Abstract
Elevated blood cholesterol and triglyceride levels induced by secondary causes are frequently observed. The identification and appropriate handling of these causes are essential for secondary dyslipidemia treatment. Major secondary causes of hypercholesterolemia and hypertriglyceridemia include an unhealthy diet, diseases and metabolic conditions affecting lipid levels, and therapeutic side effects. It is imperative to correct secondary causes prior to initiating conventional lipid-lowering therapy. Guideline-based lipid therapy can then be administered based on the subsequent lipid levels.
Keywords: Lipoproteins, Diagnosis, Drug-related side effects and adverse reactions, Food, Metabolism
CONSENSUS SUMMARY
| Nutrients, foods, and dietary patterns causing secondary dyslipidemia | • Saturated fatty acids (FAs) abundant in red meat and trans FAs increase low-density lipoprotein-cholesterol (LDL-C) levels. Unsaturated FAs lower lipid levels. |
| • Diets high in carbohydrates, added sugar, and alcohol raise blood triglyceride (TG) levels. | |
| • Ultra-processed foods are a risk factor for dyslipidemia. | |
| Recommendations: | |
| • An appropriate energy intake and healthy body weight are important for preventing secondary dyslipidemia. | |
| • Reduce saturated FAs, red and processed meats, and limit trans FA intake. | |
| • Consume foods high in dietary fiber, whole grains, fruits and vegetables, legumes, and fish. | |
| • Limit alcohol consumption. | |
| Diseases and conditions causing secondary hypercholesterolemia | • Diseases such as hypothyroidism, liver disease, primary biliary cholangitis, and nephrotic syndrome can increase LDL-C levels. |
| Recommendations: | |
| • All patients with high LDL-C levels should be screened for these conditions. | |
| • If high cholesterol levels persist after managing these underlying diseases, further lipid-lowering therapy (LLT) may be required. | |
| Drugs causing secondary hypercholesterolemia | • Cardiovascular drugs (e.g., thiazide), steroid hormones (e.g., progestogens, androgenic steroids, and glucocorticoids), dermatologic drugs, immunosuppressants (e.g., cyclosporine), anti-infective agents (e.g., protease inhibitors), and anticonvulsants can elevate LDL-C levels. |
| Recommendations: | |
| • Replacing the causative drug with an alternative is desirable. | |
| • If drug replacement is difficult, monitor the lipid levels and reassess the necessity of the causative drug. If long-term use of the drug is necessary, further guideline-based LLT may be required. | |
| Diseases and conditions causing secondary hypertriglyceridemia | • Obesity and uncontrolled diabetes, Cushing syndrome, hypothyroidism, nephrotic syndrome and chronic kidney disease, liver disease, autoimmune diseases (e.g., systemic lupus erythematosus), sepsis and critical illnesses, and pregnancy can raise blood TG levels. |
| Recommendations: | |
| • Diagnosis and treatment of secondary factors for hypertriglyceridemia are absolutely imperative before administering additional TG-lowering treatments. | |
| • Lifestyle modifications are required before any pharmacological treatment. | |
| Drugs causing secondary hypertriglyceridemia | • Cardiovascular drugs (e.g., b-blockers and thiazide), steroid hormones (e.g., estrogens, oral contraceptives, and glucocorticoids), dermatologic drugs (e.g., isotretinoin), immunosuppressants, anti-infective agents (e.g., protease inhibitors), anti-cancer drugs, and antipsychotics (e.g., olanzapine) can cause varying degrees of hypertriglyceridemia. |
| Recommendations: | |
| • When using drugs known to elevate TG levels, measuring TG levels before and after drug use can be helpful to diagnose the secondary cause. | |
| • If very severe hypertriglyceridemia occurs, discontinuation of the causative agent is necessary to prevent pancreatitis. |
INTRODUCTION
Pharmacological lipid-lowering is an essential measure for cardiovascular prevention in modern medicine [1,2]. Elevated blood cholesterol and triglyceride (TG) levels are commonly induced by secondary causes [3]. If secondary dyslipidemia is suspected, diagnosing and managing the causative factors are critical, as is lipid level monitoring. Therefore, screening for the influence of diet, disease, and drug-induced lipid changes may be an important procedure for clinicians. Furthermore, as many physicians may be insufficiently aware of the side effects of medications that they do not often prescribe [4], relevant supplemental knowledge can help differentiate secondary dyslipidemia.
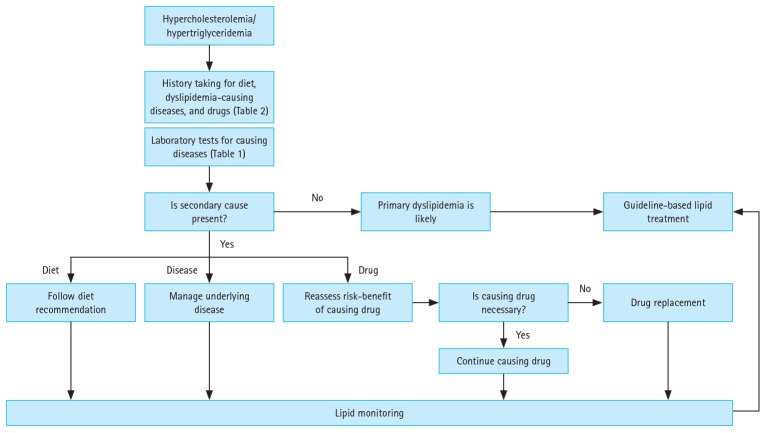
Many guidelines on lipid-lowering therapy (LLT) address secondary dyslipidemia [5,6]. Although these guidelines describe the causative conditions and the significance of their screening and treatment, these are only briefly described. Nevertheless, dyslipidemia holds a solid position in contemporary clinical medicine [7,8] and has a variety of secondary causes. Therefore, the accumulation and summary of detailed data and a recommended approach for suspected cases are required. Consequently, we reached a consensus regarding secondary dyslipidemia. For practical use by clinicians, hypercholesterolemia and hypertriglyceridemia are presented separately. In addition, three major etiologies— diet, diseases, and drugs—were explained, and recommendations were made. Secondary causes of dyslipidemia are presented in Figure 1 according to their impact on lipoprotein synthesis and clearance. The therapeutic approach for these patients is suggested in Figure 2.
Figure 1.
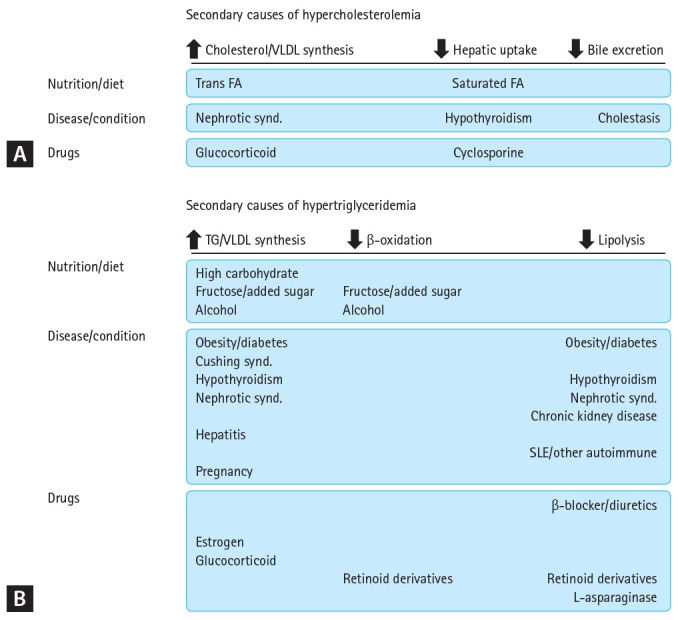
Secondary causes of hypercholesterolemia (A) and hypertriglyceridemia (B) based on their impact on lipoprotein synthesis and clearance. VLDL, very low-density lipoprotein; FA, fatty acid; TG, triglyceride; SLE, systemic lupus erythematosus.
Figure 2.
Management approach for patients suspected of secondary hypercholesterolemia or hypertriglyceridemia.
NUTRIENT, FOOD, AND DIETARY PATTERN-ASSOCIATED HYPERCHOLESTEROLEMIA AND HYPERTRIGLYCERIDEMIA
A lifestyle that accelerates obesity, including excessive calorie intake and low physical activity, commonly worsens hypertriglyceridemia [9].
Nutrients
Excessive energy intake
Obesity, especially abdominal obesity, is a major cause of secondary dyslipidemia. Therefore, an appropriate energy intake is important. The timing of daily energy intake also affects total cholesterol and low-density lipoprotein-cholesterol (LDL-C) levels. A Taiwanese study found that shifting consumption of 100 kcal from nighttime to breakfast or lunch could reduce LDL-C by 1.5 mg/dL [10]. Cholesterol synthesis in humans increases in the evening and night, possibly due to the peak activity of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase.
Saturated fatty acids (FAs)
Saturated FAs are a key factor in elevating LDL-C levels. Saturated FAs are associated with dyslipidemia in individuals whose diet includes saturated FAs at ≥ 10% of the total energy intake [11]. Specifically, saturated FAs with 12–16 carbon atoms tend to increase LDL-C levels [12]. Palm oil, including palmitic acid, elevates LDL-C levels by 9 mg/dL compared with other vegetable oils [13]. The most plausible underlying mechanism is the effect of saturated FAs on LDL receptor (LDLR) activity [14]. Polyunsaturated FAs can upregulate the mRNA and protein expression of LDLR and downregulate FA synthase. Conversely, monounsaturated FAs decrease apoC3 and apoB100 synthesis, which are the underlying mechanisms for lipid lowering by unsaturated FAs [15].
Trans FAs
Trans FA consumption increases blood LDL-C and TG levels to a greater extent than isocaloric intake of saturated FAs [16]. In human cells, elaidic acid, the most common trans FA, increases the expression of enzymes involved in cholesterol and FA biosynthesis compared with oleic acid [17].
High carbohydrate
A diet high in carbohydrates raises blood TG levels and decreases high-density lipoprotein-cholesterol (HDL-C) levels. Increased insulin concentrations resulting from a high-carbohydrate diet stimulate fat synthesis by elevating malonyl-CoA, which contributes to the conversion of FAs from oxidative to TG synthesis [18]. A low-carbohydrate diet leads to an improvement in blood TG and HDL-C levels compared with a low-fat diet [19]. A low-carbohydrate diet decreases TG levels to a greater extent than a moderate-carbohydrate diet, whereas it increases LDL-C [20]. LDL-C levels increase as carbohydrate intake decreases [21]. These results indicate the potential risks and benefits of a low-carbohydrate diet and underscore carbohydrate replacement over carbohydrate restriction.
Fructose
Adding fructose to the diet to boost energy intake increases blood TG levels. Associations between fructose and simple sugars, increased lipogenesis, and very low-density lipoprotein (VLDL) production have also been highlighted. Consuming ≥ 100 g of fructose per day or added sugars ≥ 10% of the total energy intake is known to elevate blood TG levels [9]. Fructose has been shown to increase (postprandial) TG concentrations only in hypercaloric trials [22].
Food
Red meat
Excessive consumption of red meat, a major source of saturated FAs, is associated with the risk of dyslipidemia. In a Korean cohort study, a one serving (60 g) increase in red meat intake was associated with a higher risk of hypercholesterolemia in both men and women [23].
Added sugars and sugar-sweetened beverage (SSB)
According to data from the National Health and Nutrition Examination Survey in the United States, as the percentage calorie intake from added sugar increased, the risk of hypertriglyceridemia increased [24]. An analysis of the Framingham Offspring study showed that more than one serving of SSB per day caused a 1.52 times higher risk of TG elevation than less than one serving per day [25]. High sugar intake enhances fructose metabolism, which mostly occurs in the liver. Excessive fructose accumulation increases de novo lipogenesis and inhibits FA oxidation, which in turn increases lipid supply in the liver. Elevated lipid levels promote VLDL secretion [26].
Furthermore, although non-nutritive sweeteners are often used as alternatives to sugar, the World Health Organization (WHO) has announced that the long-term consumption of non-nutritive sweeteners may be associated with the risk of non-communicable diseases [27].
Ultra-processed food
Consuming ultra-processed foods, which mainly contain free sugars, trans FAs, and saturated FAs, is a risk factor for dyslipidemia. A study that analyzed the intake of ultra-processed foods according to the NOVA classification identified the followings. Individuals in the highest tertile had a 2.7 times higher risk of hypertriglyceridemia than those in the lowest tertile [28]. In a Korean cohort study, the increased consumption of processed meat was associated with a higher risk of hypercholesterolemia and hypertriglyceridemia [23].
Alcohol
Every 1 oz of alcohol intake in non-drinkers increases blood TG levels by 5–10% [29]. Excessive alcohol intake suppresses hepatic FA oxidation, thereby promoting hepatic TG synthesis and VLDL secretion [9,30]. In particular, TG elevation can be more severe in individuals with underlying hypertriglyceridemia [9].
Dietary patterns
Dietary habits characterized by an excessive intake of refined grains, saturated fats, and sodium have a negative impact on lipid metabolism. In 2023, the American Heart Association highlighted issues with the Northeast Asian diet, including the Korean diet, that increase cardiovascular risk: the absence of dietary fiber in refined grains, such as white rice, high saturated fat and dietary cholesterol from animal protein and organ meats, and high sodium intake [31]. According to cancer screening data from the Korean National Cancer Center, Western dietary patterns, characterized by a higher intake of bread, snacks, noodles, meat, pizza, hamburgers, sugar, oil, and fat, were associated with a higher risk of total cholesterol and LDL-C levels [32].
Recommendations
Consume an appropriate energy intake to maintain a healthy body weight
Appropriate energy intake to maintain healthy weight is required. Energy needs vary according to individual’s age, sex, size, and activity [33]. Weight loss reduces cholesterol and TG levels [34]. In excessive adiposity, a 5% decrease in body weight reduces LDL-C by 3–5% [35].
Any diet that results in weight reduction, regardless of nutrient composition, can decrease blood TG levels. A study comparing four diets with different nutritional contents found that all four reduced TG levels and weight to similar extents [36]. For overweight individuals with hypertriglyceridemia, a 5–10% weight reduction is reasonable and is expected to lower TG levels by 20% [9]. Nonnutritive sweeteners are not recommended for weight control or risk reduction of these diseases.
Reduce saturated FA intake to < 7% of total energy, and limit trans FA intake
The 2020 Dietary Reference Intakes for Koreans recommends an intake of saturated FA < 7% and trans FA < 1% of total energy [37]. A greater intake of monounsaturated FAs (nuts, olive oil, etc.) and marine omega-3 polyunsaturated FAs is recommended [9]. Replacing saturated fat with unsaturated fat lowers lipid levels and cardiovascular risk [38,39], and substituting with unsaturated FAs and carbohydrates lowers LDL-C levels by 3 mg/dL [40]. In addition, substituting red meat with high-quality plant protein sources such as legumes leads to a reduction in total cholesterol and LDL-C [41].
Consume foods high in dietary fiber to reach a daily intake > 25 g
A meta-analysis revealed that high dietary fiber intake reduces total cholesterol compared with low fiber intake, with 25–29 g/day showing health benefits [42]. Dietary fiber, especially water-soluble fiber, reduced intestinal fat absorption and increased hepatic LDLR with lower VLDL secretion, thereby lowering blood cholesterol levels in animals [43]. The WHO recommends consuming at least 400 g of fruits and vegetables per day to increase dietary fiber intake [44]. Similarly, the 2020 Dietary Reference Intakes for Koreans recommends dietary fiber ≥ 30 g/day and ≥ 20 g/day for adult men and women, respectively [37].
Promote the consumption of whole grains, fruits and vegetables, legumes, and fish while reducing red and processed meat
The WHO emphasizes the consumption of whole grains, fruits and vegetables, legumes, and nuts to prevent noncommunicable diseases [44]. These foods are also recommended by international guidelines for LDL-C management and cardiovascular health [45].
The benefits of carbohydrate replacement have been emphasized over those of carbohydrate restriction. To improve dietary principles and personalized nutritional recommendations for secondary dyslipidemia, referral to a dietitian nutritionist is recommended [46]. The optimal diet for those with elevated TG levels is fiber-rich and includes < 50–60% carbohydrates, mostly consisting of whole grains, fruits, and vegetables [9].
Limit alcohol consumption to one to two glasses per day, and refrain from drinking if possible
The European Society of Cardiology recommends reducing alcohol consumption to lower TG levels [5]. The dietary guidelines for Americans limit alcohol consumption to two cups per day for men and one cup per day for women [47]. Furthermore, they recommended that people who do not consume alcohol refrain from starting to drink [33], as alcohol can exacerbate TG elevation in individuals with baseline hypertriglyceridemia; complete abstinence is recommended for these individuals.
DISEASES AND METABOLIC CAUSES CONTRIBUTING TO SECONDARY HYPERCHOLESTEROLEMIA
Hypothyroidism
Hypothyroidism is commonly accompanied by increased plasma LDL-C levels and sometimes mild TG elevation. Thyroid hormone has opposing effects on cholesterol synthesis and clearance. It induces HMG-CoA reductase expression in the liver and activates sterol regulatory element-binding protein 2. This protein binds to sterol regulatory elements in the LDLR promoter and induces hepatic LDLR expression. In addition, thyroid hormone upregulates LDLR expression by binding to the thyroid response element of the LDLR gene [48]. Therefore, hyper-LDL cholesterolemia in this condition is mainly caused by reduced hepatic LDLR and delayed LDL clearance [30]. Furthermore, FA oxidation can decrease and VLDL production is increased in individuals with hypothyroidism. Recently, thyroid-stimulating hormone has also been reported to influence lipid metabolism. It possibly promotes cholesterol synthesis and inhibit LDL clearance. This hormone could also increase TG synthesis [48].
Liver disease and primary biliary cholangitis (PBC)
As the liver is the primary site of lipoprotein metabolism, liver diseases can influence blood lipid levels in diverse ways. Cholestasis is associated with hypercholesterolemia, as bile is a major route of cholesterol excretion and cholestasis blocks this pathway. In this condition, free cholesterol is bound to phospholipids and transported to the blood as a particle called lipoprotein X, which is characterized by a scarcity of apoprotein B [30,49].
PBC is an autoimmune liver disease. Its lipid profile is characterized by elevated levels of lipoprotein X and HDL, and low LDL-C levels. However, altered lipoprotein metabolism in PBC is not associated with a high cardiovascular risk [49].
Nephrotic syndrome
Dysregulation of lipid metabolism correlates with proteinuria in nephrotic syndrome, and blood cholesterol, TG, VLDL, intermediate-density lipoprotein, and lipoprotein (a) levels are elevated. These changes are primarily due to increased VLDL production. Although the underlying mechanisms remain unclear, protein overproduction associated with hypoalbuminemia is a potential cause [30]. Dyslipidemia likely contributes to the increased risk of atherosclerosis associated with this condition. Furthermore, dyslipidemia can cause renal injury and the progression of chronic kidney disease [50]. Although statins have shown cardiovascular benefits in patients with dialysis-independent chronic kidney disease, there are no data on statin treatment in nephrotic syndrome [50].
Recommendations
All patients with high LDL-C levels should be screened for hypothyroidism, particularly those in which the elevation is otherwise clinically unexplainable. Thyroid hormone replacement therapy usually improves hypercholesterolemia [30,51]. Laboratory screening tests for diseases causing secondary hypercholesterolemia or hypertriglyceridemia are presented in Table 1.
Table 1.
Screening laboratory tests of secondary causes of hypercholesterolemia and hypertriglyceridemia
| Causes | Hypercholesterolemia | Hypertriglyceridemia | Tests |
|---|---|---|---|
| Hypothyroidism | O | O | Thyroid stimulating hormone, free T4 |
| Diabetes mellitus | O | Fasting plasma glucose, glycated hemoglobin | |
| Cushing syndrome | O | 24 h urine free cortisol, late night salivary cortisol, 1 mg overnight dexamethasone suppression test | |
| Liver disease | O | O | Transaminases, bilirubin, alkaline phosphatase, etc |
| Renal disease | O | O | Serum creatinine, urine/serum albumin, etc |
Physicians may be concerned about the hepatotoxicity of LLT in liver disease. Patients with liver disease may require LLT due to a combination of risk factors. In PBC, apoB levels may help determine who should start LLT. For patients needing such therapy, statins are the first-line agents, but caution should be exercised when administered to patients with decompensated cirrhosis [49].
DRUG-INDUCED SECONDARY HYPERCHOLESTEROLEMIA
Drugs inducing secondary hypercholesterolemia or hypertriglyceridemia are summarized in Table 2.
Table 2.
Drugs inducing secondary hypercholesterolemia and hypertriglyceridemia
| Severity (% elevation) | HyperLDL-cholesterolemia | Hypertriglyceridemia |
|---|---|---|
| Severe (≥ 50) | Steroid hormones | |
| Oral contraceptives with 2nd/3rd gen. progestogens | ||
| Dermatologic drugs | ||
| Isotretinoin, acitretin | ||
| Anti-infective agents | ||
| Ritonavir (200–300%) | ||
| Anti-cancer drugs | ||
| L-asparaginase | ||
| Moderate (10–50) | Steroid hormones | Cardiovascular drugs |
| Danazol, glucocorticoid (variable) | β-blockers | |
| Anti-infective agents | Steroid hormones | |
| Ritonavir, indinavir, nelfenavir | Unopposed estrogens, tamoxifen, glucocorticoid (variable) | |
| Immunosuppressants (variable) | Antipsychotics | |
| Cyclosporin, rapamycin | Clozapine, olanzapine, haloperidol | |
| Immunosuppressants (variable) | ||
| Cyclosporin, rapamycin | ||
| Anti-infective agents (variable) | ||
| Indinavir, nelfinavir | ||
| Mild (< 15) | Cardiovascular drugs | Cardiovascular drugs |
| Thiazide, loop diuretics | Thiazide, loop diuretics | |
| Steroid hormones | ||
| Unopposed progestogens (testosterone derivatives) | ||
| Oral contraceptives with 2nd gen progestogens | ||
| Dermatologic drugs | ||
| Isotretinoin | ||
| Anticonvulsants | ||
| Carbamazepine, phenytoin, phenobarbital |
Variable: 0–70%.
Cardiovascular drugs (e.g., thiazide and loop diuretics)
Thiazide diuretics increase LDL-C levels by 5–15% and these effects are known to be short-term. Loop diuretics have similar effects on lipid levels, whereas indapamide does not [52].
Steroid hormones (e.g., progestogens, androgenic anabolic steroids, and glucocorticoids)
Steroid hormones include estrogens, progestins, estrogen receptor modulators, and glucocorticoids. The original progestins, which are derivatives of testosterone, increase LDL-C levels. However, of the newer generations of progestins with varying androgenic effects, only dl-norgestrel increases LDL-C levels, whereas the others increase TG levels [53]. In contrast, combined estrogen/progestogen therapy decreases LDL-C levels. Oral contraceptives with second generation progestogens such as levonorgestrel mildly elevate LDL-C levels, whereas those with third generation progestogens, including desogestrel, do not. Danazol, a synthetic steroid prescribed for endometriosis, fibrocystic breast disease, and hereditary angioedema, increases LDL-C levels by 10–40%; however, these recover after discontinuation of the drug [52].
Androgenic anabolic steroids or drugs that increase their endogenous forms are sometimes used to enhance athletic performance or the sense of well-being. These drugs have been shown to increase LDL-C levels. These agents decrease lipoprotein lipase (LPL) activity and can reduce VLDL catabolism [53].
Glucocorticoids are used in various conditions. Prednisone increases LDL-C levels in a dose-dependent manner in patients who are post-transplant. In patients with systemic lupus erythematosus (SLE), prednisone increases LDL-C levels by up to 20%. Glucocorticoids demonstrate dose- and duration-dependent effects on lipid metabolism. In several studies, glucocorticoid use is accompanied by insulin resistance, which can confound the effects of steroids on lipid levels. The effect of glucocorticoids on post-heparin LPL activity varies [53]. In addition, glucocorticoids increase the hepatic production of VLDL by promoting rate-limiting enzyme activity [54].
Dermatologic drugs (e.g., isotretinoin)
Isotretinoin, a synthetic retinoid prescribed for acne vulgaris, mildly elevates LDL-C levels. An increased VLDL turnover induced by retinoids is a potential mechanism [53].
Immunosuppressants (e.g., cyclosporine and rapamycin)
Patients taking immunosuppressive drugs after transplantation may develop dyslipidemia. These agents can elevate LDL-C levels by 0–50%, with cyclosporine having the greatest increase. Moreover, the effects on lipid levels are greater in women. Immunosuppression can further increase small dense LDL particle levels. In addition, rapamycin has a prominent effect on cholesterol levels [52]. Although its mechanism of action on lipids remains unclear, cyclosporine can reduce LDL catabolism by inhibiting LDLR synthesis [55]. Furthermore, it also reduces bile acid synthesis [53].
Anti-infective protease inhibitors (e.g., ritonavir, indinavir, and nelfinavir)
These antiretroviral drugs administered against human immunodeficiency virus infection, many of which are currently less commonly used, increase LDL-C levels by 15–30% [52,56]. This effect develops after prolonged use (≥ 1 year) and is dose-responsive [57].
Anticonvulsants (e.g., carbamazepine, phenytoin, and phenobarbital)
These drugs are known to increase total cholesterol by 0–15%. Anticonvulsants, except for valproic acid, can induce enzymes in the hepatic microsomal cytochrome P pathway and compete with cholesterol metabolism via this route [52].
Others (e.g., sodium-glucose cotransporter-2 [SGLT2] inhibitors)
SGLT2 inhibitors, when used alone, minimally raise LDL-C levels by 2–4%. Increased synthesis and decreased catabolism underlie these changes. However, their effect on lipid levels is frequently altered when SGLT2 inhibitors are used in combination with statins and other diabetes medications in clinical practice [58].
Recommendations
Replacing drugs that induce hypercholesterolemia with alternative equivalents is desirable. For example, renin-angiotensin system blockers can be used as alternatives for patients with hypertension and dyslipidemia induced by cardiovascular agents. However, finding an appropriate alternative is often challenging. If a drug is necessary and difficult to replace, lipid levels must be monitored regularly. Reassessing the necessity of the causative drug and the risks and benefits of continuing dyslipidemia-inducing agents is essential. Guideline-based non-pharmacological and pharmacological LLT may be required with long-term use of causative agents. If a dyslipidemia-inducing drug is required for a short period, LLT is not necessary [52].
When using statins in post-transplant hypercholesterolemia, caution should be exercised regarding drug–drug interactions. In particular, a combination of cyclosporine and statins may increase the risk of myopathy, and statins that do not use the CYP3A4 pathway, such as fluvastatin and pravastatin, are preferred. Cyclosporine has been reported to elevate blood levels of ezetimibe, a second-line lipid-lowering agent [5].
Although research on dyslipidemia induced by protease inhibitors is limited, modifying the protease inhibitor regimen or initiating LLT are possible options. However, the risks and benefits of regimen changes and drug interactions between protease inhibitors and statins need to be carefully considered. Lovastatin and simvastatin are contraindicated in patients receiving protease inhibitors because they are metabolized by CYP3A4. Although atorvastatin is metabolized to a lesser extent by this pathway, the dose sometimes needs to be reduced. Fluvastatin, pravastatin, pitavastatin, and rosuvastatin can be used for these patients [56].
DISEASES AND METABOLIC CAUSES CONTRIBUTING TO SECONDARY HYPERTRIGLYCERIDEMIA
Obesity and uncontrolled diabetes
Among the medical conditions that cause TG elevation, obesity and poorly controlled diabetes are the most common. These conditions frequently result in elevated plasma TG, low HDL-C, variable LDL-C, and increased small density LDL levels. Large VLDL, increased TG in LDL and HDL, glycation of apoproteins, and high susceptibility of LDL to oxidation are qualitative differences in diabetes. Even if the blood LDL concentration is normal, its circulation time is longer in diabetes. VLDL production is increased, and its catabolism is decreased, whereas HDL catabolism is promoted. Postprandial hyperlipidemia is commonly observed [59]. Greater adipocyte mass and decreased insulin sensitivity of hormone-sensitive lipases result in excessive hepatic VLDL production from the overflow of free FA [9,30]. Furthermore, the increased insulin levels promote hepatic FA synthesis. In addition, insulin resistance reduces LPL activity and decreases the catabolism of chylomicrons and VLDL [9,30,60]. Insulin resistance and the subsequent decrease in LPL transcription and increase in LPL inhibitor levels may partly contribute to impaired lipolysis [30]. Furthermore, low clearance of TG-rich lipoproteins independent of LPL activity has been reported in type 2 diabetes. Moreover, insulin infusion promotes LPL expression in adipocytes [9].
Cushing syndrome
This condition commonly results in elevated TG levels and low HDL-C levels [30]. Excess glucocorticoids increase VLDL synthesis and cause hypertriglyceridemia.
Hypothyroidism
Hypothyroidism increases TG levels by affecting TG metabolism. Low thyroid hormone levels lead to increased VLDL TG production in the liver. LPL activity is downregulated, reducing the breakdown of TG-rich lipoproteins. In addition, impaired hepatic lipase activity contributes to the accumulation of these lipoproteins. Conversely, thyroid stimulating hormone promotes TG synthesis in adipocytes and hepatocytes [48].
Nephrotic syndrome
Lipid metabolism is altered in nephrotic syndrome. In particular, blood cholesterol, TG, and apoB-containing lipoprotein levels are elevated. By downregulating catabolizing enzymes such as LPL, hepatic lipase, and VLDL receptors, this disease can impair the clearance of VLDL and chylomicrons. Hypoalbuminemia increases hepatic protein synthesis, which is one of plausible underlying mechanisms [61].
Chronic kidney disease
This condition is often associated with mildly elevated TG levels. Lipolysis and clearance of remnant lipoproteins are decreased in affected patients. In these patients, the expression and activity of LPL are reduced. Furthermore, insulin resistance induced by parathyroid hormones and other conditions is a contributing factor. The clearance of VLDL particles is compromised in uremia because VLDL receptors are downregulated. Conversely, chronic kidney disease can increase the expression of enzymes involved in TG synthesis [62].
Liver disease
Hepatitis induced by infection, drugs, or alcohol commonly increases VLDL synthesis and causes mild-to-moderate TG elevation. In contrast, severe hepatic conditions such as liver failure can lead to dramatic decreases in blood cholesterol and TG levels [30].
Autoimmune diseases, including SLE
Patients with SLE have decreased LPL activity, and some produce anti-LPL antibodies [63]. Hyperchylomicronemia has also been reported in patients with a variety of other autoimmune diseases, such as polymyositis/dermatomyositis, rheumatoid arthritis, and Sjogren’s syndrome, and these patients were positive for anti-LPL antibodies [64]. However, some researchers have indicated that measuring anti-LPL antibodies is complicated. On the other hand, some affected patients have anti-GPIHBP1 antibodies [65]. Improving hypertriglyceridemia with steroid therapy can help to diagnose autoimmunity-associated hypertriglyceridemia.
Sepsis and critical illnesses
TG levels increase, whereas HDL-C and LDL-C levels decrease in these patients. Free FA levels increase, indicating enhanced tissue lipolysis, which is more prominent in patients in septic shock [66].
Pregnancy
Blood TG levels can increase up to 4-fold in the third trimester. However, they frequently remain <250 mg/dL, and are well-tolerated. An increase in estrogen and human placental lactogen levels during this period contributes to insulin resistance and TG elevation. Estrogen increases VLDL synthesis and impairs lipolysis; human placental lactogens also inhibit lipolysis [67].
Recommendations
The aim of TG lowering is to prevent acute pancreatitis and atherosclerotic cardiovascular disease. While chylomicron particles increase the risk of the former, smaller remnant particles may increase the risk of the latter [9]. Correcting secondary factors of hypertriglyceridemia is crucial before implementing other TG-lowering measures. For example, weight reduction through caloric restriction can reduce TG levels by approximately 10% [9]. Physical activity and exercise are known to reduce TG levels up to 30%. However, when managing individuals with secondary dyslipidemia, it is essential to personally target greatest contributor among secondary etiologies [46].
Anti-LPL antibodies may lead to elevated TG levels in patients with autoimmune diseases. However, measuring anti-LPL antibody levels is impossible in most clinical settings. This antibody is usually assayed by immunoblotting or enzyme-linked immunosorbent assay using purified LPL from post-heparin plasma. However, these methods have some technical limitations [63].
If TG levels are > 225 mg/dL during pregnancy, regular monitoring and lifestyle modifications are required. If TG levels are > 500 mg/dL despite implementing non-pharmacological measures, omega-3 FAs may be initiated [67].
DRUG-INDUCED SECONDARY HYPERTRIGLYCERIDEMIA
Some drugs cause mild changes in blood TG levels, whereas others cause severe changes (Table 2) [46].
Cardiovascular drugs (e.g., b-blockers, thiazide, and loop diuretics)
Mild-to-moderate TG elevation occurs after the administration of b-blockers, particularly nonselective ones (atenolol, propranolol, and metoprolol). In addition, thiazide and loop diuretics cause mild increases in TG levels [9,52]. Although the underlying mechanisms are unclear, b-blockers and diuretics without intrinsic sympathomimetic activity increase reflex a-adrenergic activity, which can inhibit LPL and increase TG levels. Diuretics have been associated with worsening insulin resistance that may contribute to lipid changes [53].
Steroid hormones (e.g., estrogen, oral contraceptives, tamoxifen, and glucocorticoids)
Unopposed estrogens used as oral hormone replacements may increase TG levels by 30–40% in a dose-dependent manner and are associated with increased VLDL production [9,52,53]. In contrast, the influence of transdermal estrogens without a first-pass effect is minimal. The effects of combined estrogen/progestogen therapy on TG levels are inconsistent. Oral contraceptives with second generation progestogens, including levonorgestrel, and third generation progestogens, such as desogestrel, increase TG levels by up to 50–75%. Although tamoxifen, a selective estrogen receptor modulator, can increase TG levels by 0–30%, severe elevations are uncommon [9,52]. The effects of tamoxifen appear to be related to lipase inhibition [53].
Post-transplant, prednisone increases TG levels, but not in a dose-dependent manner. In healthy individuals and patients with lupus, TG levels increase by up to 40% with glucocorticoid use [53]. This effect arises from increased VLDL production and the concomitant hyperinsulinemia [54].
Dermatologic drugs (e.g., isotretinoin and acitretin)
Variable degrees of TG elevation (35–144%) have been reported after the use of isotretinoin, a retinoid derivative. This results from the inhibition of FA oxidation and an increase in apoC3 [9]. Acitretin, used for severe psoriasis, can increase TG levels by up to 60% [52].
Immunosuppressants (e.g., cyclosporine and rapamycin)
These agents are commonly used after transplantation and may increase TG levels by 0–70% [52]. In general, the effect of cyclosporine on TG levels is less consistent than its effect on LDL-C levels.
Anti-infective agents-protease inhibitors (e.g., ritonavir, indinavir, and nelfinavir)
Ritonavir can elevate TG levels by 200–300%, whereas indinavir and nelfinavir cause an increase of 0–55% [52].
Anti-cancer drugs (e.g., L-asparaginase and cyclophosphamide)
L-asparaginase infrequently induces severe hypertriglyceridemia and is associated with LPL inhibition [9].
Antipsychotics (e.g., olanzapine and mirtazapine)
Second-generation antipsychotics are associated with mild-to-severe hypertriglyceridemia. Among these, clozapine and olanzapine are associated with a high risk of elevated TG levels [9]. Haloperidol causes a TG elevation of approximately 50%, although this effect is only seen in women [52].
Recommendations
Before administering drugs known to considerably increase TG levels, these should be measured both before initiating therapy and weeks later, which can be helpful for monitoring the effects of the causative agent. When very severe hypertriglyceridemia occurs, discontinuation and avoidance of the causative agents are required to prevent pancreatitis. In addition, hypertriglyceridemia is a contraindication for some drugs, including isotretinoin [52,68]. When treating patients with post-transplant hypertriglyceridemia, drug interactions between fibrate and cyclosporine must be considered [5].
CONCLUSIONS
Various causes including diet, diseases, and drugs can contribute to secondary elevation of blood cholesterol and TG. Screening and identification of potential causes are primary steps for secondary dyslipidemia. Managing secondary factors, especially focusing on the greatest contributor, needs to be preceded before additional treatment.
Acknowledgments
This paper is published in duplicate in the Korean Journal of Internal Medicine and the Journal of Lipid and Atherosclerosis.
Footnotes
CRedit authorship contributions
Hoyoun Won: methodology, writing - original draft, writing - review & editing; Jae Hyun Bae: methodology, writing - original draft, writing - review & editing; Hyunjung Lim: methodology, writing - original draft, writing - review & editing; Minji Kang: methodology, writing - original draft, writing - review & editing; Minjoo Kim: methodology, writing - original draft, writing - review & editing; Sang-Hak Lee: conceptualization, methodology, writing - original draft, writing - review & editing, visualization, project administration, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by the Korean Society of Lipid and Atherosclerosis. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Choi H, Kang SH, Jeong SW, et al. Lipid-lowering efficacy of combination therapy with moderate-intensity statin and ezetimibe versus high-intensity statin monotherapy: a randomized, open-label, non-inferiority trial from Korea. J Lipid Atheroscler. 2023;12:277–289. doi: 10.12997/jla.2023.12.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang Y, Park JM, Lee SH. Moderate-intensity rosuvastatin/ezetimibe combination versus quadruple-dose rosuvastatin monotherapy: a meta-analysis and systemic review. Yonsei Med J. 2024;65:19–26. doi: 10.3349/ymj.2023.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KY, Hong S, Kim KS, Han K, Park CY. Trends in prevalence of hypertriglyceridemia and related factors in Korean adults: a serial cross-sectional study. J Lipid Atheroscler. 2023;12:201–212. doi: 10.12997/jla.2023.12.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarim BA, Fici F, Tengiz I, et al. Do statins counteract the effect of antidiabetic drugs? Results of the SCEAD study. Yonsei Med J. 2023;64:175–180. doi: 10.3349/ymj.2022.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Jin ES, Shim JS, Kim SE, et al. Dyslipidemia fact sheet in South Korea, 2022. J Lipid Atheroscler. 2023;12:237–251. doi: 10.12997/jla.2023.12.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong S, Han K, Park JH, Yu SH, Lee CB, Kim DS. Higher non-high-density lipoprotein cholesterol was higher associated with cardiovascular disease comparing higher LDL-C in nine years follow up: cohort study. J Lipid Atheroscler. 2023;12:164–174. doi: 10.12997/jla.2023.12.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simha V. Management of hypertriglyceridemia. BMJ. 2020;371:m3109. doi: 10.1136/bmj.m3109. [DOI] [PubMed] [Google Scholar]
- 10.Chen HJ, Chuang SY, Chang HY, Pan WH. Energy intake at different times of the day: Its association with elevated total and LDL cholesterol levels. Nutr Metab Cardiovasc Dis. 2019;29:390–397. doi: 10.1016/j.numecd.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Magriplis E, Marakis G, Kotopoulou S, et al. Trans fatty acid intake increases likelihood of dyslipidemia especially among individuals with higher saturated fat consumption. Rev Cardiovasc Med. 2022;23:130. doi: 10.31083/j.rcm2304130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Neelakantan N, Wu Y, Lote-Oke R, Pan A, van Dam RM. Palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical trials. J Nutr. 2015;145:1549–58. doi: 10.3945/jn.115.210575. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr. 2005;135:2075–2078. doi: 10.1093/jn/135.9.2075. [DOI] [PubMed] [Google Scholar]
- 15.Hannon BA, Khan NA, Teran-Garcia M. Nutrigenetic contributions to dyslipidemia: a focus on physiologically relevant pathways of lipid and lipoprotein metabolism. Nutrients. 2018;10:1404. doi: 10.3390/nu10101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 17.Oteng AB, Kersten S. Mechanisms of action of trans fatty acids. Adv Nutr. 2020;11:697–708. doi: 10.1093/advances/nmz125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung CH, Choi KM. Impact of high-carbohydrate diet on metabolic parameters in patients with type 2 diabetes. Nutrients. 2017;9:322. doi: 10.3390/nu9040322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla S, Tessarolo Silva F, Amaral Medeiros S, Mekary RA, Radenkovic D. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong T, Guo M, Zhang P, Sun G, Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: a meta-analysis. PLoS One. 2020;15:e0225348. doi: 10.1371/journal.pone.0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fechner E, Smeets ETHC, Schrauwen P, Mensink RP. The effects of different degrees of carbohydrate restriction and carbohydrate replacement on cardiometabolic risk markers in humans-a systematic review and meta-analysis. Nutrients. 2020;12:991. doi: 10.3390/nu12040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David Wang D, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232:125–133. doi: 10.1016/j.atherosclerosis.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim SA, Shin S. Red meat and processed meat consumption and the risk of dyslipidemia in Korean adults: a prospective cohort study based on the Health Examinees (HEXA) study. Nutr Metab Cardiovasc Dis. 2021;31:1714–1727. doi: 10.1016/j.numecd.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA. 2010;303:1490–1497. doi: 10.1001/jama.2010.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haslam DE, Peloso GM, Herman MA, et al. Beverage consumption and longitudinal changes in lipoprotein concentrations and incident dyslipidemia in US adults: the Framingham Heart study. J Am Heart Assoc. 2020;9:e014083. doi: 10.1161/JAHA.119.014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci. 2016;53:52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Use of non-sugar sweeteners: WHO guideline [Internet]. Geneva: World Health Organization, c2023 [cited 2023 Oct 23]. Available from: https://www.who.int/publications/i/item/9789240073616.
- 28.Donat-Vargas C, Sandoval-Insausti H, Rey-García J, et al. High consumption of ultra-processed food is associated with incident dyslipidemia: a prospective study of older adults. J Nutr. 2021;151:2390–2398. doi: 10.1093/jn/nxab118. [DOI] [PubMed] [Google Scholar]
- 29.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rader DJ, Kathiresan S. Disorders of lipoprotein metabolism. In: Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo, ed. Harrison’s principles of internal medicine. 20th ed. New York: McGraw-Hill Education, 2018. [Google Scholar]
- 31.Kwan TW, Wong SS, Hong Y, et al. Epidemiology of diabetes and atherosclerotic cardiovascular disease among Asian American adults: implications, management, and future directions: a scientific statement from the American Heart Association. Circulation. 2023;148:74–94. doi: 10.1161/CIR.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Hoang T, Lee S, Kim J. Association between dietary patterns and dyslipidemia in Korean women. Front Nutr. 2022;8:756257. doi: 10.3389/fnut.2021.756257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144:e472. doi: 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 34.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401–406. [PubMed] [Google Scholar]
- 35.Kirkpatrick CF, Sikand G, Petersen KS, et al. Nutrition interventions for adults with dyslipidemia: a clinical perspective from the National Lipid Association. J Clin Lipidol. 2023;17:428–451. doi: 10.1016/j.jacl.2023.05.099. [DOI] [PubMed] [Google Scholar]
- 36.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weightloss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ministry of Health and Welfare, The Korean Nutrition Society. Application of 2020 dietary reference intakes for Koreans. Sejong: Ministry of Health and Welfare; 2021. [Google Scholar]
- 38.Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015;(6):CD011737. doi: 10.1002/14651858.CD011737. [DOI] [PubMed] [Google Scholar]
- 39.Hannon BA, Thompson SV, An R, Teran-Garcia M. Clinical outcomes of dietary replacement of saturated fatty acids with unsaturated fat sources in adults with overweight and obesity: a systematic review and meta-analysis of randomized control trials. Ann Nutr Metab. 2017;71:107–117. doi: 10.1159/000477216. [DOI] [PubMed] [Google Scholar]
- 40.Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;5:CD011737. doi: 10.1002/14651858.CD011737.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guasch-Ferré M, Satija A, Blondin SA, et al. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. 2019;139:1828–1845. doi: 10.1161/CIRCULATIONAHA.118.035225. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 43.Soliman GA. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients. 2019;11:1155. doi: 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization. Healthy diet [Internet]. Geneva: World Health Organization, c2020 [cited 2023 Oct 16]. Available from: https://www.who.int/news-room/fact-sheets/detail/ healthy-diet.
- 45.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 46.Virani SS, Morris PB, Agarwala A, et al. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;78:960–993. doi: 10.1016/j.jacc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Snetselaar LG, de Jesus JM, DeSilva DM, Stoody EE. Dietary guidelines for Americans, 2020-2025: understanding the scientific process, guidelines, and key recommendations. Nutr Today. 2021;56:287–295. doi: 10.1097/NT.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. 2022;11:e210002. doi: 10.1530/EC-21-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wah-Suarez MI, Danford CJ, Patwardhan VR, Jiang ZG, Bonder A. Hyperlipidaemia in primary biliary cholangitis: treatment, safety and efficacy. Frontline Gastroenterol. 2019;10:401–408. doi: 10.1136/flgastro-2018-101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018;14:57–70. doi: 10.1038/nrneph.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotwal A, Cortes T, Genere N, et al. Treatment of thyroid dysfunction and serum lipids: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105:dgaa672. doi: 10.1210/clinem/dgaa672. [DOI] [PubMed] [Google Scholar]
- 52.Mantel-Teeuwisse AK, Kloosterman JM, Maitland-van der Zee AH, Klungel OH, Porsius AJ, de Boer A. Drug-Induced lipid changes: a review of the unintended effects of some commonly used drugs on serum lipid levels. Drug Saf. 2001;24:443–456. doi: 10.2165/00002018-200124060-00003. [DOI] [PubMed] [Google Scholar]
- 53.Donahoo WT, Kosmiski LA, Eckel RH. Drugs causing dyslipoproteinemia. Endocrinol Metab Clin North Am. 1998;27:677–697. doi: 10.1016/s0889-8529(05)70033-5. [DOI] [PubMed] [Google Scholar]
- 54.Boots JM, Christiaans MH, van Hooff JP. Effect of immunosuppressive agents on long-term survival of renal transplant recipients: focus on the cardiovascular risk. Drugs. 2004;64:2047–2073. doi: 10.2165/00003495-200464180-00004. [DOI] [PubMed] [Google Scholar]
- 55.Winegar DA, Salisbury JA, Sundseth SS, Hawke RL. Effects of cyclosporin on cholesterol 27-hydroxylation and LDL receptor activity in HepG2 cells. J Lipid Res. 1996;37:179–191. [PubMed] [Google Scholar]
- 56.Myerson M. Lipid management in human immunodeficiency virus. Endocrinol Metab Clin North Am. 2016;45:141–169. doi: 10.1016/j.ecl.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Nduka C, Sarki A, Uthman O, Stranges S. Impact of antiretroviral therapy on serum lipoprotein levels and dyslipidemias: a systematic review and meta-analysis. Int J Cardiol. 2015;199:307–318. doi: 10.1016/j.ijcard.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 58.Lazarte J, Kanagalingam T, Hegele RA. Lipid effects of sodium-glucose cotransporter 2 inhibitors. Curr Opin Lipidol. 2021;32:183–190. doi: 10.1097/MOL.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 59.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–899. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckel RH. The complex metabolic mechanisms relating obesity to hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2011;31:1946–1948. doi: 10.1161/ATVBAHA.111.233049. [DOI] [PubMed] [Google Scholar]
- 61.Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. 2016;90:41–52. doi: 10.1016/j.kint.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moradi H, Vaziri ND. Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci (Landmark Ed) 2018;23:146–161. doi: 10.2741/4585. [DOI] [PubMed] [Google Scholar]
- 63.de Carvalho JF, Borba EF, Viana VS, Bueno C, Leon EP, Bonfá E. Anti-lipoprotein lipase antibodies: a new player in the complex atherosclerotic process in systemic lupus erythematosus? Arthritis Rheum. 2004;50:3610–3615. doi: 10.1002/art.20630. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto H, Tanaka M, Yoshiga S, Funahashi T, Shimomura I, Kihara S. Autoimmune severe hypertriglyceridemia induced by anti-apolipoprotein C-II antibody. J Clin Endocrinol Metab. 2014;99:1525–1530. doi: 10.1210/jc.2013-3619. [DOI] [PubMed] [Google Scholar]
- 65.Miyashita K, Lutz J, Hudgins LC, et al. Chylomicronemia from GPIHBP1 autoantibodies. J Lipid Res. 2020;61:1365–1376. doi: 10.1194/jlr.R120001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green P, Theilla M, Singer P. Lipid metabolism in critical illness. Curr Opin Clin Nutr Metab Care. 2016;19:111–115. doi: 10.1097/MCO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 67.Gupta M, Liti B, Barrett C, Thompson PD, Fernandez AB. Prevention and management of hypertriglyceridemia-induced acute pancreatitis during pregnancy: a systematic review. Am J Med. 2022;135:709–714. doi: 10.1016/j.amjmed.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Yoneyama K, Nakagawa M. Severe acute pancreatitis due to tamoxifen-induced hypertriglyceridemia. Breast J. 2019;25:788–789. doi: 10.1111/tbj.13279. [DOI] [PubMed] [Google Scholar]