Abstract
Medical device research and development are characterized by high costs, extended timelines, inherent risks, and the necessity for interdisciplinary knowledge and skills. It is significantly influenced by policies, making the understanding of medical device innovation both important and challenging. This paper takes a dual approach to analyze medical device innovation. We reviewed representative clinical product of bougie and stylet and summarized the common characteristics and trend of these product. Innovations in these products often involve adding depth markings, replacing material and design structure, enhancing visualization, deciding between reusable or disposable designs, and integrating multi-functional features. This underscores the delicate balance between technological advancements and medical costs for widespread clinical applicability. We explored the guiding role of policy in medical device innovation, emphasizing its impact through an analysis of medical device regulations and policies in China. By offering insights from the perspectives of medical device companies and regulators, this paper aims to elucidate the critical aspects of medical device innovation, assisting researchers in mitigating risks during product development.
Keywords: Medical device, innovation, economic and cost, regulatory assessment, policy impact
Introduction
Medical device development spans various disciplines, including biology, chemistry, mechanical design, and electronic information [1]. Research indicated that stringent regulatory requirements contribute to pre-market approval costs for medical devices. This can reach millions of dollars, coupled with a time-to-market that extends over several years [2]. This scenario introduces significant risk in medical device projects. Research and development funding are frequently constrained, impeding innovation within companies. Understanding the intricate trends in the innovation and development of medical devices is paramount for fostering a robust and swift growth of the medical device industry. Innovation is not confined to technological influences; it encompasses a multitude of factors, particularly medical device regulations and policies [3-5]. As a result, the innovation of medical device can be complex and circuitous. Amidst the diverse array of medical devices, a focused examination of representative components, such as bougie and stylet, emerges as a strategic approach to comprehending the innovative trajectory. These components play a profound role in shaping the lives and health of patients.
Tracheal intubation stands as a pivotal technique for the critical treatment of patients facing severe illness or injuries requiring clinical anesthesia and mechanical ventilation [6]. The success of this procedure directly correlates with the ability to effectively rescue the lives of critically ill or injured patients. Auxiliary devices such as bougie and stylet play integral roles in clinical tracheal intubation. Bougies, characterized by their soft, slender, and cylindrical design, are crafted to guide, position, support, and establish a pathway for the endotracheal tube [7]. Stylets are soft and slender devices that provide rigidity, stability, guidance, and support. These functions are essential for the endotracheal tube [8]. Despite the distinct design and appearance disparities between bougie and stylet, their shared objective is to facilitate and support the intubation process. Ongoing efforts to address intricate issues in clinical applications prompt continuous enhancements to these devices. Analyzing the innovation processes and unique characteristics of different bougies or stylets aids healthcare professionals in selecting more suitable tools and provides a profound insight into medical device innovation. This insight encompasses problem identification, the development of new devices tailored to specific needs, and contributes to the heightened efficiency of medical device research and development. This mitigates the risks associated with development failures [9].
The trajectory of medical device development is shaped by market dynamics and by a complex interplay of government policies, industry regulations, standards, and considerations involving the insurance sector [10-12]. Some of these elements impose constraints on corporate behavior. A thorough analysis of pertinent industry policies offers valuable insights into the course of medical device development [13]. This analytical approach enables companies to navigate resource wastage, attain a renewed understanding of market dynamics, and align with national demands for medical devices. It serves to enhance the overall effectiveness of the final products by ensuring their compliance with regulatory frameworks and industry standards.
Bougie and stylet
Tracheal intubation, a critical technique in ensuring patient safety, presents various challenges that can be effectively addressed through the utilization of appropriate auxiliary tools [14]. For instance, when confronted with challenges related to poor visibility in the airway, the deployment of a bougie becomes instrumental. Bougies serve to expand the airway, guiding the endotracheal tube during the intubation [15]. The endotracheal tube encounters difficulty with its softness, impeding smooth insertion. The stylet is meticulously designed to provide the necessary rigidity and stability. Stylets offer rigidity, helping maintain the endotracheal tube’s shape during insertion. This makes them suitable for standard intubations with clear airway anatomy and requiring visual confirmation of tube passage through the vocal cords, often aided by a laryngoscope. Both bougie and stylet play indispensable roles in clinical settings, addressing different aspects of the intubation process. Bougies are ideal for difficult airway intubations where navigating the airway’s curvature is challenging, minimizing tissue trauma due to their flexibility. Stylets, though providing stability, pose a risk of airway damage if not promptly removed after the tube passes the vocal cords. Researchers continue to innovate, developing products that improve the intubation process and introduce sophisticated features to meet evolving clinical demands. These innovations often incorporate new technologies or employ novel structures to address the intricacies of clinical scenarios, as illustrated by the diverse designs of bougie and stylet products born out of ongoing research and development efforts.
Bougie
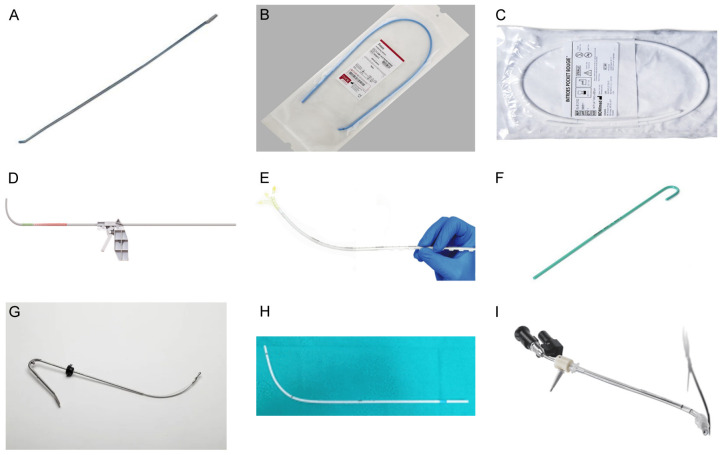
There is a wide range of bougie products, but their use can be broadly divided into the following steps. Preceding intubation, the endotracheal tube is affixed to the bougie. The bougie is meticulously inserted through the glottis, often in tandem with a direct laryngoscope or a video laryngoscope. This combined approach facilitates a comprehensive visualization of the glottis, ensuring precise information gathering and accurate insertion of the endotracheal tube into the airway. With the endotracheal tube appropriately positioned, it is deftly maneuvered through the glottis. The final step involves the removal of the bougie, marking the completion of the tracheal intubation process. Table 1 provides a summary of representative bougie products, offering insights into the diverse options available in the market and highlighting their unique features. Some of the products are shown in Figure 1.
Table 1.
Summary of the bougie product on the market
| Device Name | Depth markings | Material and design structure | Visualization | Reusable or disposable | Featured Functions |
|---|---|---|---|---|---|
| Eschmann introducer | No | A polyester core and a resin | No | Change from reusable to | Clinical gold standard |
| Frova intubating introducer | Yes | Polyethylene | No | Disposable | Tube lumen and distal side port for oxygen delivery |
| Introes pocket bougie | Yes | A unique fluoropolymer blend | No | Disposable | Allows it to fit in a pocket for quick rescue |
| Total Control Introducer | Yes | A flexible shaft, an articulating tip, and a removable pistol grip handle | Conjunction with a laryngoscope | Disposable | Suitable for use with laryngoscope, can be operated by one person |
| Flexible Tip Bougie™ | Yes | Silicone tip with fluorescent and slider | No | Disposable | Slider to adjust tip angle and silicone tip to minimize the risk of tracheal trauma |
Figure 1.
Representative product diagrams of bougie and stylet. A. Eschmann introducer, SP Services (UK) Ltd. B. Frova intubating introducer, COOK MEDICAL LLC P.O. C. Introes pocket bougie, MD Inc. D. Total Control Introducer, SOURCEMARK, LLC. E. Flexible Tip Bougie™, Lateral Medical. F. Portex stylet, Universal Specialities Limited - USL Medical. G. Truflex™ Stylet, T K India Private Limited. H. SEEKflex image from “Application of “twelve-step” approach based on SEEKflex for difficult awake tracheal intubation in patients with cervical spinal tumor (Adapted from Wenyun Xu et al, 2022)”. I. Shikani Optical Stylet, Sharn Anesthesia Inc. All images of products were cited from manufacturer’s websites.
In 1949, Sir Robert Reynolds Macintosh pioneered the design of the inaugural bougie, recognized as the “Gum Elastic Bougie [16]”. This drew inspiration from a urethral dilatation catheter. The evolution of bougie technology continued in the 1970s when P.H. Venn made significant improvements, culminating in the creation of the first purpose-made bougie [17]. This innovation was later manufactured by the UK company Eschmann and named the “Eschmann introducer”. This introducer has undergone refinements, boasting current specifications of a 60 cm length, a 5 mm outer diameter, and a distal tip angled at 40°. The introducer exhibits a unique memory function, maintaining its new shape when bent, having been made of a polyester core and a resin coating. This combination ensures an optimal balance of rigidity and flexibility, facilitating ease of insertion and withdrawal. In practical application, physicians benefit from sensory indicators such as audible “clicks” or encountered resistance to gauge the accurate placement of the endotracheal tube with the Eschmann introducer. In 2014, the “traffic-light bougie”, proposed by A Paul, introduced color coding to indicate insertion depth, preventing it from being pushed too far and causing airway trauma [18].
Building on the foundation laid by the Eschmann introducer, the introduction of the Frova Intubating Introducer [19] in the 1990s marked a pivotal evolution in the design and functionality of airway management tools. The Frova Intubating Introducer, designed with a length of 65 cm and a tip angle of 35°, distinguishes itself by facilitating enhanced sliding below the epiglottis. Crafted from polyethylene, the Frova Intubating Introducer embodies a memory effect and features a rigid, detachable internal sheath, contributing to its increased stiffness. This unique design empowers an anesthesiologist to adeptly maneuver the introducer to the ideal position during medical procedures. The Frova Intubating Introducer includes a hollow lumen for oxygen delivery and distal side ports, offering versatility for both regular and jet ventilation. This ensures the continuous supply of oxygen during the intubation process, safeguarding the patient’s life and health. The clinical application method of the Frova Intubating Introducer mirrors that of the Eschmann Introducer, providing a familiar and standardized approach across various scenarios.
The Frova intubating introducer has demonstrated significant success in clinical applications. There remain challenges regarding the portability of the device in out-of-hospital emergency scenarios. The Introes Pocket Bougie [15] stands out as a specialized tool made from a unique fluoropolymer blend, characterized by a pre-designed curved shape and remarkable plasticity with memory. Crafted with these distinctive features, the bougie is specifically designed for easy storage in airway kits, coat pockets, or bags. This design facilitates rapid deployment during airway rescue scenarios, offering convenience and accessibility for medical professionals in critical situations.
The Total Control Introducer [20], introduced in the 2010s, was designed to provide clinicians with enhanced control during intubation, particularly in difficult airway scenarios. This tool offers improved maneuverability and guidance, prioritizing control to make it easier for clinicians to navigate challenging airways. Despite its advantages, the Total Control Introducer is more expensive and requires further training for effective use, that can be a barrier to widespread adoption. The Total Control Introducer, an articulating bougie with a length of 70 cm, comprises three integral components: a flexible shaft, an articulating tip, and a removable pistol grip handle. Significant features include color-coded depth markings for precise navigation. Typically utilized with either a direct laryngoscope or a video laryngoscope, it enables a single operator to proficiently execute the procedure.
In the 2010s, the Flexible Tip Bougie™ [21] was introduced, featuring a flexible tip designed to navigate airway anatomy more easily. This balance of flexibility and rigidity enhances its versatility and effectiveness across various clinical scenarios. The flexible tip allows for smoother navigation through complex airway structures, though clinicians need to adapt to its unique handling characteristics. In extremely rigid airway situations, the flexible tip may not perform as well as more rigid alternatives. The Flexible Tip Bougie™ is a disposable, articulating bougie measuring 65 cm in length. Characterized by graduated centimeter markings from 10 to 50 cm, its smooth silicone tip is designed to minimize the risk of tracheal trauma during intubation. The fluorescent tip aids in optimal positioning. A distinctive advantage lies in its innovative slider, enabling the tip to move forward or backward. This feature facilitates easy manipulation in an up-and-down motion, allowing for the single-handed operation of the device.
Stylet
Prior to intubation, the insertion of the malleable stylet into the endotracheal tube serves a crucial function in maintaining the shape of the tube throughout the intubation process. This aids in the smooth insertion of the endotracheal tube. Upon successful passage through the vocal cords, it is imperative to promptly remove the stylet to mitigate the risk of airway damage. Table 2 provides a summary of representative stylet products.
Table 2.
Summary of the stylet product on the market
| Device Name | Depth markings | Material and design structure | Visualization | Reusable or disposable | Featured Functions |
|---|---|---|---|---|---|
| Portex Stylet | No | High-density polyethylene outer sheath and an expandable aluminum coated with high-density polyethylene | No | Disposable | Conventional Rigid stylet |
| Truflex™ Stylet | No | Stainless steels | Conjunction with a laryngoscope | Reusable | Distal end allows leveraged movement |
| SEEKflex | No | Stainless steel core, PVC tubing, ABS resin fittings, handles | Conjunction with a laryngoscope | Disposable | Shapeable, retractable, removable, versatile, inexpensive, and features a bougie |
| Shikani Optical Stylet | No | Includes a fiber optic cable, extendable and angle adjustable distal end handle | Yes | Reusable | Optical stylet |
One such product is the Portex Stylet, functioning as a traditional rigid probe. The rigidity of the Portex Stylet can be a limitation, as it may not easily navigate more complex airway anatomies. It consists of a smooth high-density polyethylene outer sheath and an expandable aluminum core coated with high-density polyethylene, allowing for shaping into any desired form. The Portex Stylet is available in a range of sizes to suit adults and children.
Building upon the foundation established by the Portex stylet, the Truflex™ Stylet [22] introduced enhanced flexibility and maintained sufficient rigidity to guide the endotracheal tube. This design allows it to navigate intricate airway more effectively. The Truflex™ Stylet is particularly beneficial in scenarios requiring a balance between flexibility and support. It has improved versatility but it may lack advanced features for extremely difficult airways and does not provide real-time visual guidance. Another stylet is the Truflex™ Stylet, designed for endotracheal tubes ranging from 6.5 to 8.5 in diameter. Featuring a flexible tip for enhanced control, it allows leveraged movement up to 30° to 60° at its distal end, contingent on the tube diameter. The stainless steels components can be sterilized by any method. The Truflex™ Stylet is equipped with a plug for securing the endotracheal tube and ergonometric handle, facilitating single-handed use.
The SEEKflex [23] (Safe Easy Endotracheal Kit-flexible) represents an evolution in stylet technology, incorporating a more refined balance of flexibility and rigidity. Its design is optimized for ease of maneuverability through complex airway structures, enhancing the clinician’s ability to navigate challenging intubations. SEEKflex often includes features such as a more ergonomic handle and improved material composition for better control. It does not provide direct visualization of the airway, relying on tactile feedback and anatomical knowledge. It represents a new single-use endotracheal intubation kit meticulously designed to enhance the intubation process with various endotracheal tubes. Ranging in length from 43 to 81 cm, the introducer features a tube tip with three large ventilation holes and three rows of small side holes. At the proximal end, the tube can be seamlessly paired with a compatible transition connector after the removal of the joint. The inner guide core is constructed from malleable steel, and the outer catheter is crafted from polyvinyl chloride (PVC). The lock connector, handle, and transition connector are fabricated from acrylonitrile-co-butadiene-co-styrene (ABS) resin material. This device, characterized by suitable materials and a distinctive design proves efficacious for challenging airways, reducing the likelihood of hypoxemia and functions as a bougie. Its flexibility in use and cost-effectiveness contributes to its appeal. Expanding on this foundation, developers have introduced a complementary intubation procedure, unlocking the device’s chances for broader clinical applications and solidifying its status as a versatile and valuable tool.
Introduced in 1999, the Shikani Optical Stylet [24] has undergone continuous enhancements to become a leading semi-rigid optical stylet that employs fiber optics for light and image transmission. This stylet represents a significant advancement in stylet technology, providing the necessary rigidity and flexibility for intubation and allowing for real-time visualization of the airway during the procedure. These improvements have significantly increased the success rate of intubations, especially in scenarios involving difficult airways. By combining visual guidance with physical support, the Shikani Optical Stylet addresses many of the limitations of previous stylets. It is more complex and expensive, necessitating additional training and maintenance. Comprising a fiber optic cable connected to a camera and video monitor, the device features an extendable distal end with an adjustable angle tailored to the patient’s anatomical structure. An adjustable stop near the proximal end and an oxygen port, designed for oxygen insufflation and to mitigate delayed desaturation, complete the ensemble. This optical stylet stands as a valuable tool for intubation, empowering healthcare professionals with the ability to visualize the airway and enhance the precision of endotracheal tube placement. The adaptability of the distal end angle and the capability to adjust the stop near the proximal end contribute to its versatility across diverse clinical scenarios. The incorporation of an oxygen port bolsters oxygenation throughout the intubation process.
Development of medical device
In the ever-evolving landscape of science, technology, and socio-economic advancements, the bougie and stylet keep undergoing continuous innovations. These advancements encompass the design of novel structures and the integration of achievements from various fields. In clinical practice, anesthesiologists select bougie and stylet by considering a blend of personal preferences, anatomical considerations, and economic factors. Despite the myriad of options available, the earlier-introduced Endotracheal Introducer and Frova Intubating Introducer persist as primary choices for many physicians [25]. By summarizing and exploring the evolutionary trends and characteristics of the bougie and stylet, we aim to provide valuable insights into the innovation within medical devices.
Addition of depth markings
The integration of depth indicators or scales has emerged as a prevalent feature in contemporary bougie products, underscoring the crucial role of precise endotracheal tube insertion depth in patient care. The choice of both physical markings and the actual insertion depth is informed by clinical expertise. The device can already visualize the airway by itself or in combination with other devices [20,24]. Factors such as economic constraints, clinical efficiency, and equipment limitations influence the prevalence of depth markings in contemporary practice [26,27]. Derived from extensive patient data, these markings play a pivotal role in ensuring the safe insertion of endotracheal tubes for the majority of patients. Advanced techniques like fiberoptic bronchoscopy or ultrasound devices offer precise determination of the tube’s position, mitigating the risk of penetration or superficial placement. These approaches pose challenges in terms of medical efficiency, resource utilization, and economic costs. In environments without access to instruments like fiberoptic bronchoscopes or ultrasound devices, the adoption of such advanced techniques may not be feasible. Clinicians grappling with difficult airways may resort to fiberoptic bronchoscopy or ultrasound devices [28]. For routine cases, the choice of insertion depth predominantly relies on clinical experience - a compromise necessitated by the imperative to manage economic costs while ensuring patient safety [29].
Material and methods
Various bougie products make deliberate material and structural choices aligned with their design philosophy. These decisions, influenced by the technological context of the time, aim to strike a balance between softness and rigidness. The material selection is meticulously calibrated to avoid extremes - neither excessively soft to impede insertion nor excessively rigid to risk tissue damage. Bougie products vary in length and in the angle of their tips, offering anesthesiologists the flexibility to choose products tailored to different clinical conditions. The replacement of materials and the design of novel structures can be iterative improvements to the previous generation of products, highlighting strengths or creating new products that increase flexibility and address challenges. The risks associated with material and structural changes are often manageable, instilling confidence in enterprises to invest in research and development [30,31]. Clinically, there is a willingness to adopt such innovations. Medical professionals find the learning curve for these medical devices relatively low, facilitating teaching and widespread clinical dissemination [32].
Visualization enhancements
Anesthesiologists play a pivotal role in monitoring various indicators during the perioperative period, with heightened attention during intubation to ensure the safe and reliable completion of the procedure. Visual technologies have emerged as effective tools in improving observation conditions for anesthesiologists, providing a more intuitive assessment of the endotracheal tube’s status [33]. Both the Total Control Introducer and SEEKflex can be used in conjunction with video laryngoscopes, while the Shikani Optical Stylet achieves direct visualization. Visualization stands out as a prominent trend in the development of bougie and stylet.
The use of visualization empowers anesthesiologists with better insights into the airway. In extreme scenarios, such as heavy bleeding or blood-contaminated lenses, challenges may arise, leading to blurred vision and hindered observation [34]. Even in cases where the decision is made to remove the tube and clean the lens, complexities such as multiple intubation attempts, increased patient trauma, prolonged procedural time, and the life-threatening risk of interrupted breathing can rise. In such circumstances, the Endotracheal Introducer demonstrates a significant advantage by providing tactile sensation or encountering resistance, offering crucial feedback to gauge the intubation process. This should not overshadow the inherent advantages of visualization. It is significant in current clinical settings, aiding anesthesiologists in better patient observation. With the ongoing development of artificial intelligence, visualization becomes a crucial data foundation and a prerequisite for future intelligent systems [35].
Decisions between reusable or disposable designs
Designed for repeated use through sterilization, the Eschmann introducer has experienced a shift in its clinical application, leaning towards becoming a disposable consumable [36]. The cost savings associated with sterilization for reuse are apparent. Several studies have raised valid concerns about hazards linked to viral infections [37]. Devices with higher economic costs, such as the Truflex™ Stylet and Shikani Optical Stylet, are often reused. This emphasizes the inherent contradiction between the one-time disposable approach that prioritizes safety and the economic considerations associated with reuse. This paradox underscores the ongoing challenge in balancing patient safety and economic considerations within clinical practices. The choice between disposable and reusable options remains a nuanced decision that necessitates careful consideration of both financial implications and safety risks.
Integration of multi-functional features
From their initial role in guiding and assisting with endotracheal tube insertion to evolving into instruments that facilitate tube replacement ensuring uninterrupted ventilation, bougie and stylet have progressed to address challenging problems through technological advancements. The SEEKflex, with its strategic material selection and innovative structural design, seamlessly integrates the functions of both bougie and stylet [23]. This innovation offers enhanced solutions for addressing difficult airway challenges while maintaining cost-effectiveness. The integration of multiple functions introduce challenges such as bulkiness, increased costs, and material waste. The Shikani Optical Stylet, driven by factors such as the imperative for visualization and the use of stainless steels material, results in a more expensive device, prompting its preference for viral sterilization and reuse. The economic and learning costs associated with the Shikani Optical Stylet may pose constraints. It is an important tool in the clinic.
In conclusion, the evolution of bougie and stylet products reflects the delicate balance between cost and functionality, operating within the confines of technological limitations of their time. As the primary objective of medical devices is to safeguard people’s health, it is imperative that these products should be accessible to a broad spectrum of individuals and reasonably priced [38]. The introduction of a new device into clinical practice involves establishing a new production line or abandoning old ones, incurring costs that are ultimately reflected in the final product’s price [39]. Recognizing the principle of marginal effects, as clinical acceptance of a new device grows, the market expands, and new production lines are established, the initially hidden costs associated with the product gradually amortize. This process is time-intensive and exhibits a degree of lag [40]. The extended development cycle of medical devices implies prolonged capital return periods for medical device companies, rendering them vulnerable to risks [41,42]. Economic costs emerge as highly sensitive factors for both production companies and patients within the medical device industry. In navigating these complexities, the balance between innovation, accessibility, and economic considerations is essential to ensure the sustainable advancement of medical practice and improved patient well-being.
Impact of policies on innovation within medical devices
As evidenced by the preceding discussion, the development of medical devices operates within the constraints of economic costs in the current technological landscape. The resolution of one issue often begets new challenges [43,44]. The distinctive characteristics of the medical device industry subject it to more stringent regulations compared to other sectors [45,46]. Industry policies exerts substantial influence over various facets of medical device development [47]. A pivotal regulation significantly shaping the medical device landscape in China is the “the Regulations on the Supervision and Administration of Medical Devices (2021 Revision) (RSAMD)”, officially implemented in 2000 and subsequently undergoing multiple revisions. Serving as the sole administrative regulation governing the Chinese medical device industry, the RSAMD spans the entire lifecycle of medical device research and production [48]. Augmenting this foundational regulation are complementary documents such as the “Special Examination Procedure for Innovative Medical Devices (SEPIMD)”, the “Administrative Measures for Medical Device Registration and Filing”, and the “Measures for the Supervision and Administration of Medical Device Production”. Together, these regulations collectively define the developmental trajectory of innovation in the medical device industry [49].
Innovative medical devices, characterized by superior performance and cutting-edge technology, showcase the technological prowess of the medical device industry and underscores its developmental success. The development of new products in the medical field is often spearheaded by startups grappling with limited funding, leading to a measured pace in the advancement of high-end innovative medical devices [50]. To catalyze innovation within the medical device industry, the RSAMD marked a significant shift by designating the promotion of the medical device industry’s development as one of its primary goals [48]. Innovative medical devices, as representatives of the industry, are granted priority in the approval process. The commitment to incentivizing innovation in the field of medical devices was solidified by the National Medical Products Administration (NMPA) through the introduction of the “Special Approval Procedure for Innovative Medical Devices (Trial)” in 2014. Building on this initiative, the procedure was officially promulgated in November 2018 as the “Special Examination Procedure for Innovative Medical Devices”, establishing a more complete regulation to support and accelerate the development of innovative medical technologies. In the year of its official promulgation, NMPA received 316 applications for special approval and completed 310 reviews. This included applications carried over from 2017, and determined that 45 products qualified to enter the special approval channel for innovative medical devices. A total of 21 innovative medical device products were approved for market release, marking an increase of 8 compared to the number approved in 2017. This initiative, including the establishment of a greenway for the priority review and approval of innovative medical devices, has significantly reduced the approval time for these devices. According to the 2023 annual report on medical device registration released by the Food and Drug Administration, NMPA received 466 applications for the special examination and approval of innovative medical devices in 2023, marking a 35.9% increase over the previous year. Of these applications, 69 were admitted into the SEPIMD. Throughout the year, a total of 61 innovative medical devices received approval, representing an 11% increase compared to 2022. There were 12 medical devices granted priority status. That is an increase of 6 compared to the previous year. This effective streamlining of the approval process has played a pivotal role in facilitating the development of core technologies for innovative medical devices by medical device enterprises.
Innovative medical devices hold paramount importance, advancements in manufacturing processes, the design of new structures and other methods can effectively enhance the clinical experience for patients and alleviate the workload for doctors. Despite incremental in nature, these innovations, especially in more mature products, play a crucial role in improving clinical outcomes, with their associated risks being more predictable and assessable [51]. The RSAMD recognize the maturity of certain devices with proven safety records and stipulate exemptions from clinical evaluations for similar devices. In 2022, the NMPA registered an initial 2,500 items, reflecting a substantial 46.2% increase compared to 2021. There were 4,224 items with registration changes, marking a significant 58.5% increase from the previous year. Innovations encompassing process improvements and parameter enhancements fall within the category of registration changes. The impact of the revised RSAMD in 2021 is evident as the medical device industry has witnessed heightened activity. In 2023, NMPA approved a total of 12,213 initial registrations, renewals, and changes in medical device registrations, marking a 2.3% increase compared to 2022. Of these, 2,728 items were registered for the first time, showing a 9.1% increase from the previous year. There were 4,788 renewals, which represented an 8.2% decrease from 2022, marking a decline for the second consecutive year. There were 4,697 changes in registrations, reflecting an 11.2% increase compared to 2022. Innovations, related to process improvements and parameter enhancements, are advancing rapidly. These innovations involve lower upfront investments and reduced R&D risks for enterprises, contributinge to shorter time-to-market and lower final selling prices. This is particularly significant for regions with relatively weaker development [52].
Regulating medical devices presents significant challenges due to their multidisciplinary nature, involving domains such as medicine, mechanics, materials, electronics, and computer science [53]. Distinguished from other industrial products, medical devices must ensure safety in clinical applications [40]. The execution of clinical trials encounters constraints stemming from ethical considerations, cost, and time constraints. The challenge is that even when large-scale, long-term clinical trials are conducted, it is difficult to comprehensively summarize the changes introduced in the clinical setting. These challenges are particularly pronounced for innovative medical devices that leverage advanced technologies. The RSAMD and associated documents, such as the “Administrative Measures for Medical Device Registration and Filing”, explicitly allow registrants or qualified medical device testing institutions to provide self-inspection reports and product inspection reports. This provision aims to infuse vitality into the medical device industry. The adoption of self-inspection for medical device registration remains limited among manufacturing enterprises. Successful cases are infrequent, with most relying on laboratory testing accredited by the China National Accreditation Service for Conformity Assessment (CNAS) [54,55]. Despite these challenges, the integration of real-world evidence (RWE) in regulatory frameworks, as exemplified by the accelerated approval of the XEN® glaucoma drainage tube, represents a significant advancement. This innovative approach facilitated the device’s approval within five months in the Boao Lecheng Pilot Zone, demonstrating how specialized regulatory environments can effectively reduce approval times and foster medical innovation, meeting urgent clinical needs while upholding safety and efficacy standards [56]. There is an ongoing trend towards shortening the review period and fortifying post-market supervision efforts [57]. This poses challenges for companies and relevant authorities, enhancing innovation-related systems can inject greater vitality into the intricate and expansive field of medical devices [58,59].
Conclusions
Since the inception of the first bougie in 1949, clinicians have witnessed a significant expansion in options for bougies and stylets, each presenting distinct features and advantages. Despite this diversity, innovations across various products predominantly center around critical aspects such as an addition of depth markings, material and design structure replacements, visualization enhancements, decisions between reusable or disposable designs, and integration of multi-functional features. With the march of technological advancements, the integration of artificial intelligence (AI) technology, particularly following visualization, is emerging as a noteworthy trend [60]. It becomes imperative for companies to support their products with reliable evidence to ensure safety and reliability. This means that medical device companies and risk analysis institutions should delve into research on model interpretability. Recognizing the constraints imposed by economic costs on the application and sales of medical devices is paramount. As policies wield significant influence on industry trajectories at a macro level, the challenge lies in determining, amid the burgeoning trends of artificial intelligence and big data, resources can be effectively integrated to foster deeper collaboration among companies in different fields, facilitating the efficient sharing of data [61]. This collaborative approach and data sharing simultaneously pose both challenges and opportunities for the future.
Acknowledgements
This study was funded by Shanghai Industrial Collaborative Innovation Project (HCXBCY-2023-041), Teaching Achievement Cultivation Project of Naval Medical University (JPY2022A15) and Shanghai Oriental Talent Extraordinary Project and School-level Basic Medical Project (2023MS013).
Disclosure of conflict of interest
None.
References
- 1.Joung YH. Development of implantable medical devices: from an engineering perspective. Int Neurourol J. 2013;17:98–106. doi: 10.5213/inj.2013.17.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galbusera F, Niemeyer F, Seyfried M, Bassani T, Casaroli G, Kienle A, Wilke HJ. Exploring the potential of generative adversarial networks for synthesizing radiological images of the spine to be used in in silico trials. Front Bioeng Biotechnol. 2018;6:53. doi: 10.3389/fbioe.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Money AG, Barnett J, Kuljis J, Craven MP, Martin JL, Young T. The role of the user within the medical device design and development process: medical device manufacturers’ perspectives. BMC Med Inform Decis Mak. 2011;11:15. doi: 10.1186/1472-6947-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moses H 3rd, Dorsey ER, Matheson DH, Thier SO. Financial anatomy of biomedical research. JAMA. 2005;294:1333–1342. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 5.Bergsland J, Elle OJ, Fosse E. Barriers to medical device innovation. Med Devices (Auckl) 2014;7:205–209. doi: 10.2147/MDER.S43369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tollman J, Ahmed Z. Efficacy of tracheal tube introducers and stylets for endotracheal intubation in the prehospital setting: a systematic review and meta-analysis. Eur J Trauma Emerg Surg. 2022;48:1723–1735. doi: 10.1007/s00068-021-01762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan JP, Wilson ME. Orotracheal intubation in patients with potential cervical spine injuries. An indication for the gum elastic bougie. Anaesthesia. 1993;48:630–633. doi: 10.1111/j.1365-2044.1993.tb07133.x. [DOI] [PubMed] [Google Scholar]
- 8.Grape S, Schoettker P. The role of tracheal tube introducers and stylets in current airway management. J Clin Monit Comput. 2017;31:531–537. doi: 10.1007/s10877-016-9879-8. [DOI] [PubMed] [Google Scholar]
- 9.Durfee WK, Iaizzo PA. Engineering in Medicine. Elsevier; 2019. The medical device innovation process; pp. 495–509. [Google Scholar]
- 10.Alfa MJ, Castillo J. Impact of FDA policy change on the reuse of single-use medical devices in Michigan hospitals. Am J Infect Control. 2004;32:337–341. doi: 10.1016/j.ajic.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Sorenson C, Kanavos P. Medical technology procurement in Europe: a cross-country comparison of current practice and policy. Health Policy. 2011;100:43–50. doi: 10.1016/j.healthpol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Um SI, Sohn UD, Jung SY, You SH, Kim C, Lee S, Lee H. Longitudinal study of the impact of three major regulations on the Korean pharmaceutical industry in the last 30 years. Health Res Policy Syst. 2022;20:4. doi: 10.1186/s12961-021-00797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AM, Behan R, Smith I, Griffin M, Keane F, Langan J, O’Rourke C, McAleenan N, Pandit A, Watson M. A multidisciplinary approach to online support for device research translation: regulatory change and clinical engagement. Health Policy Technol. 2020;10:95–103. doi: 10.1016/j.hlpt.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorbello M, Hodzovic I. Tracheal tube introducers (bougies), stylets and airway exchange catheters. In: Cook T, Kristensen MS, editors. Core Topics in Airway Management. Cambridge University Press; 2020. pp. 130–139. [Google Scholar]
- 15.Yadav P, Gupta A, Gupta N. Airway adjuncts. In: Ubaradka RS, Gupta N, Bidkar PU, Tripathy DK, Gupta A, editors. The Airway Manual: Practical Approach to Airway Management. Springer; 2023. pp. 181–194. [Google Scholar]
- 16.Macintosh RR. An aid to oral intubation. Br Med J. 1949;1:28. [Google Scholar]
- 17.Henderson JJ. Development of the ‘gum-elastic bougie’. Anaesthesia. 2003;58:103–104. doi: 10.1046/j.1365-2044.2003.296828.x. [DOI] [PubMed] [Google Scholar]
- 18.Paul A, Gibson AA, Robinson OD, Koch J. The traffic light bougie: a study of a novel safety modification. Anaesthesia. 2014;69:214–218. doi: 10.1111/anae.12522. [DOI] [PubMed] [Google Scholar]
- 19.Hodzovic I, Latto IP, Wilkes AR, Hall JE, Mapleson WW. Evaluation of Frova, single-use intubation introducer, in a manikin. Comparison with Eschmann multiple-use introducer and Portex single-use introducer. Anaesthesia. 2004;59:811–816. doi: 10.1111/j.1365-2044.2004.03809.x. [DOI] [PubMed] [Google Scholar]
- 20.Pollard J, Runnels S, Warrick C. First case report of intubation with a total control introducer and a Hyperangulated video laryngoscope. A A Pract. 2020;14:e01310. doi: 10.1213/XAA.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 21.Mahli N, Md Zain J, Mahdi SNM, Chih Nie Y, Chian Yong L, Shokri AFA, Maaya M. The performance of flexible tip Bougie™ in intubating simulated difficult airway model. Front Med (Lausanne) 2021;8:677626. doi: 10.3389/fmed.2021.677626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Qasmi A, Al-Alawi W, Malik AM, Khan RM, Kaul N. Assessment of Truflex articulating stylet versus conventional rigid Portex stylet as an intubation guide with the D-blade of C-Mac videolaryngoscope during elective tracheal intubation: study protocol for a randomized controlled trial. Trials. 2013;14:298. doi: 10.1186/1745-6215-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song L, Tan L, Ma N, Li Q, Zhou M, Hu Y, Liu Y, Chen H, Xu W, Zou Z. Reintubation during COVID-19 pandemic: a simple self-made guiding device facilitates reintubation and minimizes transmission. Am J Transl Res. 2021;13:13811–13814. [PMC free article] [PubMed] [Google Scholar]
- 24.Shikani AH. New “seeing” stylet-scope and method for the management of the difficult airway. Otolaryngol Head Neck Surg. 1999;120:113–116. doi: 10.1016/S0194-5998(99)70380-3. [DOI] [PubMed] [Google Scholar]
- 25.Evans H, Hodzovic I, Latto I. Tracheal tube introducers: choose and use with care. Anaesthesia. 2010;65:859. doi: 10.1111/j.1365-2044.2010.06432.x. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg D, Pliskin J. Adoption and use of new medical technology at the hospital level. Risk Management. 2008;10:120–134. [Google Scholar]
- 27.Medlinskiene K, Tomlinson J, Marques I, Richardson S, Stirling K, Petty D. Barriers and facilitators to the uptake of new medicines into clinical practice: a systematic review. BMC Health Serv Res. 2021;21:1198. doi: 10.1186/s12913-021-07196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murgu SD. Robotic assisted-bronchoscopy: technical tips and lessons learned from the initial experience with sampling peripheral lung lesions. BMC Pulm Med. 2019;19:89. doi: 10.1186/s12890-019-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asamani JA, Alugsi SA, Ismaila H, Nabyonga-Orem J. Balancing equity and efficiency in the allocation of health resources-where is the middle ground? Healthcare (Basel) 2021;9:1257. doi: 10.3390/healthcare9101257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grassano N, Hernandez Guevara H, Fako P, Tuebke A, Amoroso S, Georgakaki A, Napolitano L, Pasimeni F, Rentocchini F, Compano R. The 2021 eu industrial R&D investment scoreboard. EUR. 2021 [Google Scholar]
- 31.Wu X, Zeng S. R&D investment, internal control and enterprise performance-an empirical study based on the listed companies in China of the core industry of the digital economy. Sustainability. 2022;14:16700. [Google Scholar]
- 32.Price M, Lau F. The clinical adoption meta-model: a temporal meta-model describing the clinical adoption of health information systems. BMC Med Inform Decis Mak. 2014;14:43. doi: 10.1186/1472-6947-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan HS, Corey T, Luk HN, Qu JZ, Shikani A. Combined styletubation with videolaryngoscopy for tracheal intubation in patients undergoing thyroidectomy with intraoperative neuromonitoring. Anesth Res. 2023;1:8–23. [Google Scholar]
- 34.Matek J, Kolek F, Klementova O, Michalek P, Vymazal T. Optical devices in tracheal intubation-state of the art in 2020. Diagnostics (Basel) 2021;11:575. doi: 10.3390/diagnostics11030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu A, Wang Y, Shu X, Moritz D, Cui W, Zhang H, Zhang D, Qu H. Ai4vis: survey on artificial intelligence approaches for data visualization. IEEE Trans Vis Comput Graph. 2022;28:5049–5070. doi: 10.1109/TVCG.2021.3099002. [DOI] [PubMed] [Google Scholar]
- 36.Janakiraman C, Hodzovic I, Reddy S, Desai N, Wilkes AR, Latto IP. Evaluation of tracheal tube introducers in simulated difficult intubation. Anaesthesia. 2009;64:309–314. doi: 10.1111/j.1365-2044.2008.05745.x. [DOI] [PubMed] [Google Scholar]
- 37.Cook TM, Lee G, Nolan JP. The ProSeal laryngeal mask airway: a review of the literature. Can J Anaesth. 2005;52:739–760. doi: 10.1007/BF03016565. [DOI] [PubMed] [Google Scholar]
- 38.Schmutz BP, Santerre RE. Examining the link between cash flow, market value, and research and development investment spending in the medical device industry. Health Econ. 2013;22:157–167. doi: 10.1002/hec.1825. [DOI] [PubMed] [Google Scholar]
- 39.Sertkaya A, DeVries R, Jessup A, Beleche T. Estimated cost of developing a therapeutic complex medical device in the US. JAMA Netw Open. 2022;5:e2231609. doi: 10.1001/jamanetworkopen.2022.31609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maci J, Marešová P. Critical factors and economic methods for regulatory impact assessment in the medical device industry. Risk Manag Healthc Policy. 2022;15:71–91. doi: 10.2147/RMHP.S346928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drummond MF, Tarricone R, Torbica A. Economic evaluation of medical devices. 2018 [Google Scholar]
- 42.Stern AD. Innovation under regulatory uncertainty: evidence from medical technology. J Public Econ. 2017;145:181–200. doi: 10.1016/j.jpubeco.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Windecker S, Gilard M, Achenbach S, Cribier A, Delgado V, Deych N, Drossart I, Eltchaninoff H, Fraser AG, Goncalves A, Hindricks G, Holborow R, Kappetein AP, Kilmartin J, Kurucova J, Lüscher TF, Mehran R, O’Connor DB, Perkins M, Samset E, von Bardeleben RS, Weidinger F. Device innovation in cardiovascular medicine: a report from the European Society of Cardiology Cardiovascular Round Table. Eur Heart J. 2024;45:1104–1115. doi: 10.1093/eurheartj/ehae069. [DOI] [PubMed] [Google Scholar]
- 44.Kamalov F, Pourghebleh B, Gheisari M, Liu Y, Moussa S. Internet of medical things privacy and security: Challenges, solutions, and future trends from a new perspective. Sustainability. 2023;15:3317. [Google Scholar]
- 45.Huusko J, Kinnunen UM, Saranto K. Medical device regulation (MDR) in health technology enterprises - perspectives of managers and regulatory professionals. BMC Health Serv Res. 2023;23:310. doi: 10.1186/s12913-023-09316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maresova P, Rezny L, Peter L, Hajek L, Lefley F. Do regulatory changes seriously affect the medical devices industry? Evidence from the Czech Republic. Front Public Health. 2021;9:666453. doi: 10.3389/fpubh.2021.666453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang HH, Xu J. Research on the impact of policy and regulation changes on medical device industry. Medical and Health Equipment. 2018;39:77–81. [Google Scholar]
- 48.Jiang HH, Fang Y. On the revision and impact of the medical device supervision and administration regulations in 2021. Medical and Health Equipment. 2022;43:1–5. [Google Scholar]
- 49.Cheong ST, Li J, Ung COL, Tang D, Hu H. Building an innovation system of medical devices in China: drivers, barriers, and strategies for sustainability. SAGE Open Med. 2020;8:2050312120938218. doi: 10.1177/2050312120938218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan AV, Baim DS, Smith JJ, Feigal DA, Simons M, Jefferys D, Fogarty TJ, Kuntz RE, Leon MB. Medical device development: from prototype to regulatory approval. Circulation. 2004;109:3068–3072. doi: 10.1161/01.CIR.0000134695.65733.64. [DOI] [PubMed] [Google Scholar]
- 51.Jacinto MJ, Oliveira P, Canhão H. Innovations developed by patients and informal caregivers for needs associated to rheumatic diseases. Front Med (Lausanne) 2021;8:647388. doi: 10.3389/fmed.2021.647388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malkin RA. Barriers for medical devices for the developing world. Expert Rev Med Devices. 2007;4:759–763. doi: 10.1586/17434440.4.6.759. [DOI] [PubMed] [Google Scholar]
- 53.He J. Concept of smart regulation on medical devices under new situation. Zhongguo Yi Liao Qi Xie Za Zhi. 2019;43:43–47. doi: 10.3969/j.issn.1671-7104.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Zhu J, Fang Y, Yang H. Risk management analysis of medical device registration self-inspection. Zhongguo Yi Liao Qi Xie Za Zhi. 2023;47:545–549. doi: 10.3969/j.issn.1671-7104.2023.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Xing F, Peng G, Zhang B, Li S, Liang X. Socio-technical barriers affecting large-scale deployment of AI-enabled wearable medical devices among the ageing population in China. Technol Forecast Soc Change. 2021;166:120609. [Google Scholar]
- 56.Li J, Liu L, Cao H, Yang M, Sun X. Use of real-world evidence to support regulatory decisions on medical devices in China and a unique opportunity to gain accelerated approval in “Boao Lecheng Pilot Zone”. Cost Eff Resour Alloc. 2023;21:7. doi: 10.1186/s12962-022-00412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji Q, Chen M, Zhang S. Introduction and revelation of conditional approval of medical devices in the United States. China Pharmacovigilance. 2023;20:1007–1010. 1016. [Google Scholar]
- 58.Zhong D, Kirwan MJ, Duan X. Regulatory barriers blocking standardization of interoperability. JMIR Mhealth Uhealth. 2013;1:e13. doi: 10.2196/mhealth.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun XY, Yao J. Research on the current situation of operation and development of medical device industry and response strategy. 2022. 2:172–174. [Google Scholar]
- 60.Lim K, Heo TY, Yun J. Trends in the approval and quality management of artificial intelligence medical devices in the Republic of Korea. Diagnostics (Basel) 2022;12:355. doi: 10.3390/diagnostics12020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Y. Reflections on the development of China’s medical device industry under the background of new medical reform. China Medical Device Information. 2022;28:46–48. [Google Scholar]