Abstract
The properties and kinetic characteristics of a non-GSH NADPH-dependent cofactor system activating rat hepatic and renal 5'-deiodinase (5'-DI), which we have previously demonstrated with partially purified cytosol Fractions A and B [Sawada, Hummel & Walfish (1986) Biochem. J. 234, 391-398], were examined further. Although microsomal fractions prepared from either rat liver or kidneys could be activated by crude cytosol Fractions A and B from those tissues as well as from rat brain and heart, a homologous hepatic or renal system was the most potent in producing 5'-deiodination of reverse tri-iodothyronine (rT3). At nanomolar concentrations both rT3 and thyroxine (T4) were deiodinated but with a much greater substrate preference for rT3 than for T4. However, at micromolar concentrations of these substrates no activation of 5'-DI could be detected. In this deiodinative system, T4 and tri-iodothyronine (T3) competitively inhibited 5'-deiodination of rT3. Dicoumarol, iopanoate, arsenite and diamide were also inhibitory to the activation of hepatic or renal 5'-deiodination by this cofactor system. Purification of cofactor components in hepatic crude cytosolic Fractions A and B to near homogeneity, as assessed by their enzymic and physical properties, indicated that these co-purified with and were therefore identical with thioredoxin reductase and thioredoxin respectively, and accounted almost entirely for the observed activation of rT3 5'-DI. When highly purified liver cytosolic thioredoxin reductase and thioredoxin were utilized to determine the kinetic characteristics of the reaction, evidence for a sequential mechanism operative at nanomolar but not micromolar concentrations of rT3 and T4 was obtained. The Km for rT3 was 1.4 nM. Inhibition by 6-n-propyl-2-thiouracil (Ki 6.7 microM) was competitive with respect to thioredoxin and non-competitive with respect to rT3, whereas inhibition by T4 (Ki 1.3 microM) was competitive. Since rT3 is a potent inhibitor of T4 5'-deiodination, this thioredoxin system activating deiodination of rT3 may play an important role in regulating the rate of intracellular production of T3 from T4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Chopra U., Wu S. Y., Fisher D. A., Nakamura Y. Pathways of metabolism of thyroid hormones. Recent Prog Horm Res. 1978;34:521–567. doi: 10.1016/b978-0-12-571134-0.50018-1. [DOI] [PubMed] [Google Scholar]
- Das A. K., Hummel B. C., Gleason F. K., Holmgren A., Walfish P. G. Bacterial and mammalian thioredoxin systems activate iodothyronine 5'-deiodination. Biochem Cell Biol. 1988 May;66(5):460–464. doi: 10.1139/o88-057. [DOI] [PubMed] [Google Scholar]
- Das A. K., Hummel B. C., Walfish P. G. NADPH-dependent generation of a cytosolic dithiol which activates hepatic iodothyronine 5'-deiodinase. Demonstration by alkylation with iodoacetamide. Biochem J. 1986 Dec 1;240(2):559–566. doi: 10.1042/bj2400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström N. E., Holmgren A., Larsson A., Söderhäll S. Isolation and characterization of calf liver thioredoxin. J Biol Chem. 1974 Jan 10;249(1):205–210. [PubMed] [Google Scholar]
- Fekkes D., Hennemann G., Visser T. J. One enzyme for the 5'-deiodination of 3,3',5'-triiodothyronine and 3',5'-diiodothyronine in rat liver. Biochem Pharmacol. 1982 May 1;31(9):1705–1709. doi: 10.1016/0006-2952(82)90672-4. [DOI] [PubMed] [Google Scholar]
- Fekkes D., Hennemann G., Visser T. J. Properties of detergent-dispersed iodothyronine 5- and 5'-deiodinase activities from rat liver. Biochim Biophys Acta. 1983 Jan 26;742(2):324–333. doi: 10.1016/0167-4838(83)90318-7. [DOI] [PubMed] [Google Scholar]
- Goswami A., Leonard J. L., Rosenberg I. N. Inhibition by coumadin anticoagulants of enzymatic outer ring monodeiodination of iodothyronines. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1231–1238. doi: 10.1016/0006-291x(82)91382-1. [DOI] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Iodothyronine 5'-deiodinase in brown adipose tissue: thiol activation and propylthiouracil inhibition. Endocrinology. 1986 Aug;119(2):916–923. doi: 10.1210/endo-119-2-916. [DOI] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Iodothyronine 5'-deiodinase in rat kidney microsomes. Kinetic behavior at low substrate concentrations. J Clin Invest. 1984 Dec;74(6):2097–2106. doi: 10.1172/JCI111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Iodothyronine 5'-deiodinase in rat kidney microsomes. Kinetic behavior at low substrate concentrations. J Clin Invest. 1984 Dec;74(6):2097–2106. doi: 10.1172/JCI111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Purification and characterization of a cytosolic protein enhancing GSH-dependent microsomal iodothyronine 5'-monodeiodination. J Biol Chem. 1985 May 25;260(10):6012–6019. [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Stimulation of iodothyronine outer ring monodeiodinase by dihydrolipoamide. Endocrinology. 1983 Apr;112(4):1180–1187. doi: 10.1210/endo-112-4-1180. [DOI] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Thioredoxin stimulates enzymatic outer ring monodeiodination of reverse triiodothronine. Endocrinology. 1987 Dec;121(6):1937–1945. doi: 10.1210/endo-121-6-1937. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. The role of thyroid hormone deiodination in the regulation of hypothalamo-pituitary function. Neuroendocrinology. 1984 Mar;38(3):254–260. doi: 10.1159/000123900. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson K., Eriksson V., Mannervik B. Thioltransferase from human placenta. Methods Enzymol. 1985;113:520–524. doi: 10.1016/s0076-6879(85)13070-3. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Characterization of essential enzyme sulfhydryl groups of thyroxine 5'-deiodinase from rat kidney. Endocrinology. 1980 Feb;106(2):444–451. doi: 10.1210/endo-106-2-444. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Iodothyronine 5'-deiodinase from rat kidney: substrate specificity and the 5'-deiodination of reverse triiodothyronine. Endocrinology. 1980 Nov;107(5):1376–1383. doi: 10.1210/endo-107-5-1376. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Thyroxine 5'-deiodinase activity of rat kidney: observations on activation by thiols and inhibition by propylthiouracil. Endocrinology. 1978 Dec;103(6):2137–2144. doi: 10.1210/endo-103-6-2137. [DOI] [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
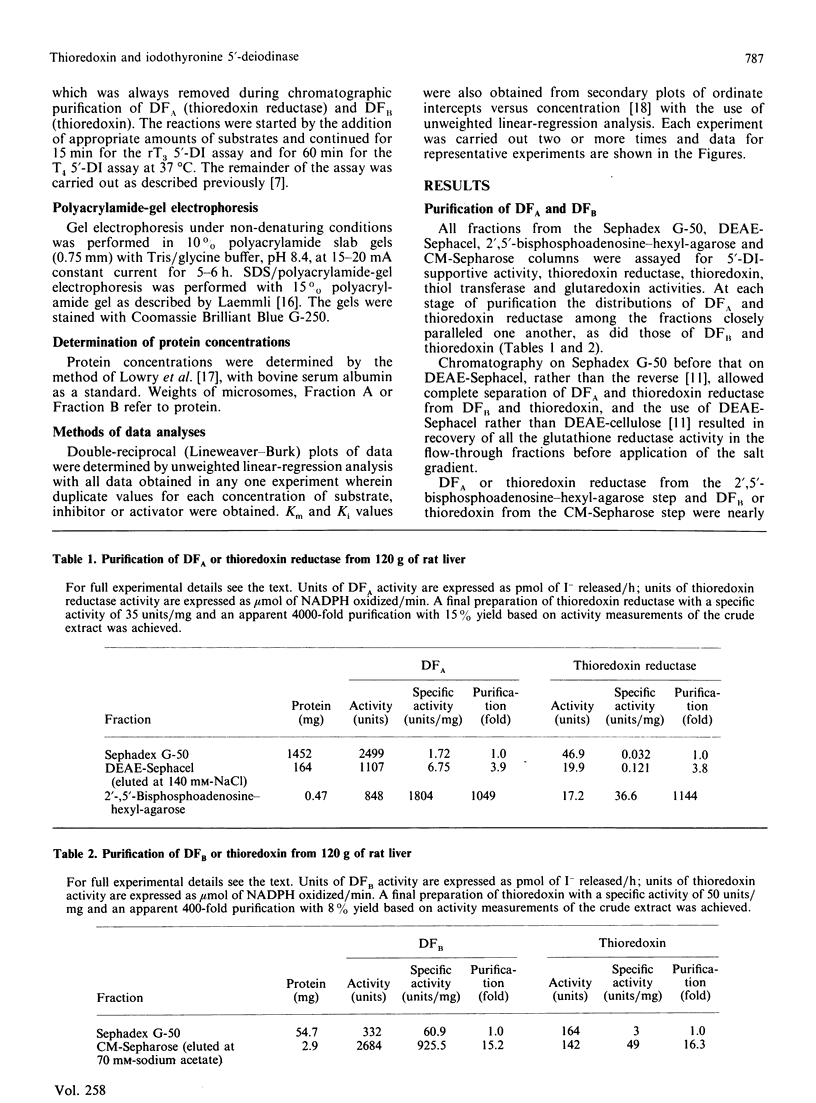
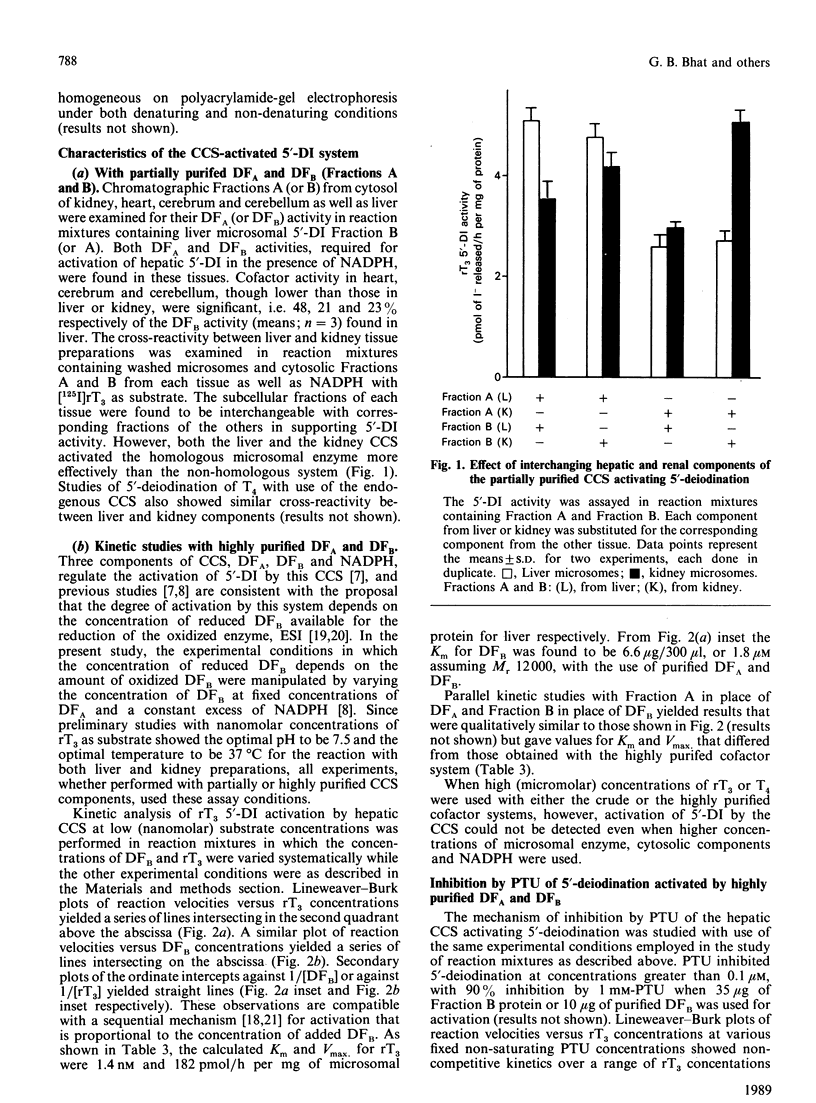
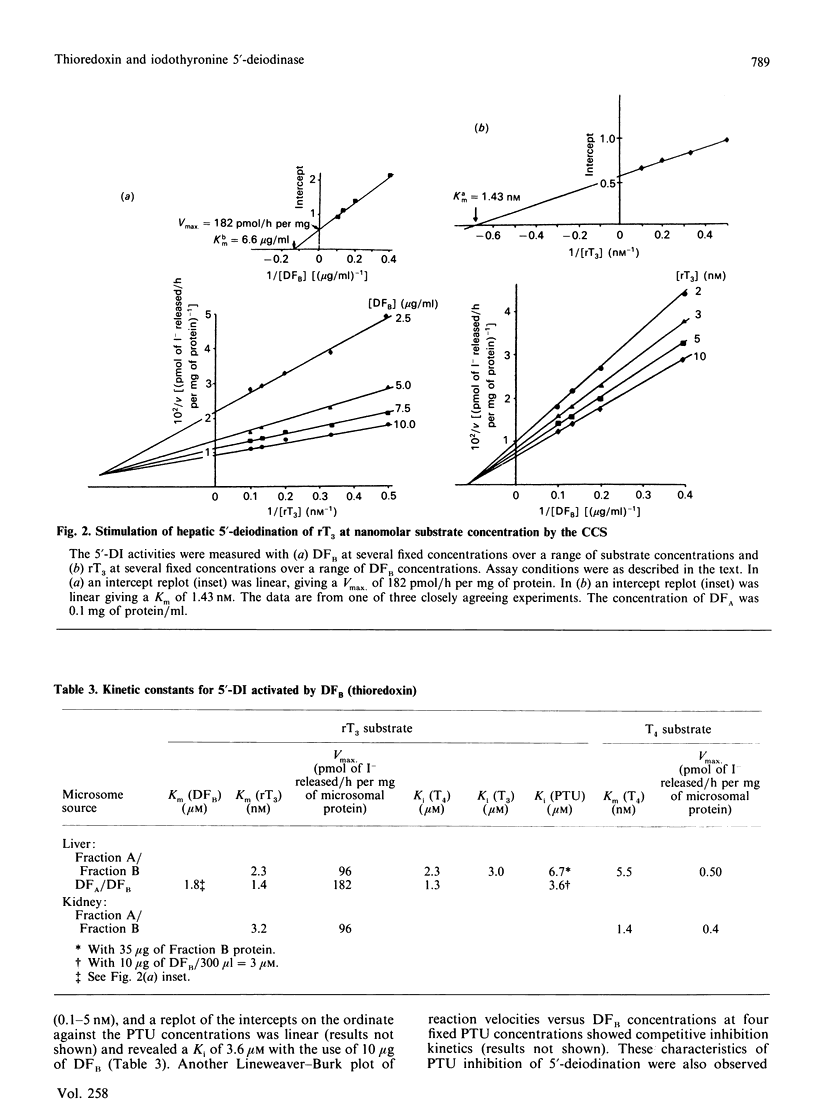
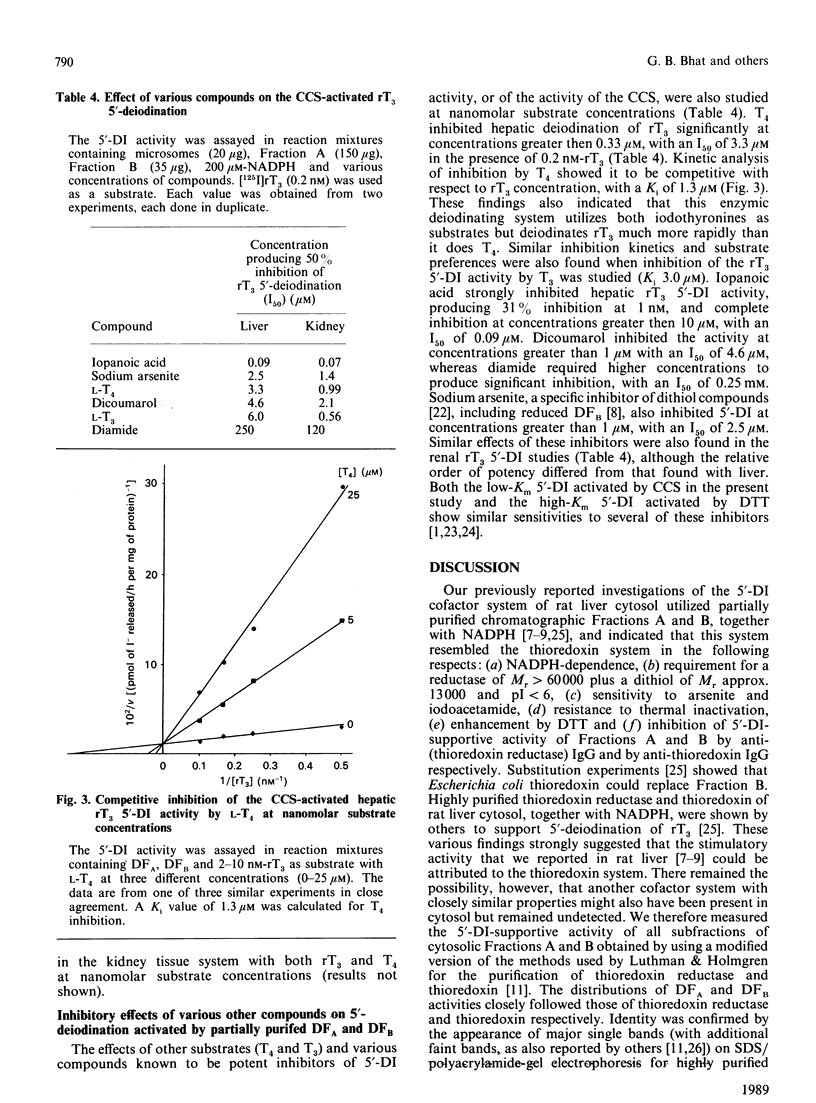
- Sawada K., Hummel B. C., Walfish P. G. Intermediate Mr cytosolic components potentiate hepatic 5'-deiodinase activation by thiols. Biochem J. 1986 Sep 15;238(3):787–791. doi: 10.1042/bj2380787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Hummel B. C., Walfish P. G. Properties of cytosolic components activating rat hepatic 5' [corrected]-deiodination in the presence of NADPH. Biochem J. 1986 Mar 1;234(2):391–398. doi: 10.1042/bj2340391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J. A tentative review of recent in vitro observations of the enzymatic deiodination of iodothyronines and its possible physiological implications. Mol Cell Endocrinol. 1978 May;10(3):241–247. doi: 10.1016/0303-7207(78)90039-4. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Does-Tobé I., Docter R., Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into tri-iodothyronine and possible involvement of essential thiol groups. Biochem J. 1976 Aug 1;157(2):479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Kaplan M. M., Leonard J. L., Larsen P. R. Evidence for two pathways of iodothyronine 5'-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983 Apr;71(4):992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Kinetic evidence suggesting two mechanisms for iodothyronine 5'-deiodination in rat cerebral cortex. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J. Mechanism of action of iodothyronine-5'-deiodinase. Biochim Biophys Acta. 1979 Aug 15;569(2):302–308. doi: 10.1016/0005-2744(79)90066-4. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Cleland W. W. A specific and sensitive assay for disulfides. J Biol Chem. 1968 Feb 25;243(4):716–719. [PubMed] [Google Scholar]


