inhibits glioma survival and invasion via suppression of the β-catenin/TCF-4 signaling pathway. J. Neuro Oncol. 2018;140:15–26. doi: 10.1007/s11060-018-2927-0. [DOI] [PubMed] [Google Scholar]
- 45.Hattori E.Y., Masuda T., Mineharu Y., Mikami M., Terada Y., Matsui Y., Kubota H., Matsuo H., Hirata M., Kataoka T.R., et al. A RUNX-targeted gene switch-off approach modulates the BIRC5/PIF1-p21 pathway and reduces glioblastoma growth in mice. Commun. Biol. 2022;5:939. doi: 10.1038/s42003-022-03917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahajan-Thakur S., Bien-Möller S., Marx S., Schroeder H., Rauch B.H. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme-A systematic review. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18112448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyers R.M., Bryan J.G., McFarland J.M., Weir B.A., Sizemore A.E., Xu H., Dharia N.V., Montgomery P.G., Cowley G.S., Pantel S., et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet. 2017;49:1779–1784. doi: 10.1038/ng.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun R., Han R., McCornack C., Khan S., Tabor G.T., Chen Y., Hou J., Jiang H., Schoch K.M., Mao D.D., et al. TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X.-Q., Tao B.-B., Li B., Wang X.-H., Zhang W.-C., Wan L., Hua X.-M., Li S.-T. Overexpression of TREM2 enhances glioma cell proliferation and invasion: a therapeutic target in human glioma. Oncotarget. 2016;7:2354–2366. doi: 10.18632/oncotarget.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuespert K., Pils S., Hauck C.R. CEACAMs: their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gur C., Maalouf N., Gerhard M., Singer B.B., Emgård J., Temper V., Neuman T., Mandelboim O., Bachrach G. The Helicobacter pylori HopQ outermembrane protein inhibits immune cell activities. OncoImmunology. 2019;8 doi: 10.1080/2162402X.2018.1553487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duxbury M.S., Matros E., Clancy T., Bailey G., Doff M., Zinner M.J., Ashley S.W., Maitra A., Redston M., Whang E.E. CEACAM6 is a novel biomarker in pancreatic adenocarcinoma and PanIN lesions. Ann. Surg. 2005;241:491–496. doi: 10.1097/01.sla.0000154455.86404.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strickland L.A., Ross J., Williams S., Ross S., Romero M., Spencer S., Erickson R., Sutcliffe J., Verbeke C., Polakis P., et al. Preclinical evaluation of carcinoembryonic cell adhesion molecule (CEACAM) 6 as potential therapy target for pancreatic adenocarcinoma. J. Pathol. 2009;218:380–390. doi: 10.1002/path.2545. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L., Wu J., Ruan M., Xiao Y., Lan H., Wu Q., Yu C.-W., Zhang Q. The loss of B7-H4 expression in breast cancer cells escaping from T cell cytotoxicity contributes to epithelial-to-mesenchymal transition. Breast Cancer Res. 2023;25:115. doi: 10.1186/s13058-023-01721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tringler B., Liu W., Corral L., Torkko K.C., Enomoto T., Davidson S., Lucia M.S., Heinz D.E., Papkoff J., Shroyer K.R. B7-H4 overexpression in ovarian tumors. Gynecol. Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 56.Gao A., Zhang L., Chen X., Chen Y., Xu Z., Liu Y., Zhu W. Effect of VTCN1 on progression and metastasis of ovarian carcinoma in vitro and vivo. Biomed. Pharmacother. 2015;73:129–134. doi: 10.1016/j.biopha.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 57.Wang X., Wang T., Xu M., Xiao L., Luo Y., Huang W., Zhang Y., Geng W. B7-H4 overexpression impairs the immune response of T cells in human cervical carcinomas. Hum. Immunol. 2014;75:1203–1209. doi: 10.1016/j.humimm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Telford E.J.A., Jiang W.G., Martin T.A. HAVcR-1 involvement in cancer progression. Histol. Histopathol. 2017;32:121–128. doi: 10.14670/HH-11-817. [DOI] [PubMed] [Google Scholar]
- 59.Guo Y.-Y., Yin C.-J., Zhao M., Guo L.-T., Su R.-F., Fu X.-X., Dong W.-L., Tan X.-B. Effect of RMT1-10 on the immunological characteristics of dendritic cells cultured in vitro and corneal transplantation in vivo. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9150–9162. doi: 10.26355/eurrev_201911_19405. [DOI] [PubMed] [Google Scholar]
- 60.Evans J.P., Liu S.-L. Multifaceted Roles of TIM-Family Proteins in Virus-Host Interactions. Trends Microbiol. 2020;28:224–235. doi: 10.1016/j.tim.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du P., Xiong R., Li X., Jiang J. Immune Regulation and Antitumor Effect of TIM-1. J. Immunol. Res. 2016;2016 doi: 10.1155/2016/8605134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilà M.R., Kaplan G.G., Feigelstock D., Nadal M., Morote J., Porta R., Bellmunt J., Meseguer A. Hepatitis A virus receptor blocks cell differentiation and is overexpressed in clear cell renal cell carcinoma. Kidney Int. 2004;65:1761–1773. doi: 10.1111/j.1523-1755.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 63.Chrétien I., Marcuz A., Courtet M., Katevuo K., Vainio O., Heath J.K., White S.J., Du Pasquier L. CTX, a Xenopus thymocyte receptor, defines a molecular family conserved throughout vertebrates. Eur. J. Immunol. 1998;28:4094–4104. doi: 10.1002/(SICI)1521-4141(199812)28:12<4094::AID-IMMU4094>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 64.Prasad D.V.R., Nguyen T., Li Z., Yang Y., Duong J., Wang Y., Dong C. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 65.Vogt L., Schmitz N., Kurrer M.O., Bauer M., Hinton H.I., Behnke S., Gatto D., Sebbel P., Beerli R.R., Sonderegger I., et al. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J. Clin. Invest. 2006;116:2817–2826. doi: 10.1172/JCI25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen C.Q., Cornelius J.G., Cooper L., Neff J., Tao J., Lee B.H., Peck A.B. Identification of possible candidate genes regulating Sjögren’s syndrome-associated autoimmunity: a potential role for TNFSF4 in autoimmune exocrinopathy. Arthritis Res. Ther. 2008;10:R137. doi: 10.1186/ar2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams B.B., Tebbutt N.C., Buchert M., Putoczki T.L., Doggett K., Bao S., Johnstone C.N., Masson F., Hollande F., Burgess A.W., et al. Glycoprotein A33 deficiency: a new mouse model of impaired intestinal epithelial barrier function and inflammatory disease. Dis. Model. Mech. 2015;8:805–815. doi: 10.1242/dmm.019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Opstelten R., de Kivit S., Slot M.C., van den Biggelaar M., Iwaszkiewicz-Grześ D., Gliwiński M., Scott A.M., Blom B., Trzonkowski P., Borst J., et al. GPA33: A Marker to Identify Stable Human Regulatory T Cells. J. Immunol. 2020;204:3139–3148. doi: 10.4049/jimmunol.1901250. [DOI] [PubMed] [Google Scholar]
- 69.Opstelten R., Suwandi J.S., Slot M.C., Morgana F., Scott A.M., Laban S., Nikolic T., Turksma A.W., Kroeze A., Voermans C., et al. GPA33 is expressed on multiple human blood cell types and distinguishes CD4+ central memory T cells with and without effector function. Eur. J. Immunol. 2021;51:1377–1389. doi: 10.1002/eji.202048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katsukura N., Watanabe S., Shirasaki T., Hibiya S., Kano Y., Akahoshi K., Tanabe M., Kirimura S., Akashi T., Kitagawa M., et al. Intestinal phenotype is maintained by Atoh1 in the cancer region of intraductal papillary mucinous neoplasm. Cancer Sci. 2021;112:932–944. doi: 10.1111/cas.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishiyama N., Lee S.-H., Liu S., Li G.-Y., Smith M.J., Reichardt L.F., Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Gessner R., Tauber R. Intestinal cell adhesion molecules. Liver-intestine cadherin. Ann. N. Y. Acad. Sci. 2000;915:136–143. doi: 10.1111/j.1749-6632.2000.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 73.Ordóñez N.G. Cadherin 17 is a novel diagnostic marker for adenocarcinomas of the digestive system. Adv. Anat. Pathol. 2014;21:131–137. doi: 10.1097/PAP.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 74.Qiu H.-B., Zhang L.-Y., Ren C., Zeng Z.-L., Wu W.-J., Luo H.-Y., Zhou Z.-W., Xu R.-H. Targeting CDH17 suppresses tumor progression in gastric cancer by downregulating Wnt/β-catenin signaling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abouelkhair M.B., Mabrouk S.H., Zaki S.S.A., Nada O.H., Hakim S.A. The Diagnostic Value of Cadherin 17 and CDX2 Expression as Immunohistochemical Markers in Colorectal Adenocarcinoma. J. Gastrointest. Cancer. 2021;52:960–969. doi: 10.1007/s12029-020-00513-w. [DOI] [PubMed] [Google Scholar]
- 76.Yang B., Verkman A.S. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 77.Sun M.-C., Honey C.R., Berk C., Wong N.L.M., Tsui J.K.C. Regulation of aquaporin-4 in a traumatic brain injury model in rats. J. Neurosurg. 2003;98:565–569. doi: 10.3171/jns.2003.98.3.0565. [DOI] [PubMed] [Google Scholar]
- 78.Taniguchi M., Yamashita T., Kumura E., Tamatani M., Kobayashi A., Yokawa T., Maruno M., Kato A., Ohnishi T., Kohmura E., et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res. Mol. Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 79.Aoki K., Uchihara T., Tsuchiya K., Nakamura A., Ikeda K., Wakayama Y. Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol. 2003;106:121–124. doi: 10.1007/s00401-003-0709-y. [DOI] [PubMed] [Google Scholar]
- 80.Saadoun S., Papadopoulos M.C., Davies D.C., Krishna S., Bell B.A. Aquaporin-4 expression is increased in oedematous human brain tumours. J. Neurol. Neurosurg. Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu H., Yao H.-T., Zhang W.-P., Zhang L., Ding W., Zhang S.-H., Chen Z., Wei E.-Q. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J. Zhejiang Univ. - Sci. B. 2005;6:33–37. doi: 10.1631/jzus.2005.B0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verkman A.S. Aquaporin water channels and endothelial cell function. J. Anat. 2002;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y., Gao F., Jiang R., Liu H., Hou J., Yi Y., Kang L., Liu X., Li Y., Yang M. Down-Regulation of AQP4 Expression via p38 MAPK Signaling in Temozolomide-Induced Glioma Cells Growth Inhibition and Invasion Impairment. J. Cell. Biochem. 2017;118:4905–4913. doi: 10.1002/jcb.26176. [DOI] [PubMed] [Google Scholar]
- 84.Lan Y.-L., Chen C., Wang X., Lou J.-C., Xing J.-S., Zou S., Hu J.-L., Lyu W., Zhang B. Gamabufotalin induces a negative feedback loop connecting ATP1A3 expression and the AQP4 pathway to promote temozolomide sensitivity in glioblastoma cells by targeting the amino acid Thr794. Cell Prolif. 2020;53 doi: 10.1111/cpr.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amagai M., Klaus-Kovtun V., Stanley J.R. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 86.Hanakawa Y., Amagai M., Shirakata Y., Yahata Y., Tokumaru S., Yamasaki K., Tohyama M., Sayama K., Hashimoto K. Differential effects of desmoglein 1 and desmoglein 3 on desmosome formation. J. Invest. Dermatol. 2002;119:1231–1236. doi: 10.1046/j.1523-1747.2002.19648.x. [DOI] [PubMed] [Google Scholar]
- 87.Rötzer V., Hartlieb E., Winkler J., Walter E., Schlipp A., Sardy M., Spindler V., Waschke J. Desmoglein 3-Dependent Signaling Regulates Keratinocyte Migration and Wound Healing. J. Invest. Dermatol. 2016;136:301–310. doi: 10.1038/JID.2015.380. [DOI] [PubMed] [Google Scholar]
- 88.Viehweger F., Azem A., Gorbokon N., Uhlig R., Lennartz M., Dwertmann Rico S., Kind S., Reiswich V., Kluth M., Hube-Magg C., et al. Desmoglein 3 (Dsg3) expression in cancer: A tissue microarray study on 15,869 tumors. Pathol. Res. Pract. 2022;240 doi: 10.1016/j.prp.2022.154200. [DOI] [PubMed] [Google Scholar]
- 89.Wang L., Liu T., Wang Y., Cao L., Nishioka M., Aguirre R.L., Ishikawa A., Geng L., Okada N. Altered expression of desmocollin 3, desmoglein 3, and beta-catenin in oral squamous cell carcinoma: correlation with lymph node metastasis and cell proliferation. Virchows Arch. 2007;451:959–966. doi: 10.1007/s00428-007-0485-5. [DOI] [PubMed] [Google Scholar]
- 90.Fang W.-K., Gu W., Liao L.-D., Chen B., Wu Z.-Y., Wu J.-Y., Shen J., Xu L.-Y., Li E.-M. Prognostic significance of desmoglein 2 and desmoglein 3 in esophageal squamous cell carcinoma. Asian Pac. J. Cancer Prev. APJCP. 2014;15:871–876. doi: 10.7314/apjcp.2014.15.2.871. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y.-Q., Zou H.-Y., Xie J.-J., Fang W.-K. Paradoxical Roles of Desmosomal Components in Head and Neck Cancer. Biomolecules. 2021;11 doi: 10.3390/biom11060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abula Y., Su Y., Tuniyazi D., Yi C. Desmoglein 3 contributes to tumorigenicity of pancreatic ductal adenocarcinoma through activating Src-FAK signaling. Anim. Cell Syst. 2021;25:195–202. doi: 10.1080/19768354.2021.1943707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown L., Waseem A., Cruz I.N., Szary J., Gunic E., Mannan T., Unadkat M., Yang M., Valderrama F., O’Toole E.A., Wan H. Desmoglein 3 promotes cancer cell migration and invasion by regulating activator protein 1 and protein kinase C-dependent-Ezrin activation. Oncogene. 2014;33:2363–2374. doi: 10.1038/onc.2013.186. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y.-J., Chang J.T., Lee L., Wang H.-M., Liao C.-T., Chiu C.-C., Chen P.-J., Cheng A.-J. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene. 2007;26:467–476. doi: 10.1038/sj.onc.1209802. [DOI] [PubMed] [Google Scholar]
- 95.Walia V., Ding M., Kumar S., Nie D., Premkumar L.S., Elble R.C. hCLCA2 Is a p53-Inducible Inhibitor of Breast Cancer Cell Proliferation. Cancer Res. 2009;69:6624–6632. doi: 10.1158/0008-5472.CAN-08-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Connon C.J., Kawasaki S., Yamasaki K., Quantock A.J., Kinoshita S. The quantification of hCLCA2 and colocalisation with integrin β4 in stratified human epithelia. Acta Histochem. 2005;106:421–425. doi: 10.1016/j.acthis.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Shinmura K., Igarashi H., Kato H., Kawanishi Y., Inoue Y., Nakamura S., Ogawa H., Yamashita T., Kawase A., Funai K., Sugimura H. CLCA2 as a novel immunohistochemical marker for differential diagnosis of squamous cell carcinoma from adenocarcinoma of the lung. Dis. Markers. 2014;2014 doi: 10.1155/2014/619273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alabiad M.A., Harb O.A., Abozaid M., Embaby A., Mandour D., Hemeda R., Shalaby A.M. The Diagnostic and Prognostic Roles of Combined Expression of Novel Biomarkers in Lung Adenocarcinoma and Lung Squamous Cell Carcinoma: An Immunohistochemical Study. Iran. J. Pathol. 2021;16:162–173. doi: 10.30699/IJP.2020.130944.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liang Z., Dong J., Yang N., Li S.-D., Yang Z.-Y., Huang R., Li F.-J., Wang W.-T., Ren J.-K., Lei J., et al. Tandem CAR-T cells targeting FOLR1 and MSLN enhance the antitumor effects in ovarian cancer. Int. J. Biol. Sci. 2021;17:4365–4376. doi: 10.7150/ijbs.63181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clarke D.J.B., Jeon M., Stein D.J., Moiseyev N., Kropiwnicki E., Dai C., Xie Z., Wojciechowicz M.L., Litz S., Hom J., et al. Appyters: Turning Jupyter Notebooks into data-driven web apps. Patterns (N Y) 2021;2 doi: 10.1016/j.patter.2021.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong Y., Li S., Sun X., Wang Y., Lu T., Wo Y., Leng X., Kong D., Jiao W. Desmoglein 3 and Keratin 14 for Distinguishing Between Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Onco. Targets. Therapy. 2020;13:11111–11124. doi: 10.2147/OTT.S270398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Funahashi S.-I., Kawai S., Fujii E., Taniguchi K., Nakano K., Ishikawa S., Aburatani H., Suzuki M. Generation of an anti-desmoglein 3 antibody without pathogenic activity of pemphigus vulgaris for therapeutic application to squamous cell carcinoma. J. Biochem. 2018;164:471–481. doi: 10.1093/jb/mvy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galindo I., Gómez-Morales M., Díaz-Cano I., Andrades Á., Caba-Molina M., Miranda-León M.T., Medina P.P., Martín-Padron J., Fárez-Vidal M.E. The value of desmosomal plaque-related markers to distinguish squamous cell carcinoma and adenocarcinoma of the lung. Ups. J. Med. Sci. 2020;125:19–29. doi: 10.1080/03009734.2019.1692101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salceda S., Tang T., Kmet M., Munteanu A., Ghosh M., Macina R., Liu W., Pilkington G., Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 105.Liang L., Jiang Y., Chen J.-S., Niu N., Piao J., Ning J., Zu Y., Zhang J., Liu J. B7-H4 expression in ovarian serous carcinoma: a study of 306 cases. Hum. Pathol. 2016;57:1–6. doi: 10.1016/j.humpath.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Purrington K.S., Knight J., 3rd, Dyson G., Ali-Fehmi R., Schwartz A.G., Boerner J.L., Bandyopadhyay S. CLCA2 expression is associated with survival among African American women with triple negative breast cancer. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma J., Xu X., Fu C., Xia P., Tian M., Zheng L., Chen K., Liu X., Li Y., Yu L., et al. CDH17 nanobodies facilitate rapid imaging of gastric cancer and efficient delivery of immunotoxin. Biomater. Res. 2022;26:64. doi: 10.1186/s40824-022-00312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rachid Zaim S., Kenost C., Berghout J., Vitali F., Zhang H.H., Lussier Y.A. Evaluating single-subject study methods for personal transcriptomic interpretations to advance precision medicine. BMC Med. Genom. 2019;12:96. doi: 10.1186/s12920-019-0513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:1–14. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A., et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lachmann A., Schilder B.M., Wojciechowicz M.L., Torre D., Kuleshov M.V., Keenan A.B., Ma'ayan A. Geneshot: search engine for ranking genes from arbitrary text queries. Nucleic Acids Res. 2019;47:W571–W577. doi: 10.1093/nar/gkz393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berger S.I., Posner J.M., Ma’ayan A. Genes2Networks: connecting lists of gene symbols using mammalian protein interactions databases. BMC Bioinf. 2007;8:372. doi: 10.1186/1471-2105-8-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao L., Lee V.H.F., Ng M.K., Yan H., Bijlsma M.F. Molecular subtyping of cancer: current status and moving toward clinical applications. Briefings Bioinf. 2019;20:572–584. doi: 10.1093/bib/bby026. [DOI] [PubMed] [Google Scholar]
- 114.McInnes L., Healy J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv. 2018 doi: 10.48550/arXiv.1802.03426. Preprint at. [DOI] [Google Scholar]
- 115.Traag V.A., Waltman L., van Eck N.J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-41695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Binder J.X., Pletscher-Frankild S., Tsafou K., Stolte C., O’Donoghue S.I., Schneider R., Jensen L.J. COMPARTMENTS: unification and visualization of protein subcellular localization evidence. Database. 2014;2014 doi: 10.1093/database/bau012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thul P.J., Lindskog C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018;27:233–244. doi: 10.1002/pro.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsherniak A., Vazquez F., Montgomery P.G., Weir B.A., Kryukov G., Cowley G.S., Gill S., Harrington W.F., Pantel S., Krill-Burger J.M., et al. Defining a Cancer Dependency Map. Cell. 2017;170:564–576.e16. doi: 10.1016/j.cell.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carvalho-Silva D., Pierleoni A., Pignatelli M., Ong C., Fumis L., Karamanis N., Carmona M., Faulconbridge A., Hercules A., McAuley E., et al. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res. 2019;47:D1056–D1065. doi: 10.1093/nar/gky1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wolf F.A., Angerer P., Theis F.J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Virtanen P., Gommers R., Oliphant T.E., Haberland M., Reddy T., Cournapeau D., Burovski E., Peterson P., Weckesser W., Bright J., et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bergstra J., Komer B., Eliasmith C., Yamins D., Cox D.D. Hyperopt: a Python library for model selection and hyperparameter optimization. Comput. Sci. Discov. 2015;8 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyzes publicly accessible data. Accessions are listed in the Key Resources Table.
-
•
The Multiomics2Targets website can be accessed at: https://multiomics2targets.maayanlab.cloud/
The source code for the Multiomics2Targets site can be accessed at https://github.com/MaayanLab/Multiomics2Targets. A snapshot of the Multiomics2Targets code was deposited into Figshare at https://figshare.com/articles/software/Multiomics2Targets_source_code_snapshot_from_07082024/26199887, with https://doi.org/10.6084/m9.figshare.26199887. The source code for the eXpression2Kinases analysis can be accessed at https://github.com/MaayanLab/x2k-pathway-analysis.
-
•
Any additional information needed to re-analyze the data reported in this paper is available from the lead contact upon request.