Full text
PDF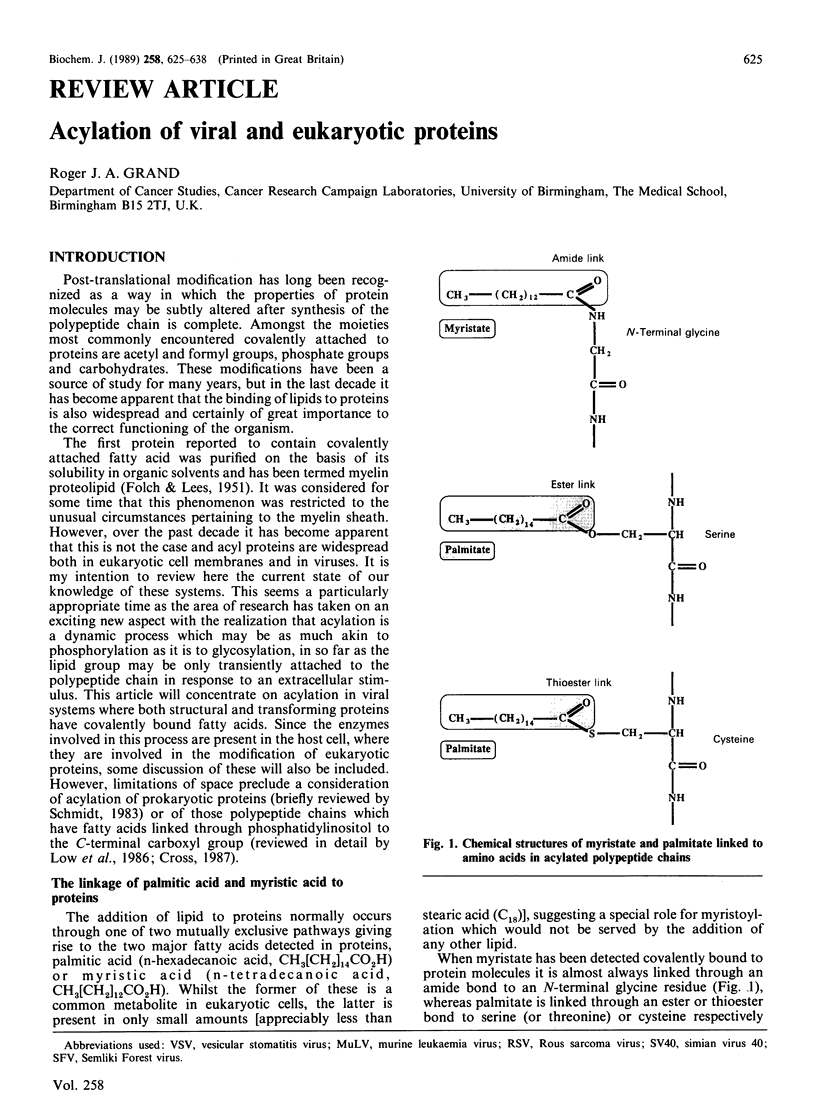













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Aderem A. A., Keum M. M., Pure E., Cohn Z. A. Bacterial lipopolysaccharides, phorbol myristate acetate, and zymosan induce the myristoylation of specific macrophage proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5817–5821. doi: 10.1073/pnas.83.16.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal H. C., Randle C. L., Agrawal D. In vivo acylation of rat brain myelin proteolipid protein. J Biol Chem. 1982 Apr 25;257(8):4588–4592. [PubMed] [Google Scholar]
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Allan J. S., Coligan J. E., Lee T. H., McLane M. F., Kanki P. J., Groopman J. E., Essex M. A new HTLV-III/LAV encoded antigen detected by antibodies from AIDS patients. Science. 1985 Nov 15;230(4727):810–813. doi: 10.1126/science.2997921. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Coleman R. A. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem. 1980;49:459–487. doi: 10.1146/annurev.bi.49.070180.002331. [DOI] [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Cell-free fatty acid acylation of Semliki Forest viral polypeptides with microsomal membranes from eukaryotic cells. J Biol Chem. 1984 Jun 10;259(11):7245–7252. [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Identification of acyl donors and acceptor proteins for fatty acid acylation in BHK cells infected with Semliki Forest virus. EMBO J. 1984 Apr;3(4):713–719. doi: 10.1002/j.1460-2075.1984.tb01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Protein fatty acyltransferase is located in the rough endoplasmic reticulum. FEBS Lett. 1985 Aug 5;187(2):289–294. doi: 10.1016/0014-5793(85)81261-8. [DOI] [PubMed] [Google Scholar]
- Bernards R., Van der Eb A. J. Adenovirus: transformation and oncogenicity. Biochim Biophys Acta. 1984 Dec 14;783(3):187–204. doi: 10.1016/0167-4781(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Kornfeld R. Evidence for an alpha-mannosidase in endoplasmic reticulum of rat liver. J Biol Chem. 1983 Jul 10;258(13):7907–7910. [PubMed] [Google Scholar]
- Bizzozero O. A., McGarry J. F., Lees M. B. Acylation of endogenous myelin proteolipid protein with different acyl-CoAs. J Biol Chem. 1987 Feb 15;262(5):2138–2145. [PubMed] [Google Scholar]
- Bizzozero O. A., McGarry J. F., Lees M. B. Autoacylation of myelin proteolipid protein with acyl coenzyme A. J Biol Chem. 1987 Oct 5;262(28):13550–13557. [PubMed] [Google Scholar]
- Burn P., Burger M. M. The cytoskeletal protein vinculin contains transformation-sensitive, covalently bound lipid. Science. 1987 Jan 23;235(4787):476–479. doi: 10.1126/science.3099391. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Direct identification of palmitic acid as the lipid attached to p21ras. Mol Cell Biol. 1986 Jan;6(1):116–122. doi: 10.1128/mcb.6.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Jarvis D. L. The plasma-membrane-associated form of SV40 large tumor antigen: biochemical and biological properties. Biochim Biophys Acta. 1986 Oct 28;865(2):171–195. doi: 10.1016/0304-419x(86)90027-2. [DOI] [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Fatty acid modification of Newcastle disease virus glycoproteins. J Virol. 1982 Jul;43(1):342–347. doi: 10.1128/jvi.43.1.342-347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Q., Ulsh L. S., DuBois G., Shih T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J Virol. 1985 Nov;56(2):607–612. doi: 10.1128/jvi.56.2.607-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Deppert W., Walter G. Domains of simian virus 40 large T-antigen exposed on the cell surface. Virology. 1982 Oct 15;122(1):56–70. doi: 10.1016/0042-6822(82)90377-4. [DOI] [PubMed] [Google Scholar]
- Deppert W., Walter G. Simian virus to (SV40) tumor-specific proteins in nucleus and plasma membrane of HeLa cells infected by adenovirus 2-SV40 hybrid virus Ad2+ND2. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2505–2509. doi: 10.1073/pnas.73.7.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Broach J. R. Fatty acylation is important but not essential for Saccharomyces cerevisiae RAS function. Mol Cell Biol. 1987 Jul;7(7):2344–2351. doi: 10.1128/mcb.7.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Fries E., Urbani L. J., Rothman J. E. Early and late functions associated with the Golgi apparatus reside in distinct compartments. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7453–7457. doi: 10.1073/pnas.78.12.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Featherstone C., Griffiths G., Warren G. Newly synthesized G protein of vesicular stomatitis virus is not transported to the Golgi complex in mitotic cells. J Cell Biol. 1985 Dec;101(6):2036–2046. doi: 10.1083/jcb.101.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallick G. E., Arlinghaus R. B. Incorporation of lipids into variants of Moloney sarcoma virus which produce gag-mos fusion proteins. Virology. 1984 Feb;133(1):228–232. doi: 10.1016/0042-6822(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983 Apr;46(1):162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Krueger J. G., Hanafusa H., Goldberg A. R. Only membrane-associated RSV src proteins have amino-terminally bound lipid. Nature. 1983 Mar 10;302(5904):161–163. doi: 10.1038/302161a0. [DOI] [PubMed] [Google Scholar]
- Glover C. J., Goddard C., Felsted R. L. N-myristoylation of p60src. Identification of a myristoyl-CoA:glycylpeptide N-myristoyltransferase in rat tissues. Biochem J. 1988 Mar 1;250(2):485–491. doi: 10.1042/bj2500485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J., Gallimore P. H. Adenovirus type 12 early region 1 proteins: a study of their subcellular localization and protein-protein interactions. J Gen Virol. 1984 Dec;65(Pt 12):2149–2166. doi: 10.1099/0022-1317-65-12-2149. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Roberts C., Gallimore P. H. Acylation of adenovirus type 12 early region 1b 18-kDa protein. Further evidence for its localisation in the cell membrane. FEBS Lett. 1985 Feb 25;181(2):229–235. doi: 10.1016/0014-5793(85)80265-9. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Smith K. J., Gallimore P. H. Purification and characterisation of the protein encoded by the activated human N-ras gene and its membrane localisation. Oncogene. 1987;1(3):305–314. [PubMed] [Google Scholar]
- Grand R. J. The structure and functions of the adenovirus early region 1 proteins. Biochem J. 1987 Jan 1;241(1):25–38. doi: 10.1042/bj2410025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinna L. S., Robbins P. W. Glycoprotein biosynthesis. Rat liver microsomal glucosidases which process oligosaccharides. J Biol Chem. 1979 Sep 25;254(18):8814–8818. [PubMed] [Google Scholar]
- Hedo J. A., Collier E., Watkinson A. Myristyl and palmityl acylation of the insulin receptor. J Biol Chem. 1987 Jan 25;262(3):954–957. [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Lange-Mutschler J. Tightly associated lipids may anchor SV40 large T antigen in plasma membrane. Nature. 1983 Oct 20;305(5936):736–738. doi: 10.1038/305736a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Jakoi E. R., Ross P. E., Ping Ting-Beall H., Kaufman B., Vanaman T. C. Ligatin: a peripheral membrane protein with covalently bound palmitic acid. J Biol Chem. 1987 Jan 25;262(3):1300–1304. [PubMed] [Google Scholar]
- Jing S. Q., Trowbridge I. S. Identification of the intermolecular disulfide bonds of the human transferrin receptor and its lipid-attachment site. EMBO J. 1987 Feb;6(2):327–331. doi: 10.1002/j.1460-2075.1987.tb04758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Rous sarcoma virus transforming protein lacking myristic acid phosphorylates known polypeptide substrates without inducing transformation. Cell. 1986 Apr 11;45(1):105–112. doi: 10.1016/0092-8674(86)90542-8. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Krangel M. S., Strominger J. L. Cysteines in the transmembrane region of major histocompatibility complex antigens are fatty acylated via thioester bonds. J Biol Chem. 1984 Jun 10;259(11):7230–7238. [PubMed] [Google Scholar]
- Kellie S., Wigglesworth N. M. The cytoskeletal protein vinculin is acylated by myristic acid. FEBS Lett. 1987 Mar 23;213(2):428–432. doi: 10.1016/0014-5793(87)81536-3. [DOI] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylation: a new post-translational modification specific for plasma membrane-associated simian virus 40 large T-antigen. FEBS Lett. 1983 Jan 24;151(2):257–259. doi: 10.1016/0014-5793(83)80081-7. [DOI] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Evidence for transmembrane orientation of acylated simian virus 40 large T antigen. J Virol. 1985 Nov;56(2):541–548. doi: 10.1128/jvi.56.2.541-548.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Staufenbiel M., Deppert W. Membrane interactions of simian virus 40 large T-antigen: influence of protein sequences and fatty acid acylation. Mol Cell Biol. 1984 Aug;4(8):1542–1550. doi: 10.1128/mcb.4.8.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. Ia-associated invariant chain is fatty acylated before addition of sialic acid. Biochemistry. 1985 Oct 22;24(22):6185–6190. doi: 10.1021/bi00343a023. [DOI] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. The HLA-D-associated invariant chain binds palmitic acid at the cysteine adjacent to the membrane segment. J Biol Chem. 1986 Mar 5;261(7):3434–3440. [PubMed] [Google Scholar]
- Kotwal G. J., Ghosh H. P. Role of fatty acid acylation of membrane glycoproteins. Absence of palmitic acid in glycoproteins of two serotypes of vesicular stomatitis virus. J Biol Chem. 1984 Apr 25;259(8):4699–4701. [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Lacal P. M., Pennington C. Y., Lacal J. C. Transforming activity of ras proteins translocated to the plasma membrane by a myristoylation sequence from the src gene product. Oncogene. 1988 Jun;2(6):533–537. [PubMed] [Google Scholar]
- Lange-Mutschler J. Acylated fibronectin: a new type of posttranslational modification of cellular fibronectin. FEBS Lett. 1986 Jun 9;201(2):210–214. doi: 10.1016/0014-5793(86)80610-x. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. The ras gene family. Cancer Surv. 1986;5(2):275–289. [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Koyama A. H., Malfer C., Wen D., Schlesinger M. J. Release of fatty acids from virus glycoproteins by hydroxylamine. Biochim Biophys Acta. 1984 Apr 10;798(2):156–166. doi: 10.1016/0304-4165(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Malvoisin E., Wild F., Zwingelstein G. 12-O-tetradecanoyl phorbol 13-acetate stimulates the myristylation of an approximately 82 kDa protein in HL-60 cells. FEBS Lett. 1987 May 4;215(1):175–178. doi: 10.1016/0014-5793(87)80136-9. [DOI] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters S. B., Stroud R. M., Bourne H. R. Family of G protein alpha chains: amphipathic analysis and predicted structure of functional domains. Protein Eng. 1986 Oct-Nov;1(1):47–54. [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade C. J., Tremblay M. L., Yee S. P., Ross R., Branton P. E. Acylation of the 176R (19-kilodalton) early region 1B protein of human adenovirus type 5. J Virol. 1987 Oct;61(10):3227–3234. doi: 10.1128/jvi.61.10.3227-3234.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlhinney R. A., Pelly S. J., Chadwick J. K., Cowley G. P. Studies on the attachment of myristic and palmitic acid to cell proteins in human squamous carcinoma cell lines: evidence for two pathways. EMBO J. 1985 May;4(5):1145–1152. doi: 10.1002/j.1460-2075.1985.tb03752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar C. M., Prange R., Gallwitz D. A carboxyl-terminal cysteine residue is required for palmitic acid binding and biological activity of the ras-related yeast YPT1 protein. EMBO J. 1988 Apr;7(4):971–976. doi: 10.1002/j.1460-2075.1988.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H., Klenk H. D. Coronavirus glycoprotein E1, a new type of viral glycoprotein. J Mol Biol. 1981 Dec 25;153(4):993–1010. doi: 10.1016/0022-2836(81)90463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P. J., St Jules R. S., Reddy T. S., Bazan N. G., Zatz M. Acylation of disc membrane rhodopsin may be nonenzymatic. J Biol Chem. 1987 Apr 15;262(11):5210–5215. [PubMed] [Google Scholar]
- O'Brien P. J., Zatz M. Acylation of bovine rhodopsin by [3H]palmitic acid. J Biol Chem. 1984 Apr 25;259(8):5054–5057. [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P. Alpha and beta subunits of the nicotinic acetylcholine receptor contain covalently bound lipid. J Biol Chem. 1984 May 10;259(9):5364–5367. [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Fatty acylation of cellular proteins. Temporal and subcellular differences between palmitate and myristate acylation. J Biol Chem. 1986 Feb 15;261(5):2458–2466. [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Ozols J., Carr S. A., Strittmatter P. Identification of the NH2-terminal blocking group of NADH-cytochrome b5 reductase as myristic acid and the complete amino acid sequence of the membrane-binding domain. J Biol Chem. 1984 Nov 10;259(21):13349–13354. [PubMed] [Google Scholar]
- Pal R., Barenholz Y., Wagner R. R. Vesicular stomatitis virus membrane proteins and their interactions with lipid bilayers. Biochim Biophys Acta. 1987 Jun 24;906(2):175–193. doi: 10.1016/0304-4157(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. V., Schultz A., Pincus S. E., Oroszlan S., Wimmer E. Capsid protein VP4 of poliovirus is N-myristoylated. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7827–7831. doi: 10.1073/pnas.84.22.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. 1985 Mar 28-Apr 3Nature. 314(6009):374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987 May;61(5):1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. doi: 10.1128/jvi.42.3.905-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Myristoylation and the post-translational acquisition of hydrophobicity by the membrane immunoglobulin heavy-chain polypeptide in B lymphocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7654–7658. doi: 10.1073/pnas.84.21.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L., Branton P. E. Intracellular localization of adenovirus type 5 tumor antigens in productively infected cells. Virology. 1983 Sep;129(2):456–468. doi: 10.1016/0042-6822(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Schmidt J. W., Catterall W. A. Palmitylation, sulfation, and glycosylation of the alpha subunit of the sodium channel. Role of post-translational modifications in channel assembly. J Biol Chem. 1987 Oct 5;262(28):13713–13723. [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acid binding: a new kind of posttranslational modification of membrane proteins. Curr Top Microbiol Immunol. 1983;102:101–129. doi: 10.1007/978-3-642-68906-2_3. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Lambrecht B. On the structure of the acyl linkage and the function of fatty acyl chains in the influenza virus haemagglutinin and the glycoproteins of Semliki Forest virus. J Gen Virol. 1985 Dec;66(Pt 12):2635–2647. doi: 10.1099/0022-1317-66-12-2635. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Tsai S. C., Kung H. F., Oroszlan S., Moss J., Vaughan M. Hydroxylamine-stable covalent linkage of myristic acid in G0 alpha, a guanine nucleotide-binding protein of bovine brain. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1234–1239. doi: 10.1016/0006-291x(87)90780-7. [DOI] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Sharma S., Rodgers L., Brandsma J., Gething M. J., Sambrook J. SV40 T antigen and the exocytotic pathway. EMBO J. 1985 Jun;4(6):1479–1489. doi: 10.1002/j.1460-2075.1985.tb03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Takagi A., Liau Y. H., Jozwiak Z., Slomiany A. In vitro acylation of rat gastric mucus glycoprotein with [3H]palmitic acid. J Biol Chem. 1984 Oct 10;259(19):11997–12000. [PubMed] [Google Scholar]
- Stadler J., Gerisch G., Bauer G., Deppert W. In vivo acylation of Dictyostelium actin with palmitic acid. EMBO J. 1985 May;4(5):1153–1156. doi: 10.1002/j.1460-2075.1985.tb03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M. Ankyrin-bound fatty acid turns over rapidly at the erythrocyte plasma membrane. Mol Cell Biol. 1987 Aug;7(8):2981–2984. doi: 10.1128/mcb.7.8.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Lazarides E. Ankyrin is fatty acid acylated in erythrocytes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):318–322. doi: 10.1073/pnas.83.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C. H., Griffin B. E. Myristic acid is coupled to a structural protein of polyoma virus and SV40. Nature. 1987 Apr 9;326(6113):619–622. doi: 10.1038/326619a0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taparowsky E., Shimizu K., Goldfarb M., Wigler M. Structure and activation of the human N-ras gene. Cell. 1983 Sep;34(2):581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Myristoyl CoA:protein N-myristoyltransferase activities from rat liver and yeast possess overlapping yet distinct peptide substrate specificities. J Biol Chem. 1988 Feb 5;263(4):1784–1790. [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]
- Towler D., Glaser L. Protein fatty acid acylation: enzymatic synthesis of an N-myristoylglycyl peptide. Proc Natl Acad Sci U S A. 1986 May;83(9):2812–2816. doi: 10.1073/pnas.83.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Blose S. H., Stillman B. W. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984 Dec;4(12):2865–2875. doi: 10.1128/mcb.4.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Norris K., Papageorge A. G., Hubbert N. L., Lowy D. R. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984 Nov;3(11):2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]
- van Berlo M. F., van den Brink W. J., Horzinek M. C., van der Zeijst B. A. Fatty acid acylation of viral proteins in murine hepatitis virus-infected cells. Brief report. Arch Virol. 1987;95(1-2):123–128. doi: 10.1007/BF01311339. [DOI] [PMC free article] [PubMed] [Google Scholar]



