Abstract
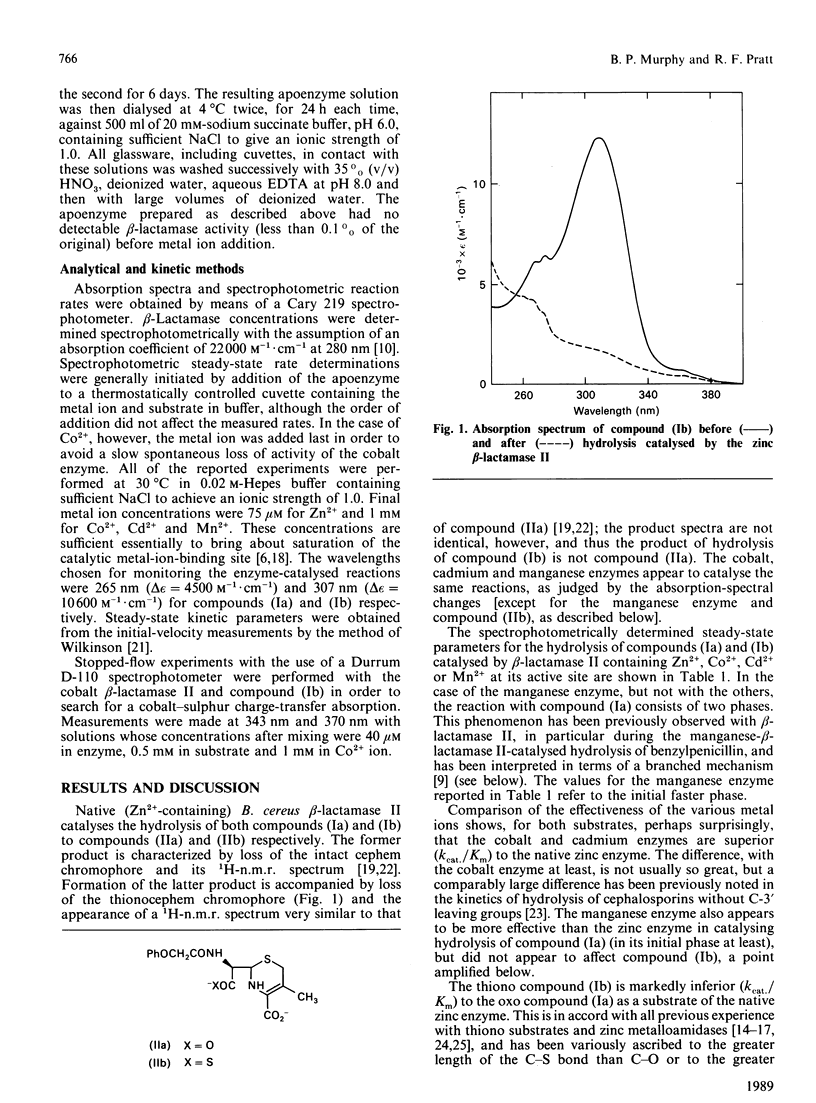
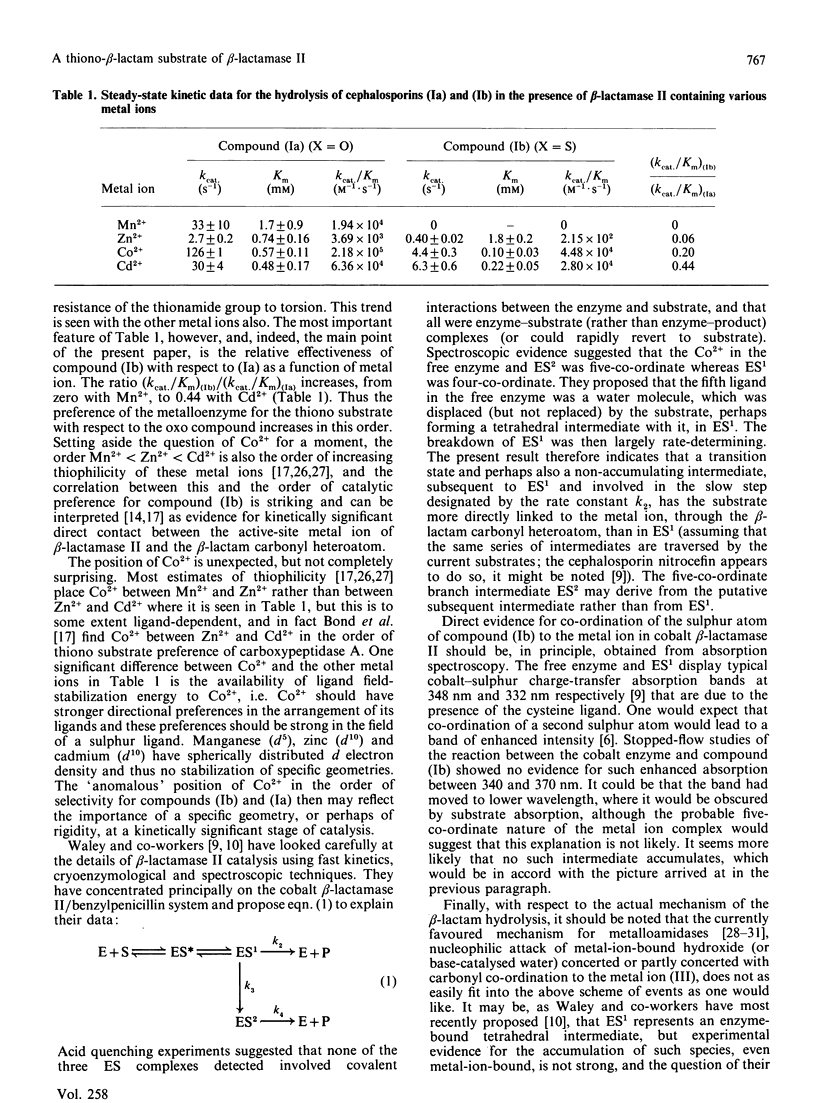
An 8-thionocephalosporin was shown to be a substrate of the beta-lactamase II of Bacillus cereus, a zinc metalloenzyme. Although it is a poorer substrate, as judged by the Kcat./Km parameter, than the corresponding 8-oxocephalosporin, the discrimination against sulphur decreased when the bivalent metal ion in the enzyme active site was varied in the order Mn2+ (the manganese enzyme catalysed the hydrolysis of the oxo compound but not that of the thiono compound), Zn2+, Co2+ and Cd2+. This result is taken as evidence for kinetically significant direct contact between the active-site metal ion of beta-lactamase II and the beta-lactam carbonyl heteroatom. No evidence was obtained, however, for accumulation of an intermediate with such co-ordination present.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. S., Galdes A., Hill H. A., Smith B. E., Waley S. G., Abraham E. P. Histidine residues of zinc ligands in beta-lactamase II. Biochem J. 1978 Nov 1;175(2):441–447. doi: 10.1042/bj1750441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Galdes A., Hill H. A., Waley S. G., Abraham E. P. A spectroscopic study of metal ion and ligand binding to beta-lactamase II. J Inorg Biochem. 1980 Nov;13(3):189–204. doi: 10.1016/s0162-0134(00)80068-9. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Waley S. G., Abraham E. P. Identification of histidine residues that act as zinc ligands in beta-lactamase II by differential tritium exchange. Biochem J. 1979 Jun 1;179(3):459–463. doi: 10.1042/bj1790459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P. A., Spear K. L., Jacobsen N. E. A thioamide substrate of carboxypeptidase A. Biochemistry. 1982 Mar 30;21(7):1608–1611. doi: 10.1021/bi00536a022. [DOI] [PubMed] [Google Scholar]
- Beattie R. E., Elmore D. T., Williams C. H., Guthrie D. J. The behaviour of leucine aminopeptidase towards thionopeptides. Biochem J. 1987 Jul 1;245(1):285–288. doi: 10.1042/bj2450285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Knott-Hunziker V., Waley S. G. The pH-dependence of class B and class C beta-lactamases. Biochem J. 1983 Jul 1;213(1):61–66. doi: 10.1042/bj2130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Schäffer A., Waley S. G., Auld D. S. Changes in the coordination geometry of the active-site metal during catalysis of benzylpenicillin hydrolysis by Bacillus cereus beta-lactamase II. Biochemistry. 1986 Nov 4;25(22):7208–7215. doi: 10.1021/bi00370a066. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Waley S. G. Cryoenzymology of Bacillus cereus beta-lactamase II. Biochemistry. 1985 Nov 19;24(24):6876–6887. doi: 10.1021/bi00345a021. [DOI] [PubMed] [Google Scholar]
- Bond M. D., Holmquist B., Vallee B. L. Thioamide substrate probes of metal-substrate interactions in carboxypeptidase A catalysis. J Inorg Biochem. 1986 Oct-Nov;28(2-3):97–105. doi: 10.1016/0162-0134(86)80074-5. [DOI] [PubMed] [Google Scholar]
- Christianson D. W., David P. R., Lipscomb W. N. Mechanism of carboxypeptidase A: hydration of a ketonic substrate analogue. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1512–1515. doi: 10.1073/pnas.84.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdes A., Hill H. A., Baldwin G. S., Waley S. G., Abraham E. P. The 1H nuclear-magnetic-resonance spectroscopy of cobalt(II)-beta-lactamase II. Biochem J. 1980 Jun 1;187(3):789–795. doi: 10.1042/bj1870789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer D. G., Monzingo A. F., Matthews B. W. An interactive computer graphics study of thermolysin-catalyzed peptide cleavage and inhibition by N-carboxymethyl dipeptides. Biochemistry. 1984 Nov 20;23(24):5730–5741. doi: 10.1021/bi00319a011. [DOI] [PubMed] [Google Scholar]
- Jaffe E. K., Cohn M. Diastereomers of the nucleoside phosphorothioates as probes of the structure of the metal nucleotide substrates and of the nucleotide binding site of yeast hexokinase. J Biol Chem. 1979 Nov 10;254(21):10839–10845. [PubMed] [Google Scholar]
- Little C., Emanuel E. L., Gagnon J., Waley S. G. Identification of an essential glutamic acid residue in beta-lactamase II from Bacillus cereus. Biochem J. 1986 Jan 15;233(2):465–469. doi: 10.1042/bj2330465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock W. L., Chen J. T., Tsang J. W. Hydrolysis of a thiopeptide by cadmium carboxypeptidase A. Biochem Biophys Res Commun. 1981 Sep 16;102(1):389–396. doi: 10.1016/0006-291x(81)91533-3. [DOI] [PubMed] [Google Scholar]
- Murphy B. P., Pratt R. F. Evidence for an oxyanion hole in serine beta-lactamases and DD-peptidases. Biochem J. 1988 Dec 1;256(2):669–672. doi: 10.1042/bj2560669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Finland M. Thiol-group binding of zinc to a beta-lactamase of Bacillus cereus: differential effects on enzyme activity with penicillin and cephalosporins as substrates. J Bacteriol. 1968 May;95(5):1513–1519. doi: 10.1128/jb.95.5.1513-1519.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton B. J., Artymiuk P. J., Cordero-Borboa A. E., Little C., Phillips D. C., Waley S. G. An X-ray-crystallographic study of beta-lactamase II from Bacillus cereus at 0.35 nm resolution. Biochem J. 1987 Nov 15;248(1):181–188. doi: 10.1042/bj2480181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


