Abstract
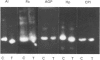
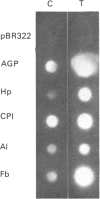
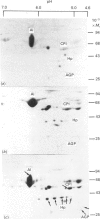
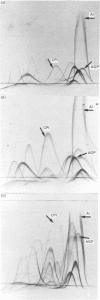
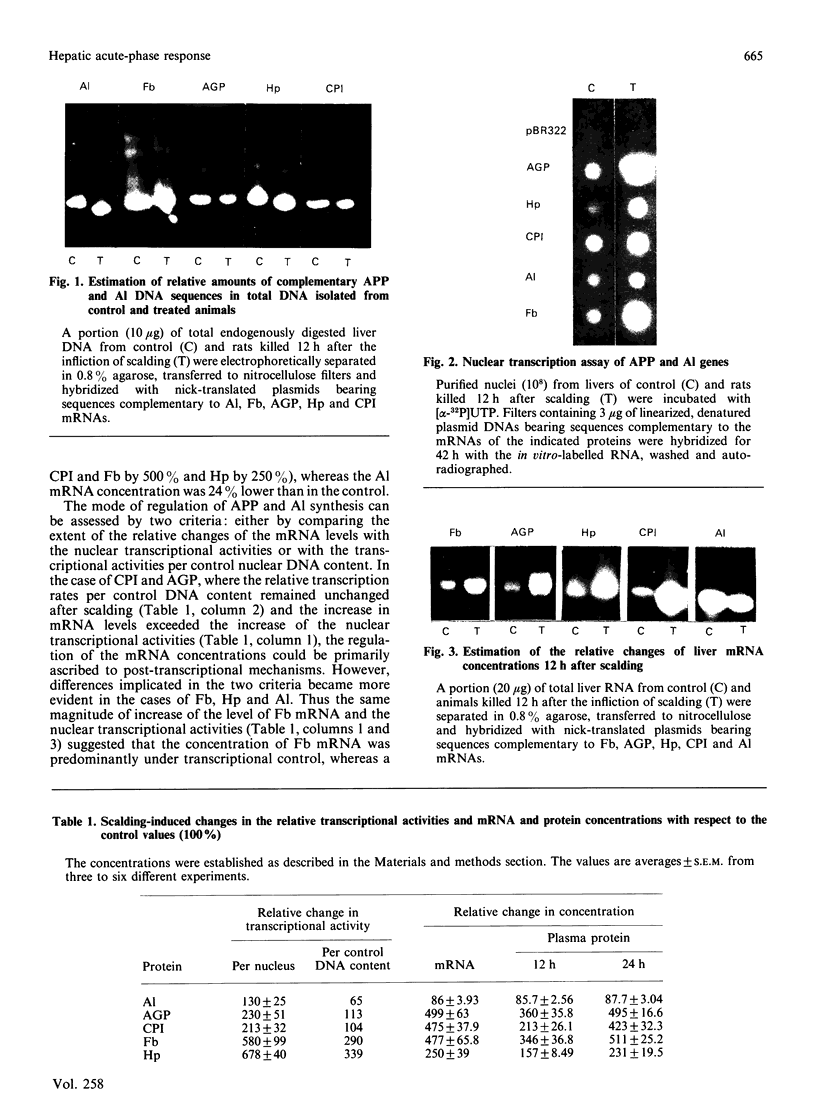
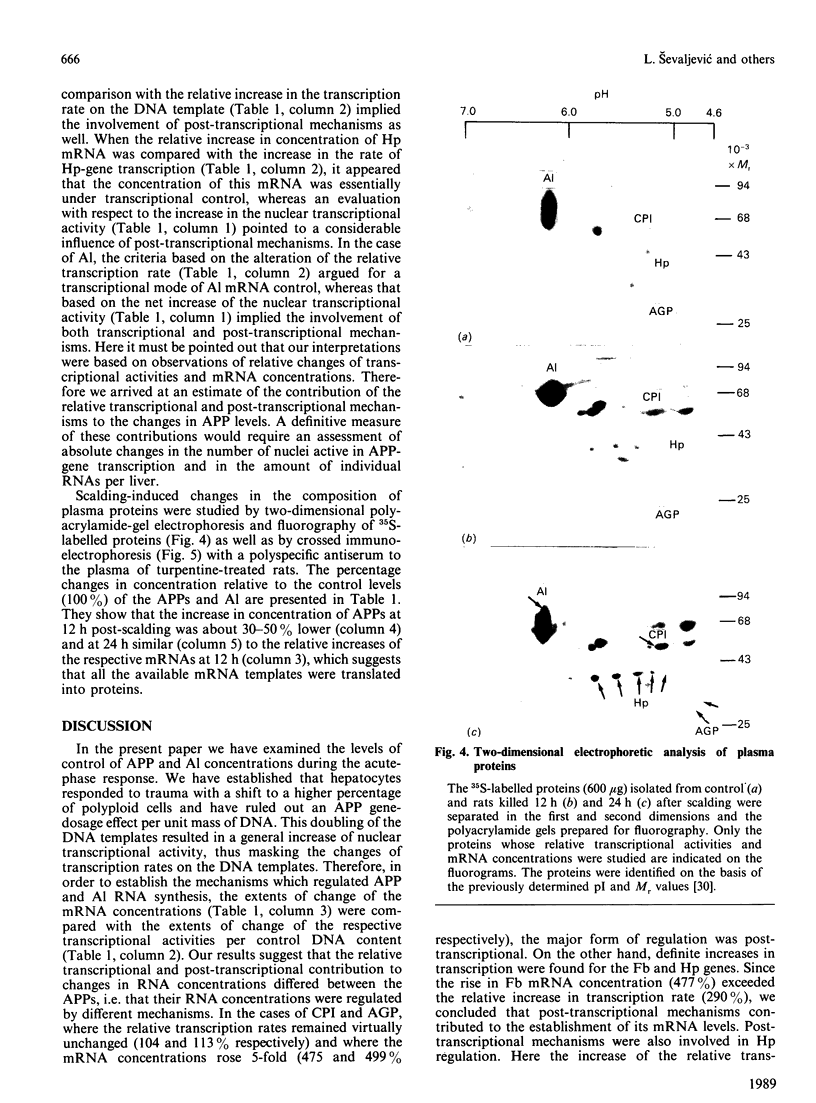
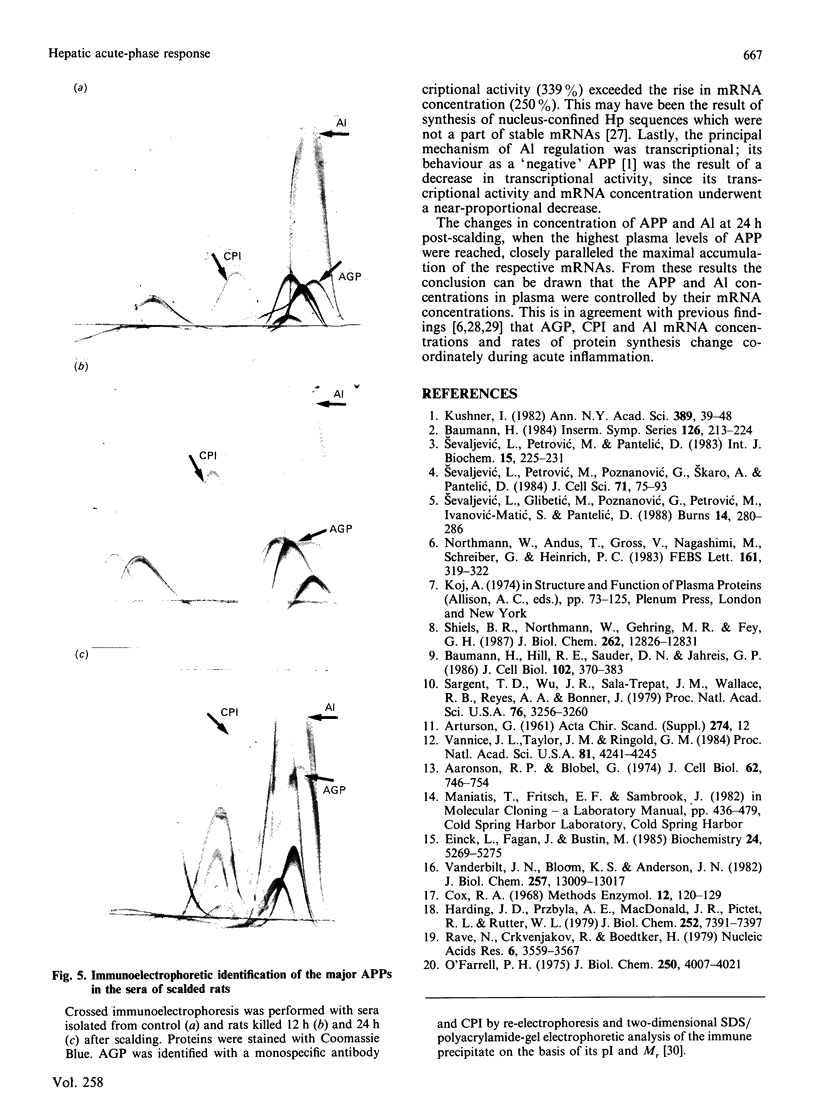
At 12 h after scalding of rats a doubling of the hepatocyte nuclear DNA content, which arose from the presence of additional complete genomes and not from amplification of genes coding for the major acute-phase proteins or albumin, was observed. Examination of relative transcription rates per control DNA mass revealed that alpha 1-acid-glycoprotein and cysteine-proteinase-inhibitor genes remained constitutive, alpha- and gamma-fibrinogen and haptoglobin genes underwent transcriptional activation for 290 and 339% respectively, whereas the relative transcription rate of albumin decreased to 65% of the control level. Along with these changes, the alpha 1-acid glycoprotein, cysteine-proteinase inhibitor and the fibrinogen mRNA concentrations increased about 500%, haptoglobin mRNA 250%, whereas the albumin mRNA concentration fell to 86% of the control. The regulation of the mRNA levels was assessed by comparing the relative change in transcription rates expressed per control DNA content with the relative changes of mRNA concentrations. We arrived at the conclusion that the concentrations of alpha 1-acid-glycoprotein and cysteine-proteinase-inhibitor mRNAs were predominantly regulated by a post-transcriptional mechanism, albumin mRNA by a transcriptional mechanism, and the fibrinogen and haptoglobin mRNAs by a combination of both. The degree of change of the serum levels of the examined proteins was similar to that of their mRNA concentrations and was the result of the complete use of the available RNA templates in protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. On the attachment of the nuclear pore complex. J Cell Biol. 1974 Sep;62(3):746–754. doi: 10.1083/jcb.62.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Kurdowska A., Tran-Thi T. A., Budek W., Koj A., Decker K., Heinrich P. C. Biosynthesis and secretion of alpha 1 acute-phase globulin in primary cultures of rat hepatocytes. Eur J Biochem. 1985 Jan 15;146(2):347–352. doi: 10.1111/j.1432-1033.1985.tb08659.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Held W. A., Berger F. G. The acute phase response of mouse liver. Genetic analysis of the major acute phase reactants. J Biol Chem. 1984 Jan 10;259(1):566–573. [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cole T., Inglis A. S., Roxburgh C. M., Howlett G. J., Schreiber G. Major acute phase alpha 1-protein of the rat is homologous to bovine kininogen and contains the sequence for bradykinin: its synthesis is regulated at the mRNA level. FEBS Lett. 1985 Mar 11;182(1):57–61. doi: 10.1016/0014-5793(85)81153-4. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Einck L., Fagan J., Bustin M. Chromatin structure of a 3-methylcholanthrene-induced cytochrome P-450 gene. Biochemistry. 1985 Sep 10;24(19):5269–5275. doi: 10.1021/bi00340a047. [DOI] [PubMed] [Google Scholar]
- Gambal D. Simple rapid procedure for isolating serum albumin. Biochim Biophys Acta. 1971 Oct;251(1):54–56. doi: 10.1016/0005-2795(71)90058-4. [DOI] [PubMed] [Google Scholar]
- Harding J. D., MacDonald R. J., Przybyla A. E., Chirgwin J. M., Pictet R. L., Rutter W. J. Changes in the frequency of specific transcripts during development of the pancreas. J Biol Chem. 1977 Oct 25;252(20):7391–7397. [PubMed] [Google Scholar]
- Koj A., McFarlane A. S. Effect of endotoxin on plasma albumin and fibrinogen synthesis rates in rabbits as measured by the [14C] carbonate method. Biochem J. 1968 Jun;108(1):137–146. doi: 10.1042/bj1080137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Ljiljana S., Miodrag P., Dragoljub P. Thermal injury response of rat liver nuclei. Int J Biochem. 1983;15(2):225–231. doi: 10.1016/0020-711x(83)90069-1. [DOI] [PubMed] [Google Scholar]
- Northemann W., Andus T., Gross V., Nagashima M., Schreiber G., Heinrich P. C. Messenger RNA activities of four acute phase proteins during inflammation. FEBS Lett. 1983 Sep 19;161(2):319–322. doi: 10.1016/0014-5793(83)81033-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevaljević L., Glibetić M., Poznanović G., Petrović M., Matić S., Pantelić D. Thermal injury-induced expression of acute-phase proteins in rat liver. Burns Incl Therm Inj. 1988 Aug;14(4):280–286. doi: 10.1016/0305-4179(88)90067-8. [DOI] [PubMed] [Google Scholar]
- Sevaljević L., Petrović M., Poznanović G., Skaro A., Pantelić D. Structural and biochemical aspects of liver cell nuclear organization in normal and scalded rats. J Cell Sci. 1984 Oct;71:75–93. doi: 10.1242/jcs.71.1.75. [DOI] [PubMed] [Google Scholar]
- Shiels B. R., Northemann W., Gehring M. R., Fey G. H. Modified nuclear processing of alpha 1-acid glycoprotein RNA during inflammation. J Biol Chem. 1987 Sep 15;262(26):12826–12831. [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]