Abstract
Acute lung injury (ALI) is defined as the acute onset of diffuse bilateral pulmonary infiltration, leading to PaO2/FiO2 ≤ 300 mmHg without clinical evidence of left atrial hypertension. Acute respiratory distress syndrome (ARDS) involves more severe hypoxemia (PaO2/FiO2 ≤ 200 mmHg). Treatment of ALI and ARDS has received renewed attention as the incidence of ALI caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has increased. Itaconate and its derivatives have shown therapeutic potential against ALI. This review provides an in-depth summary of the mechanistic research of itaconate in the field of acute lung injury, including inducing autophagy, preventing ferroptosis and pyroptosis, shifting macrophage polarization to an anti-inflammatory M2 phenotype, inhibiting neutrophil activation, regulating epigenetic modifications, and repressing aerobic glycolysis. These compounds merit further consideration in clinical trials. We anticipate that the clinical translation of itaconate-based drugs can be accelerated.
Keywords: Itaconate, inflammation, macrophage polarization, neutrophil, acute lung injury
Introduction
Acute lung injury (ALI) is a serious condition characterized by the progressive breakdown of alveolar permeability and the accumulation of severe alveolar edema [1,2]. It is typically caused by viral or bacterial infections, such as sepsis, leading to inflammation and damage in the lungs. If left untreated, ALI can progress to acute respiratory distress syndrome (ARDS), a life-threatening condition with a high mortality rate [3]. The recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has heightened concerns about the risk of mortality from ARDS [4]. Current treatment strategies for ALI include restrictive fluid management, respiratory support therapy, and pharmacological interventions [5]. While glucocorticoids and pulmonary vasodilators have been used in clinical settings, their effectiveness is limited, prompting the need for more potent drugs to combat ALI. Inflammatory responses, immune cells, and the integrity of the pulmonary epithelial vascular barrier play crucial roles in the development and progression of ALI.
Although the exact cause of ALI remains unclear, research has revealed that various signaling pathways and molecules are implicated in its development. These include phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), NLRP3 inflammasome, nuclear factor-κB (NF-κB), JAK/STAT, and P38 mitogen-activated protein kinase (MAPK). In ALI, the regulation of autophagy through the PI3K/AKT/mTOR signaling pathway plays a crucial role in reducing lung injury and facilitating lung tissue repair [6]. This pathway can also activate the antioxidant pathway to reduce oxidative stress. Overactivation of the NLR family pyrin domain 3 (NLRP3) inflammasome increases the permeability of alveolar epithelial cells, promotes the formation of pulmonary edema, exacerbates lung tissue injury, and influences the infiltration and activation of neutrophils by regulating the production of inflammatory mediators [7]. NF-κB is activated by cell surface receptor signaling, leading to the phosphorylation and degradation of IκB (NF-κB inhibitory protein). This releases NF-κB into the nucleus where it binds to DNA and initiates the transcription of inflammatory genes, including TNF-α, IL-6, and IL-8 [8,9]. Promotion of the expression of inflammatory factors exacerbates the lung inflammatory response. Activation of the JAK/STAT signaling pathway induces an inflammatory response in acute lung injury. JAK1, a key component of the JAK-STAT signaling pathway, negatively regulates inflammation by controlling JAK1 [10]. The p38 MAPK signaling pathway is activated to release cytokines, recruit inflammatory cells, and promote apoptosis in ALI [11]. Inhibition of the p38 MAPK pathway may reduce the inflammatory response of ALI. It is evident that a variety of signaling pathways, molecules, and genes are activated during acute lung injury, forming a complex network of regulation. Targeting inflammation, immune cells, and the pulmonary epithelial vascular barrier, along with corresponding pathways, molecules, and genes using modulatory drugs, has emerged as a strategy for treating ALI.
Itaconate, a metabolic intermediate produced in the mitochondrial matrix by the enzyme aconitate decarboxylase encoded by immune-responsive gene 1 (Irg1), has recently garnered significant attention for its diverse applications and potential therapeutic prospects in clinical settings [12]. This immune metabolite serves as a signaling molecule that impacts various cellular metabolic and functional processes, including glycolysis and the tricarboxylic acid (TCA) cycle [13,14]. Recent research has highlighted its anti-inflammatory and antioxidant properties, with key cells involved being macrophages, neutrophils, and lung epithelial endothelial cells. Important physiologic processes influenced by itaconate include autophagy, pyroptosis, ferroptosis, epigenetic modifications, and glycolysis. The molecular mechanisms at play involve the nuclear factor (NF)-κB inhibitor Zeta (IκBζ), the cyclic adenosine monophosphate (AMP)-dependent transcription factor ATF-3 [8], the kelch-like epichloropropane (ECH)-associated protein 1 (KEAP1), the nuclear factor erythroid 2-related factor 2 (NRF2) [15], interferon gene stimulating factor (STING) [16], and the neutralization of reactive oxygen species (ROS) [17]. Given the mechanism of ALI and the role of itaconate, compounds containing itaconate and its derivatives may represent a promising therapeutic approach for ALI.
Currently, various itaconate derivatives, such as 4-octyl itaconate, dimethyl itaconate, and 4-ethyl itaconate, have shown promise in preclinical studies for treating ALI, although they have not yet advanced to clinical trials. For instance, these derivatives have demonstrated effectiveness in reducing the viral load of SARS-CoV-2 in human airway epithelial cells [18]. The therapeutic potential of itaconate and its derivatives is uncertain, requiring further research efforts to facilitate their clinical application. Therefore, a timely summary of recent mechanistic research on itaconate and its derivatives is crucial for advancing ALI treatment. This review begins by outlining the inflammatory response mechanism in ALI progression, evaluates the therapeutic potential of different compounds, and provides perspectives on treating ALI. Lastly, the review delves into the latest insights on the mechanisms of action and efficacy of itaconate and its derivatives acting against ALI.
Inflammation plays a crucial role in the development of ALI
The pathogenesis of ALI involves a complex interplay of various cells and molecules. Research has shown that lung inflammation, triggered by inhibited autophagy [19], pyroptosis [20], and ferroptosis [21], as well as epigenetic modifications [9], glycolysis [22], and the activation of macrophages [20,23] and neutrophils [20,24], plays a critical role in the development of ALI. Inducing autophagy can help remove harmful substances and reduce cell damage and inflammation, while inhibiting pyroptosis and ferroptosis can decrease cytokine release and alleviate lung injury [25]. Additionally, promoting macrophage activation and inhibiting neutrophil activation can help to suppress inflammation and mitigate lung injury [26].
Inflammation is a key early response to lung infection, injury or irritation. When the lungs are injured or infected, innate immune cells in the lung, such as macrophages, neutrophils and lymphocytes, recognize pathogen-associated molecular patterns (PAMPs) or microbial-associated molecular patterns (MAMPs) through surface or intracellular pattern recognition receptors (PPP) in order to defend against foreign pathogens [27]. PPPs include, for example, Toll-like receptors (TLR) [28], NOD-like receptors (NLR) [29] and the intracellular DNA sensor-associated Cyclic GMP-AMP (cGAMP) synthase (cGAS)-STING signaling pathway [30]. In this process, these immune cells kill pathogens or remove necrotic tissue by phagocytosis, release cytokines, and produce ROS, but at the same time, they damage the alveolar-capillary barrier [31], resulting in necrosis, hemorrhage and edema of lung tissue. Various inflammatory mediators released after the activation of inflammatory cells (such as cytokines, chemokines, proteases, free radicals) can amplify the inflammatory response, regulate the permeability of blood vessels, attract more inflammatory cells, and increase the inflammatory load on the lungs [4,32].
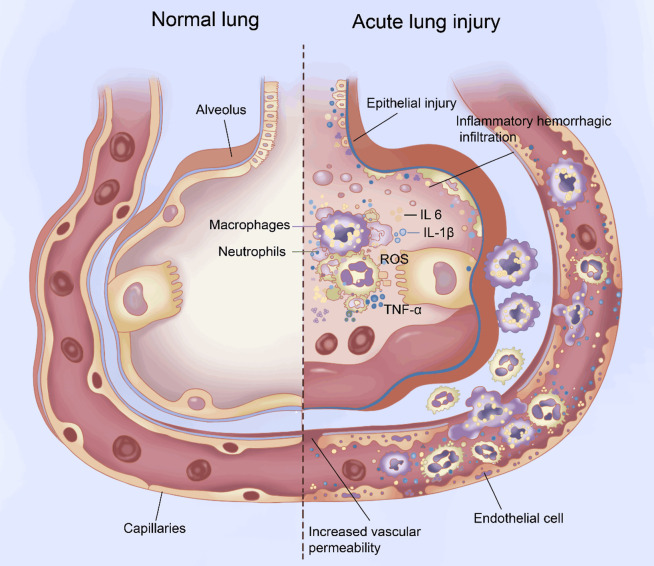
Under normal circumstances, the inflammatory response is typically limited and reversible. When the inflammatory stimulus is removed, the inflammatory cells and mediators gradually diminish, allowing the lung tissue to return to its normal state, sometimes with scarring [33]. However, in certain cases, the inflammatory response becomes imbalanced, persistent, or recurrent, leading to the disruption of the alveolar-capillary barrier. This damage affects the structure and function of the lungs, further progressing to ALI and possibly even ARDS. Excessive lung inflammation and the release of inflammatory factors such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, and ROS play crucial roles in the pathophysiologic changes seen in ALI, along with the disruption of the alveolar-capillary barrier (Figure 1).
Figure 1.
Pathophysiologic changes during acute lung injury (ALI). Changes involve oxidative stress due to reactive oxygen species (ROS); leukocyte activation; excessive and uncontrolled inflammatory responses mediated by interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α; and injury of the pulmonary epithelium and vascular endothelium.
Lung inflammation and immune cells
Macrophages, neutrophils, and other immune cells in the lung are critical for maintaining homeostasis. Macrophages and neutrophils defend against microbial invasion. Macrophages make up 90-95% of immune cells in the lung that maintain homeostasis [34]. Macrophages are able to recognize and endocytose pathogens, dead cells, and foreign bodies through phagocytic receptors on their surface, thereby removing debris from the body. Neutrophils are another important innate immune cells in the lung [24]. Activated neutrophils cause inflammatory responses and tissue damage by producing toxic molecules and cytokines.
Macrophages can be classified as either alveolar or interstitial macrophages, based on their location in the lung. Alveolar macrophages serve as first-line defense cells and play a crucial role in initiating the immune response. When receptors detect damage-associated molecular patterns (DAMPs) or PAMPs, alveolar macrophages and monocytes recruited from the peripheral blood are polarized into M1 macrophages through the activation of specific signaling pathways that regulate NF-κB, mitogen-activated protein kinase, and NLRP3, as well as through the promotion of glycolysis [35]. Activation of the NLRP3 inflammasome can lead to pyroptosis. Factors secreted by M1 macrophages, such as macrophage inflammatory protein-2 (MIP-2), TNF-α, IL-1β, and IL-8, can recruit monocytes and neutrophils, promote lung inflammation, and ultimately contribute to the development of ALI and ARDS. In contrast, M2 macrophages in the later stages of ALI secrete anti-inflammatory and pro-angiogenic factors, as well as phagocytic cells, to facilitate tissue remodeling. Therefore, inhibiting the pro-inflammatory macrophage response may be an effective strategy for reducing pulmonary inflammation. This can be achieved by inhibiting NLRP3 inflammasome activation to prevent pyroptosis, promoting the polarization of macrophages from M1 to M2, and inhibiting glycolysis.
In the early stage of pulmonary inflammation, neutrophils are the first cells recruited to the site of alveolar inflammation through the endothelial and epithelial cell barriers. Activated neutrophils release cytotoxic and immune cell activators, such as chemokines [36], cationic peptides, ROS, and serine proteases; or they form neutrophil extracellular traps (NETs) to capture pathogens. NET formation induces lung epithelial damage, leading to severe lung inflammation. NET also promotes ARDS by regulating macrophage polarization. Neutrophil-derived exosome miR-30d-5p was shown to promote sepsis-associated ALI by activating NF-κB signaling, inducing macrophage M1 polarization and triggering macrophage pyroptosis [20]. NET levels in ARDS patients are positively correlated with M1 macrophage polarization. NET inhibitors can significantly downregulate M1 macrophage markers such as inducible nitric oxide synthase, as well as macrophage M2 markers like CD206 and arginase1 (Arg1). However, at later stages after lung injury, neutrophils can promote lung tissue repair by phagocytizing cell debris, promoting tissue neovascularization, and secreting lipolysis mediators that alleviate lung inflammation. Therefore, when considering the role of itaconate in the regulation of neutrophils in ALI, the timing of itaconate intervention must be carefully considered. It should be used at the early stage of inflammation.
Lung inflammation and the alveolar-capillary barrier
The alveolar-capillary barrier is composed of the alveolar epithelium, the alveolar capillary endothelium, and the alveolar basement membrane, which is rich in collagen and laminins [37]. Endothelial cells play a crucial role in this barrier as they act as transport devices for immune cells and provide a mechanical barrier against invaders. Additionally, endothelial cells have a paracrine function by expressing adhesion molecules and chemokines to facilitate the recruitment of immune cells and regulate the extravasation of leukocytes into inflamed areas [37].
Normal resting endothelial cells inhibit neutrophil activation, chemotaxis, and adhesion by expressing and secreting anti-inflammatory factors, such as nitric oxide, prostaglandins, IL-10, and transforming growth factor-beta (TGF-β), which inhibit neutrophil activation, chemotaxis, and adhesion [37]. The alveolar-capillary barrier is crucial for maintaining the integrity of the respiratory system. Normal resting endothelial cells play a key role in this barrier by ensuring the tight junctions and adhesive linkages are intact, which prevents the crossing of neutrophils. Additionally, endothelial cells have the ability to remove damaged or over-activated cells through autophagy, thus preventing the release of inflammatory mediators and cellular debris that could trigger an inflammatory response. These endothelial cells also regulate clotting, control blood flow, and facilitate the movement of proteins from the blood into tissues. By maintaining these functions, normal resting endothelial cells help to inhibit inflammation and maintain the overall health of the alveolar-capillary barrier [37]. The dysfunction of pulmonary vascular endothelial cells eventually leads to massive infiltration of monocytes and neutrophils, tissue damage, and organ dysfunction.
In the early stages of inflammation in ALI, activated monocytes and neutrophils, as well as a disrupted alveolar-capillary barrier, play a significant role in pro-inflammatory responses, leading to lung inflammation and injury. Additionally, intracellular inhibition of autophagy, excessive pyroptosis and ferroptosis, epigenetic modifications, glycolysis, macrophage M1 polarization, and activated neutrophils also contribute to ALI and lung dysfunction. Therefore, targeting the function of these immune cells and the associated inflammatory molecules may be a promising approach for the treatment of ALI.
Functions of itaconate and its derivatives
Introduction to itaconate
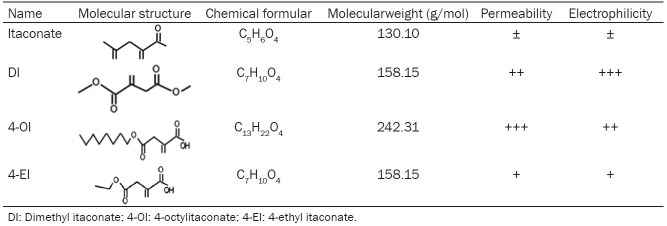
Itaconate is a five-carbon dicarboxylic acid (C5H6O4) with an α,β-unsaturated bond that can accept an electron pair to bond with a nucleophile, facilitating derivatization [38]. Itaconate inactivates proteins by covalently bonding to their cysteine residues through Michael addition. Its structure and chemical formula are shown in Table 1.
Table 1.
Chemical structure and properties of itaconate and its derivatives
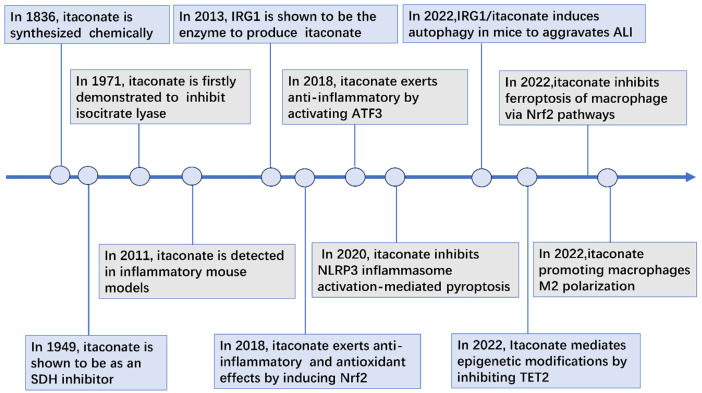
Itaconate was first discovered as a product of citrate distillation through chemical method in 1836 [12]. Until in the early 1970s, itaconate was found to be an anti-bacteria effect through inhibition bacterial enzyme isocitrate lyase (ICL), the key enzyme of an essential pathway (glyoxylate shunt) for bacterial growth, on Mycobacteria and Salmonella in vivo [39-42]. Since 1995, the role of itaconate in innate immunity was identified in Mycobacterium tuberculosis-infected murine lungs and in lipopolysaccharide -stimulated macrophages [43-46]. Later, in 2013, aconitate decarboxylase 1 (ACOD1) was confirmed as the enzyme catalyzed the production of itaconate via the decarboxylation of cis-aconitate [43]. Its antioxidant and anti-inflammatory effects through activation of NRF2 were demonstrated in 2018 [15]. Its anti-inflammatory effects by activating ATF3 were also confirmed in 2018 [8]. Subsequently, itaconate induced autophagy [47], inhibited NLRP3 inflammasome activation-mediated pyroptosis [48], inhibited ferroptosis of macrophage by NRF2 pathways [49] and mediated epigenetic modifications by inhibiting TET2 [9]. Additionally, itaconate promoting macrophages M2 polarization was also confirmed in 2022 [50]. The main findings are presented in Figure 2.
Figure 2.
A timeline of significant discoveries in itaconate. Itaconate was first found to be a citrate distillation product through chemical methods in 1836 [12]. Itaconate was demonstrated to be as an SDH inhibitor in 1949 [105] and posess antimicrobial properties by inhibiting isocitrate lyase in 1971 [40]. Then in 2011 [46], itaconate was subsequently discovered in some inflammatory models, and the enzyme IRG1 was finally confirmed to produce itaconate in 2013 [43]. Its antioxidant and anti-inflammatory effects through activation of NRF2 were demonstrated in 2018 [15]. Its anti-inflammatory effects by activating ATF3 were also confirmed in 2018 [8]. Subsequently, itaconate induced autophagy [47], inhibited NLRP3 inflammasome activation-mediated pyroptosis [48], inhibited ferroptosis of macrophage via NRF2 pathways [49] and mediated epigenetic modifications by inhibiting TET2 [9]. Additionally, itaconate promoting macrophages M2 polarization was also confirmed in 2022 [50].
Itaconate derivatives
The relatively strong polarity and weak electrophilicity of the parental itaconate prevents it from crossing cell membranes, making its derivatives dimethyl itaconate, 4-octylitaconate, and 4-ethyl itaconate potentially more useful as drugs. Dimethyl itaconate is recognized as the “powered-up” version of itaconate [51,52] and its molecular structure is C7H10O4 (Table 1). Dimethyl itaconate is esterified at the carboxyl group at position 1, which strengthens electrophilicity and membrane permeability [53]. Dimethyl itaconate can prevent lipopolysaccharide from inducing expression of IκBζ, an inhibitor of NF-κB transcription factors, and from depleting intracellular levels of glutathione [8]. Its usefulness as a drug is reduced by the fact that it does not convert to itaconate within cells and is rapidly degraded [54,55].
The derivative 4-ethyl itaconate is more weakly electrophilic than dimethyl itaconate and crosses cell membranes less easily, of which molecular structure is C13H22O4 (Table 1) [56]. Because only the 4-carboxyl group is esterified, the derivative does not inhibit IκBζ [8]. More similar to itaconate is 4-octylitaconate, whose long carbon chain makes it weakly electrophilic and able to cross cell membranes like dimethyl itaconate [56]. It resists degradation by esterases, while its hydrolysis by other enzymes produces itaconate in the presence or absence of lipopolysaccharide [57].
4-ethyl itaconate is also a high membrane permeability itaconate derivative, of which molecular structure is C7H10O4 (Table 1) [16]. 4-ethyl itaconate has a similar structure to dimethyl itaconate, but with lower electrophilicity and higher polarity. Because only 4-carboxyl of dimethyl itaconate is esterified, it has no inhibitory effect on IκBζ [31]. To date, there are few literatures mentioning 4-ethyl itaconate [29].
Itaconate and its derivatives play a significant role in the treatment of inflammatory and immune-related diseases. Conditions such as ALI, psoriasis, rheumatoid arthritis, systemic lupus erythematosus (SLE), multiple sclerosis (MS), and inflammatory bowel disease (IBD) can benefit from the therapeutic properties of itaconate [58,59]. This study specifically focuses on the mechanisms underlying its role in ALI.
Therapeutic mechanisms of itaconate and its derivatives against ALI
Activating autophagy
Inducing autophagy can mitigate tissue injury associated with ALI [6]. The entire process of autophagy, through which damaged cellular components are destroyed in order to maintain cellular integrity, is regulated by different autophagy associated proteins [19,60]. Microtubule-associated protein 1A/1B- light chain 3 (LC3) is a protein involved in autophagy: it couples with phosphatidylethanolamine to interact with the membrane and participate in the formation and maturation of autophagosomes [19]. LC3-I is the soluble form of LC3 found in the cytoplasm, while LC3-II is the lipidized membrane-bound form of LC3 located on the inner and outer membranes of the autophagosome. The transition between LC3-I and LC3-II is a crucial process in autophagy and serves as a distinct indicator of autophagic activity [60]. It is widely accepted that changes in the LC3-II/I ratio can either stimulate or suppress autophagy activity. Additionally, p62 is recognized as a marker for the autophagy degradation process. In the autophagy process, misfolded proteins labeled with ubiquitin are linked to p62, forming aggregates that are then engulfed and broken down. Consequently, the buildup of p62 indicates a hindrance in the autophagy degradation process.
Inducing autophagy can enhance the phagocytic function of macrophages and reduce lung inflammation [19]. For example, an inhibitor of the anti-apoptotic protein Bcl-2, ABT-263, enhanced bacterial phagocytosis of macrophages in mice by inducing beclin-1 dependent autophagy, thereby preventing sepsis in mice [61]. In addition, autophagy can promote the polarization of macrophages from M1 type into M2 type and reduce the release of inflammatory factors in the acute phase [62]. Itaconate has been shown to induce autophagy in macrophages. In a study involving ALI mice, itaconate was found to induce autophagy in microglia that had been treated with lipopolysaccharide to mimic inflammatory processes. The compound upregulated proteins that promote autophagy, such as LC3 and beclin-1, while downregulating the autophagy inhibitor p62 [47]. In an animal model of osteoarthritis, the itaconate derivative 4-octylitaconate was found to induce autophagy through a mechanism involving the downregulation of p62. This compound inhibited the PI3K/AKT/mTOR signaling pathway, leading to the upregulation of LC3 and beclin-1 while simultaneously downregulating p62 in chondrocytes [6].
There is still much to clarify about how itaconate and its derivatives induce autophagy. Itaconate is known to activate transcription factor EB (TFEB) by directly alkylating a cysteine in the protein (Cys212 in humans, Cys270 in mice), which prevents phosphorylation of a specific serine (Ser211 in humans, Ser269 in mice) [63,64]. This prevents the kinase mTORC1 from phosphorylating the serine to inactivate TFEB. Consequently, the constitutively active transcription factor induces the expression of several genes that promote the biogenesis and activity of lysosomes, thereby enhancing lysosomal autophagy [63,64].
Inhibiting pyroptosis
Pyroptosis, another non-apoptotic form of cell death, also plays a role in the development and progression of ALI [20,65]. One driver of pyroptosis is the activation of inflammatory responses involving the NLRP3 inflammasome. Itaconate and its derivative, 4-octylitaconate, have been shown to inhibit the NLRP3 inflammasome in macrophages, thereby reducing the production of pro-inflammatory cytokines [48]. In the case of 4-octylitaconate, the inhibition of pyroptosis appears to involve the alkylation of a specific cysteine in NLRP3 (Cys548 in HEK293T cells), which prevents its binding to NIMA-related kinase 7 and subsequently reduces the expression of the pro-inflammatory cytokine IL-1β [66].
The derivative 4-octylitaconate has also been shown to inhibit pyroptosis through downregulation of signaling involving NF-κB and MAP kinases in RAW264.7 and mouse bone marrow-derived macrophages [67] or signaling involving STING and IRF3 in a mouse model of ARDS [68]. Li et al. [16] showed that 4-octylitaconate could alkylate cysteine147 in STING in HEK293T cells, inhibiting STING phosphorylation and reducing the production of the pro-inflammatory factors IFN-β, TNF-α, IL-1b, and IL-6. The derivative was also found to alkylate cysteine 91 in STING and limit STING activation by blocking its palmitoylation [69]. Other work showed that it inhibited the protective effects of caspase1/gasdermin D (GSDMD) and TNF-α-induced caspase3/gasdermin E (GSDME)-mediated pyroptosis in a mouse model of acute colitis [67]. GSDMD and GSDME help drive pyroptosis. Bambuskova et al. demonstrated that itaconate prevents the activation of caspase-1 and the processing of GSDMD by regulating the cysteine77 in GSDMD in macrophages, preventing pyroptosis [70].
Several additional mechanisms through which itaconate and its derivatives act on the NLRP3 inflammasome have been reported. For example, the derivative 4-octylitaconate has been shown to induce the transcription factor NRF2, which in turn activates the expression of genes controlled by the antioxidant response element. These genes include those encoding glutathione peroxidase 4, NAD(P)H dehydrogenase, and heme oxygenase, effectively neutralizing mitochondrial ROS [71]. Under normal conditions, NRF2 is maintained inactive in the cytoplasm through binding to Kelch-like ECH-associated protein 1. However, 4-octylitaconate alkylates this protein at cysteine 257, 288, and 273 in the human protein, leading to the activation of NRF2 and its translocation into the nucleus [15]. Under normal conditions, NRF2 is maintained at low levels by its interaction with KEAP1, resulting in its degradation and the liberation of NRF2. The free NRF2 then translocates into the nucleus, where it activates genes that express proteins responsible for reducing oxidative stress and inflammation. In a mouse model of allergic airway inflammation, itaconate was found to inhibit the activation of the NLRP3 inflammasome, decrease mitochondrial ROS release, and regulate mitochondrial fusion/fission, ultimately reducing the inflammatory response in the airway by modulating mitochondrial metabolism and morphology [72].
Inhibiting ferroptosis
Ferroptosis may be associated with the development of ALI [73,74], and inhibiting it with ferrostatin-1 significantly mitigates lung injury in the human bronchial epithelial cell line BEAS-2B [75]. In ferroptosis, cells die as a result of iron-dependent lipid peroxidation and accumulation of ROS [76], which damage membranes. Exposing a mouse model of ALI to lipopolysaccharide exacerbated these processes in bronchial epithelium by increasing iron levels in lung tissues and reducing levels of glutathione and activity of glutathione peroxidase 4 [75], which normally neutralize ROS and repair membrane damage [77,78].
Itaconate has been shown to alleviate ALI by inhibiting ferroptosis. This mechanism is believed to be related to the activation of the NRF2 pathway, an increase in glutathione levels, inhibition of ROS production, reduction of lipid peroxidation, and a decrease in the release of inflammatory factors [79]. For example, when human THP-1 monocytes were treated with 4-octylitaconate followed by lipopolysaccharide, there was an upregulation of glutathione peroxidase 4, the transcription factor NRF2, and the cysteine/glutamate transporter SLC7A11. This suggests a greater capacity to mitigate ferroptosis injury, while also decreasing levels of ROS and the oxidative stress marker malondialdehyde [49]. In a study where Nrf2 was silenced, glutathione peroxidase 4 (GPX4) was downregulated, further supporting the role of NRF2 in this process. Additionally, research has shown that granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce the expression of ACOD1 in neutrophils through the transcription factor C/EBPb, leading to the upregulation of NRF2-dependent antioxidant response genes such as Gpx4, Gclc, and Nqo1, which mediate ferroptosis resistance [80]. These findings collectively suggest that itaconate inhibits ferroptosis by activating the NRF2 pathway.
Its structural similarity to succinate means that itaconate can competitively inhibit succinate dehydrogenase (SDH) and thereby reduce production of ROS in mitochondria [81]. The derivative 4-octylitaconate can also reduce such production by preventing mitochondrial DNA from escaping into the cytosol in alveolar macrophages in response to oxidative stress to improve macrophage pyroptosis and alleviate ARDS in a mouse model [68]. The ability of 4-octylitaconate to reduce levels of ROS in macrophages appears to involve inhibition of signaling p38MAPK [82]. Itaconate may also inhibit ferroptosis by inducing autophagy [47,83]. On the other hand, elevated levels of itaconate may induce ferroptosis by triggering a particular type of autophagy called ferritinophagy [79]. Future studies should clarify whether, and under what conditions, itaconate inhibits or induces ferroptosis and how these effects may contribute to ALI.
Promoting polarization of macrophages to an anti-inflammatory M2 phenotype
Itaconate and its derivatives have been found to shift macrophage polarization from an M1 to M2 phenotype [50,82]. In a mouse model of osteoarthritis, 4-octylitaconate was shown to downregulate the M1 macrophage marker CD68 while upregulating the M2 markers Arg-1 and CD206. These changes were associated with less severe cartilage degeneration and synovial inflammation [50]. In macrophage cultures, 4-octylitaconate was found to downregulate the M1 markers CD86 and inducible nitric oxide synthase while upregulating the M2 markers CD206 and Arg-1 [71].
However, Runtsch et al. [84] reported that derivative 4-octylitaconate could inhibit JAK1 phosphorylation in M2 phenotype macrophages, thereby inhibiting its enzyme activity. The specific mechanism is that derivative 4-octylitaconate can directly modify JAK1 cysteine residues at the main sites of 715, 816, 943 and 1130. Blanco et al. [85] reported that the derivative 4-octylitaconate has been shown to reduce the severity of rat lupus. This effect may be attributed to the increase in CD8+ T cells and Treg cells, as well as the decrease in type I IFN and pro-inflammatory cytokines. By influencing both M1 and M2 macrophages, it is possible that itaconate plays a crucial role in modulating the immune response in acute lung injury (ALI), thereby contributing to the improvement of ALI. Further research is required to elucidate the mechanisms through which itaconate influences macrophage polarization.
Inhibiting neutrophil activation
Neutrophils play a crucial role in promoting acute lung injury. The interactions between itaconate, its derivatives, and neutrophils are currently under investigation. For instance, during trauma, mature circulating neutrophils exhibit high inflammatory activity and have the ability to produce itaconate. They can also transport endogenous itaconate to the bone marrow, thereby stimulating the production of granulocyte/monocyte immune cells in this tissue [86]. Exogenous itaconate can reduce inflammation and heterotopic ossification at injured sites and promote tendon differentiation and recovery [86]. In a mouse model of pulmonary infection by Staphylococcus aureus, itaconate inhibited neutrophil glycolysis and accelerated neutrophil death, it targeted nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and inhibited oxidative phosphorylation of neutrophils [87], and it inhibited neutrophil glycolysis and oxidative phosphorylation [87]. The derivative 4-octylitaconate inhibited the formation of NETs in a normal or fat mouse model by upregulating the expression of NRF2/Heme Oxygenase (HO-1) while downregulating hypoxia-inducible factor 1α (HIF-1α) [88].
Regulating epigenetic modifications
Epigenetic modifications refer to heritable changes in gene expression despite no change in DNA sequence, such as DNA methylation, histone modification. Epigenetic modifications play a significant role in the pathophysiology of ALI/ARDS [89,90]. The derivative 4-octylitaconate prevents the epigenetic regulator enzyme “ten-eleven translocation methylcytosine dioxygenase 2” (TET2) from converting 5-methylcytosine to 5-hydroxymethylcytosine in DNA [9,91]. This modification upregulates the expression of genes involving inflammatory responses [92], so blocking it exerts anti-inflammatory effects, such as downregulation of genes turned on by cytokines through the transcription factors NF-κB [9] and STAT1 [93], releasing less inflammatory cytokines (IL-6, Cxcl9, Cxcl10 and Cxcl11). Aso et al. reported that itaconate plays a significant role in regulating T cell subsets through epigenetic modifications. It inhibits Th17 cell differentiation while promoting Treg cell differentiation. It induces histone demethylation primarily by inhibiting the activity of methionine adenosine transferase and isocitrate dehydrogenase 1 and 2 (IDH1/2) enzymes, ultimately altering the chromatin accessibility of essential transcription factors at Il17a and Foxp3 sites [94]. Additionally, Domínguez-André also reported that itaconate influences histone 3 lysine 27 acetylation [95].
In addition, dysregulated histone deacetylation alters gene expression in ways that compromise the integrity of lung epithelium in ALI, exacerbating injury [96]. Inhibiting histone deacetylases has been shown to alleviate lipopolysaccharide-induced injury to lung endothelial cultures [97]. Valproic acid, for example, inhibits histone deacetylases in ways that lead to Irg1 upregulation, leading in turn to greater production of itaconate, which protects tissue from inflammation and oxidative stress [98]. Future studies should explore whether supplementation with itaconate can similarly protect lung endothelium in ALI.
Inhibiting glycolysis
Appropriate inhibition of glycolysis can slow progression of ALI [99]. For example, treating a mouse model of ALI with the glycolysis inhibitor 2-deoxyglucose significantly alleviated tissue pathology and neutrophil accumulation in the lungs, while downregulating pro-inflammatory factors [99]. Itaconate and its derivatives can inhibit aerobic glycolysis by inhibiting glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the rate-limiting enzyme in the entire glycolytic pathway, as well as inhibiting aldolase A and lactate dehydrogenase [13,100,101]. The inhibition of each enzyme involves the alkylation of cysteine 245 in mouse GAPDH, cysteine 73, and cysteine 339 in mouse aldolase A, and cysteine 84 in mouse lactate dehydrogenase.
Molecular pathway-related genes and their interrelationship
Itaconate has been shown to play important roles in the treatment of acute lung injury through several genes and molecular pathways. One of the key genes involved is Nrf2 (nuclear factor erythroid 2-related factor 2), which is activated by itaconate [102]. Activation of Nrf2 leads to the upregulation of antioxidant genes, helping to reduce oxidative stress and inflammation in the lungs [103]. Another gene is STAT3 (signal transducer and activator of transcription 3), which can be modulated by itaconate to regulate inflammatory responses [104].
In terms of molecular pathways, itaconate can affect the NLRP3 (NOD-like receptor family pyrin domain-containing 3) inflammasome pathway. By inhibiting NLRP3 activation, itaconate can suppress the production of pro-inflammatory cytokines and reduce lung inflammation [105,106]. It can also interact with the Keap1 (Kelch-like ECH-associated protein 1)-Nrf2 pathway to enhance antioxidant defense mechanisms [107]. Additionally, itaconate may impact on mitochondrial function and metabolism, which are crucial in maintaining cellular homeostasis and responding to lung injury [108]. These genes and molecular pathways work together to mediate the therapeutic effects of itaconate in acute lung injury, providing possible targets for therapeutic intervention and a better understanding of the underlying mechanisms.
Conclusion and perspectives
The candidate targets and mechanisms of itaconate and its derivatives suggest their use as a treatment for ALI. Furthermore, the endogenous nature of itaconate in mammalian cells provides an advantage as a novel drug candidate. Numerous studies have demonstrated the therapeutic effects of itaconate in relevant mouse disease models. In both animal and in vitro models, itaconate and its derivatives have been shown to counteract various disease pathways in ALI, including regulated cell autophagy, pyroptosis, ferroptosis, inflammation, oxidative stress, macrophage M2 polarization, neutrophil activation, epigenetic modifications, and glycolysis (as summarized in Figure 3 and Table 2). However, to expedite the progression of these compounds into clinical trials, several key questions must be addressed. The safety profile of itaconate and its derivatives for clinical use requires thorough evaluation. Additionally, the optimal concentration range, timing, and duration of administration need to be investigated. Given that itaconate and its derivatives have the potential to target multiple proteins, there is a heightened risk of toxicity and adverse reactions, underscoring the importance of elucidating their pharmacokinetics and pharmacodynamics in future studies.
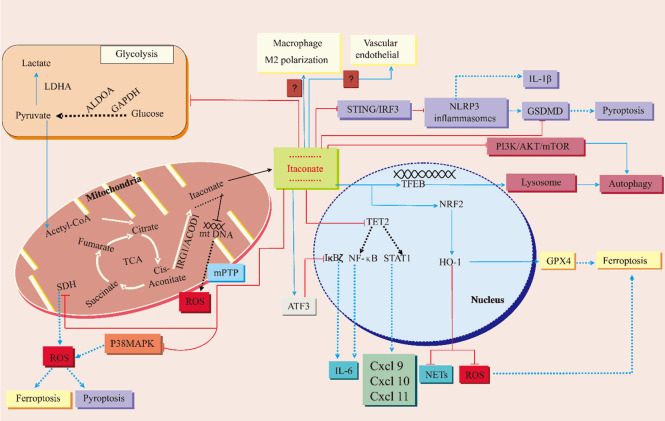
Figure 3.
Biosynthesis of itaconate and its therapeutic mechanisms against acute lung injury. The enzyme aconitate decarboxylase (AOCD1), encoded by the immune response gene 1 (Igr1), is responsible for generating itaconate through the decarboxylation of cis-aconitate in the mitochondrial matrix. Itaconate has been shown to induce autophagy by inhibiting signaling pathways involving phosphoinositide 3-kinase (PI3K), AKT, and mTOR, as well as by activating the transcription factor EB (TFEB). Additionally, itaconate inhibits the STING/IRF3 pathway, leading to the inhibition of the NLRP3 inflammasome and ultimately suppressing pyroptosis. It also reduces pyroptosis by decreasing the accumulation of reactive oxygen species (ROS) and downregulating gasdermin D (GSDMD). Itaconate further inhibits ferroptosis by upregulating the transcription factor NRF2, which in turn increases the expression of heme oxygenase (HO)-1 and glutathione peroxidase 4 (GPX4). It inhibits succinate dehydrogenase (SDH) to reduce ROS production in mitochondria, suppresses p38 MAP kinase signaling, and prevents mitochondrial DNA (mtDNA) from escaping into the cytosol through mitochondrial permeability transition pores (mPTP). Itaconate also plays a role in shifting macrophage polarization from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype, although the exact mechanisms involved are not fully understood. This polarization helps mitigate injury to the vascular endothelium in the lung. Furthermore, it exerts anti-inflammatory effects by activating the transcription factor ATF3, which inhibits IκBζ and downregulates IL-6, by inhibiting NLRP3 inflammasome activation and subsequent downregulation of IL-1β, and by upregulating NRF2 and HO-1 to suppress the formation of neutrophil extracellular traps (NETs). Itaconate downregulates the expression of IL-6, Cxcl9, Cxcl10, and Cxcl11, as well as the glycolytic enzymes GAPDH, ALDOA, and LDHA, by inhibiting the “ten-eleven translocation methylcytosine dioxygenase 2” (TET2) and histone deacetylases. The red terminator indicates that the event is prevented. The blue dashed line indicates the post-termination event effect. The solid blue line shows the post-event effect.
Table 2.
Summary of targets of action of itaconate and its derivatives
| Compound name | Physiologic processes involved | Cells or molecules involved | Targets or mechanisms of action | Effect | References |
|---|---|---|---|---|---|
| Itaconate/4-OI | Autophage | PI3K/AKT/mTOR | Inhibited PI3K/AKT/mTOR pathway | Induced autophagy | [6] |
| TFEB | Alkylated TFEB at cysteine 212 in humans, cysteine 269 in mice | Enhanced lysosomal autophagy | [63,64] | ||
| 4-OI | Pyroptosis | NLRP3 inflammasome | Alkylated NLRP3 inflammasome at cysteine 548 in HEK293T cells | Inhibited pyroptosis | [66] |
| STING | Alkylated STING at cysteine 147 in HEK293T cells | [16] | |||
| Alkylated STING at cysteine 91 | [69] | ||||
| Caspase1/GSDMD | Alkylated GSDMD at cysteine77 | [70] | |||
| 4-OI | Inflammation, pyroptosis, ferroptosis | KEAP1/Nrf2 | Alkylated KEAP1 at cysteine 257, 288, and 273 in the human protein to activate Nrf2 | Released less inflammatory cytokines | [15,79] |
| SDH | Inhibited SDH, released less ROS | Inhibited pyroptosis, ferroptosis | [68,78,81] | ||
| Itaconate/4-OI | Epigenetic modifications | TET2 | Regulated the transcription factors NF-κB and STAT1 | Released less inflammatory cytokines | [8,9,93] |
| Histone | Induced histone demethylation by inhibiting the activity of methionine adenosine transferase and IDH1/2 enzyme | [94] | |||
| Histone 3 | Influence the histone 3 lysine 27 acetylation | [95] | |||
| Histone | Inhibits histone deacetylases | ||||
| Itaconate/4-OI | Glycolysis | GAPDH | Alkylated GADPH at cysteine 245 | Inhibited glycolysis | [13,100,101] |
| Aldolase A | Alkylated aldolase A at cysteine 73 and 339 | ||||
| Lactate dehydrogenase | Alkylated lactate dehydrogenase at cysteine 84 | ||||
| 4-OI | M2 macrophage | Modified JAK1 at cysteine 715, 816, 943 and 1130 | Regulated M2 macrophages | [84] | |
| Itaconate/4-OI | Neutrophils | Targeted NADPH oxidase | Inhibited neutrophils activation | [87,88] | |
| Inhibited neutrophil glycolysis and oxidative phosphorylation | |||||
| Inhibited the formation of NETs |
Given the ability of itaconate supplementation to mitigate ALI in preclinical models, future work should explore whether endogenous itaconate can be upregulated through manipulation of the Irg1 gene. Future studies should also explore the therapeutic use of itaconate and its derivatives against other diseases. For example, itaconate has been linked to cancer [109,110], yet depending on the context, the metabolite may promote [111] or inhibit tumor growth [109]. Research should continue to explore the full therapeutic possibilities of itaconate and its derivatives.
In addition, it is important to note some limitations of this review. Firstly, the focus is solely on the effects of itaconate and its derivatives on ALI, without considering other applications or effects in different inflammatory conditions. The mechanisms of action discussed in this review are largely based on findings from other inflammatory diseases and may require further experimental validation specifically in the context of ALI. Furthermore, recent discoveries of natural isomers of itaconic acid with similar effects as itaconate have not been adequately addressed in this review. Additionally, while itaconate is discussed as a single compound, it is important to recognize that itaconate and its derivatives are distinct compounds with complex metabolic pathways. Future research should aim to differentiate and analyze these compounds individually to better explaintheir specific roles and mechanisms of action.
Disclosure of conflict of interest
None.
References
- 1.Mason C, Dooley N, Griffiths M. Acute respiratory distress syndrome. Clin Med (Lond) 2017;17:439–443. doi: 10.7861/clinmedicine.17-5-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care. 2014;20:3–9. doi: 10.1097/MCC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 3.Batra R, Whalen W, Alvarez-Mulett S, Gomez-Escobar LG, Hoffman KL, Simmons W, Harrington J, Chetnik K, Buyukozkan M, Benedetti E, Choi ME, Suhre K, Schenck E, Choi AMK, Schmidt F, Cho SJ, Krumsiek J. Multi-omic comparative analysis of COVID-19 and bacterial sepsis-induced ARDS. PLoS Pathog. 2022;18:e1010819. doi: 10.1371/journal.ppat.1010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2021;74:168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 6.Pan X, Shan H, Bai J, Gao T, Chen B, Shen Z, Zhou H, Lu H, Sheng L, Zhou X. Four-octyl itaconate improves osteoarthritis by enhancing autophagy in chondrocytes via PI3K/AKT/mTOR signalling pathway inhibition. Commun Biol. 2022;5:641. doi: 10.1038/s42003-022-03592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao N, Di B, Xu LL. The NLRP3 inflammasome and COVID-19: activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021;61:2–15. doi: 10.1016/j.cytogfr.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, Korenfeld D, Mathyer ME, Kim H, Huang LH, Duncan D, Bregman H, Keskin A, Santeford A, Apte RS, Sehgal R, Johnson B, Amarasinghe GK, Soares MP, Satoh T, Akira S, Hai T, de Guzman Strong C, Auclair K, Roddy TP, Biller SA, Jovanovic M, Klechevsky E, Stewart KM, Randolph GJ, Artyomov MN. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018;556:501–504. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LL, Morcelle C, Cheng ZL, Chen X, Xu Y, Gao Y, Song J, Li Z, Smith MD, Shi M, Zhu Y, Zhou N, Cheng M, He C, Liu KY, Lu G, Zhang L, Zhang C, Zhang J, Sun Y, Qi T, Lyu Y, Ren ZZ, Tan XM, Yin J, Lan F, Liu Y, Yang H, Qian M, Duan C, Chang X, Zhou Y, Shen L, Baldwin AS, Guan KL, Xiong Y, Ye D. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat Cell Biol. 2022;24:353–363. doi: 10.1038/s41556-022-00853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue C, Yao Q, Gu X, Shi Q, Yuan X, Chu Q, Bao Z, Lu J, Li L. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023;8:204. doi: 10.1038/s41392-023-01468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Huang GD, Duan GC, Qin HJ. MicroRNA-147b alleviates inflammation and apoptosis in acute lung injury via inhibition of p38 MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25:1974–1981. doi: 10.26355/eurrev_202102_25098. [DOI] [PubMed] [Google Scholar]
- 12.Hooftman A, O’Neill LAJ. The immunomodulatory potential of the metabolite itaconate. Trends Immunol. 2019;40:687–698. doi: 10.1016/j.it.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Qin W, Qin K, Zhang Y, Jia W, Chen Y, Cheng B, Peng L, Chen N, Liu Y, Zhou W, Wang YL, Chen X, Wang C. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat Chem Biol. 2019;15:983–991. doi: 10.1038/s41589-019-0323-5. [DOI] [PubMed] [Google Scholar]
- 14.Cordes T, Metallo CM. Itaconate alters succinate and coenzyme a metabolism via inhibition of mitochondrial complex II and methylmalonyl-CoA mutase. Metabolites. 2021;11:117. doi: 10.3390/metabo11020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa ASH, Higgins M, Hams E, Szpyt J, Runtsch MC, King MS, McGouran JF, Fischer R, Kessler BM, McGettrick AF, Hughes MM, Carroll RG, Booty LM, Knatko EV, Meakin PJ, Ashford MLJ, Modis LK, Brunori G, Sévin DC, Fallon PG, Caldwell ST, Kunji ERS, Chouchani ET, Frezza C, Dinkova-Kostova AT, Hartley RC, Murphy MP, O’Neill LA. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Li Y, Kang J, Jiang H, Gong W, Chen L, Wu C, Liu M, Wu X, Zhao Y, Ren J. 4-octyl itaconate as a metabolite derivative inhibits inflammation via alkylation of STING. Cell Rep. 2023;42:112145. doi: 10.1016/j.celrep.2023.112145. [DOI] [PubMed] [Google Scholar]
- 17.Tian F, Wang Z, He J, Zhang Z, Tan N. 4-Octyl itaconate protects against renal fibrosis via inhibiting TGF-β/Smad pathway, autophagy and reducing generation of reactive oxygen species. Eur J Pharmacol. 2020;873:172989. doi: 10.1016/j.ejphar.2020.172989. [DOI] [PubMed] [Google Scholar]
- 18.Olagnier D, Farahani E, Thyrsted J, Blay-Cadanet J, Herengt A, Idorn M, Hait A, Hernaez B, Knudsen A, Iversen MB, Schilling M, Jorgensen SE, Thomsen M, Reinert LS, Lappe M, Hoang HD, Gilchrist VH, Hansen AL, Ottosen R, Nielsen CG, Moller C, van der Horst D, Peri S, Balachandran S, Huang J, Jakobsen M, Svenningsen EB, Poulsen TB, Bartsch L, Thielke AL, Luo Y, Alain T, Rehwinkel J, Alcami A, Hiscott J, Mogensen TH, Paludan SR, Holm CK. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437–453. doi: 10.1016/j.immuni.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao Y, Zhang T, Zhang C, Ji H, Tong X, Xia R, Wang W, Ma Z, Shi X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit Care. 2021;25:356. doi: 10.1186/s13054-021-03775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu T, Sun S. Role and mechanism of ferroptosis in acute lung injury. Cell Cycle. 2023;22:2119–2129. doi: 10.1080/15384101.2023.2278328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Chen X, Zhang H, Xiao J, Yang C, Chen W, Wei Z, Chen X, Liu J. 4-Octyl Itaconate alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting oxidative stress and inflammation. Drug Des Devel Ther. 2020;14:5547–5558. doi: 10.2147/DDDT.S280922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JW, Chun W, Lee HJ, Min JH, Kim SM, Seo JY, Ahn KS, Oh SR. The role of macrophages in the development of acute and chronic inflammatory lung diseases. Cells. 2021;10:897. doi: 10.3390/cells10040897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zemans RL, Matthay MA. What drives neutrophils to the alveoli in ARDS? Thorax. 2017;72:1–3. doi: 10.1136/thoraxjnl-2016-209170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Li C, Zhou J, Song Y, Fang X, Ou J, Li J, Bai C. Autophagy protects against ischemia/reperfusion-induced lung injury through alleviating blood-air barrier damage. J Heart Lung Transplant. 2015;34:746–755. doi: 10.1016/j.healun.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Xie QM, Chen N, Song SM, Zhao CC, Ruan Y, Sha JF, Liu Q, Jiang XQ, Fei GH, Wu HM. Itaconate suppresses the activation of mitochondrial NLRP3 inflammasome and oxidative stress in allergic airway inflammation. Antioxidants (Basel) 2023;12:489. doi: 10.3390/antiox12020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Xu P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. 2023;33:630–648. doi: 10.1016/j.tcb.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Barlow JL, McKenzie ANJ. Innate lymphoid cells of the lung. Annu Rev Physiol. 2019;81:429–452. doi: 10.1146/annurev-physiol-020518-114630. [DOI] [PubMed] [Google Scholar]
- 32.He YQ, Zhou CC, Yu LY, Wang L, Deng JL, Tao YL, Zhang F, Chen WS. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol Res. 2021;163:105224. doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 35.Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015;70:1189–1196. doi: 10.1136/thoraxjnl-2015-207020. [DOI] [PubMed] [Google Scholar]
- 36.Williams AE, José RJ, Mercer PF, Brealey D, Parekh D, Thickett DR, O’Kane C, McAuley DF, Chambers RC. Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax. 2017;72:66–73. doi: 10.1136/thoraxjnl-2016-208597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 38.Robert T, Friebel S. Itaconic acid - a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016;18:2922–2934. [Google Scholar]
- 39.Rittenhouse JW, McFadden BA. Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate. Arch Biochem Biophys. 1974;163:79–86. doi: 10.1016/0003-9861(74)90456-1. [DOI] [PubMed] [Google Scholar]
- 40.Williams JO, Roche TE, McFadden BA. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. 1971;10:1384–1390. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]
- 41.McFadden BA, Purohit S. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. J Bacteriol. 1977;131:136–144. doi: 10.1128/jb.131.1.136-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel TR, McFadden BA. Caenorhabditis elegans and Ascaris suum: inhibition of isocitrate lyase by itaconate. Exp Parasitol. 1978;44:262–268. doi: 10.1016/0014-4894(78)90107-8. [DOI] [PubMed] [Google Scholar]
- 43.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin JH, Yang JY, Jeon BY, Yoon YJ, Cho SN, Kang YH, Ryu DH, Hwang GS. (1)H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J Proteome Res. 2011;10:2238–2247. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto M, Sakagami H, Yokote Y, Onuma H, Kaneko M, Mori M, Sakaguchi Y, Soga T, Tomita M. Non-targeted metabolite profiling in activated macrophage secretion. Metabolomics. 2012;8:624–633. [Google Scholar]
- 46.Strelko CL, Lu W, Dufort FJ, Seyfried TN, Chiles TC, Rabinowitz JD, Roberts MF. Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc. 2011;133:16386–16389. doi: 10.1021/ja2070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu JH, Zhang L, Li KX, Zhang QH, Fan KR, Chen K, Jiang Y, Liu G. Deficiency of IRG1/itaconate aggravates endotoxemia-induced acute lung injury by inhibiting autophagy in mice. Exp Anim. 2023;72:164–172. doi: 10.1538/expanim.22-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyle C, Green JP, Allan SM, Brough D, Lemarchand E. Itaconate and fumarate derivatives inhibit priming and activation of the canonical NLRP3 inflammasome in macrophages. Immunology. 2022;165:460–480. doi: 10.1111/imm.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He R, Liu B, Xiong R, Geng B, Meng H, Lin W, Hao B, Zhang L, Wang W, Jiang W, Li N, Geng Q. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022;8:43. doi: 10.1038/s41420-021-00807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni L, Lin Z, Hu S, Shi Y, Jiang Z, Zhao J, Zhou Y, Wu Y, Tian N, Sun L, Wu A, Pan Z, Zhang X, Wang X. Itaconate attenuates osteoarthritis by inhibiting STING/NF-κB axis in chondrocytes and promoting M2 polarization in macrophages. Biochem Pharmacol. 2022;198:114935. doi: 10.1016/j.bcp.2022.114935. [DOI] [PubMed] [Google Scholar]
- 51.Sano M, Tanaka T, Ohara H, Aso Y. Itaconic acid derivatives: structure, function, biosynthesis, and perspectives. Appl Microbiol Biotechnol. 2020;104:9041–9051. doi: 10.1007/s00253-020-10908-1. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt T, Dai Z, Drexler HJ, Baumann W, Jäger C, Pfeifer D, Heller D. Novel contributions to the mechanism of the enantioselective hydrogenation of dimethyl itaconate with rhodium complexes. Chemistry. 2008;14:4469–4471. doi: 10.1002/chem.200800389. [DOI] [PubMed] [Google Scholar]
- 53.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, Artyomov MN. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swain A, Bambouskova M, Kim H, Andhey PS, Duncan D, Auclair K, Chubukov V, Simons DM, Roddy TP, Stewart KM, Artyomov MN. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2:594–602. doi: 10.1038/s42255-020-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ElAzzouny M, Tom CT, Evans CR, Olson LL, Tanga MJ, Gallagher KA, Martin BR, Burant CF. Dimethyl itaconate is not metabolized into itaconate intracellularly. J Biol Chem. 2017;292:4766–4769. doi: 10.1074/jbc.C117.775270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin J, Ren J, Gao DS, Dai Y, Yu L. The emerging application of itaconate: promising molecular targets and therapeutic opportunities. Front Chem. 2021;9:669308. doi: 10.3389/fchem.2021.669308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O’Neill LA, Mills EL. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat Metab. 2019;1:16–33. doi: 10.1038/s42255-018-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang W, Wang Y, Tao K, Li R. Metabolite itaconate in host immunoregulation and defense. Cell Mol Biol Lett. 2023;28:100. doi: 10.1186/s11658-023-00503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang R, Siddique MNAA. Control of immune cell signaling by the immuno-metabolite itaconate. Front Immunol. 2024;15:1352165. doi: 10.3389/fimmu.2024.1352165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Tang LH, Lu J, Xu LM, Cheng BL, Xiong JY. ABT-263 enhanced bacterial phagocytosis of macrophages in aged mouse through Beclin-1-dependent autophagy. BMC Geriatr. 2021;21:225. doi: 10.1186/s12877-021-02173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu T, Wang L, Liang P, Wang X, Liu Y, Cai J, She Y, Wang D, Wang Z, Guo Z, Bates S, Xia X, Huang J, Cui J. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell Mol Immunol. 2021;18:2431–2442. doi: 10.1038/s41423-020-00567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martina JA, Puertollano R. The IRG1/itaconate/TFEB axis: a new weapon in macrophage antibacterial defense. Mol Cell. 2022;82:2732–2734. doi: 10.1016/j.molcel.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z, Chen C, Yang F, Zeng YX, Sun P, Liu P, Li X. Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mol Cell. 2022;82:2844–2857. e10. doi: 10.1016/j.molcel.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196. doi: 10.1038/s41392-022-01046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, Ruzek MC, Slivka PF, McGettrick AF, Banahan K, Hughes MM, Irvine AD, Fischer R, O’Neill LAJ. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32:468–478. e7. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang W, Wang Y, Wang T, Li C, Shi L, Zhang P, Yin Y, Tao K, Li R. Protective effects of IRG1/itaconate on acute colitis through the inhibition of gasdermins-mediated pyroptosis and inflammation response. Genes Dis. 2022;10:1552–1563. doi: 10.1016/j.gendis.2022.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu YT, Xu WT, Zheng L, Wang S, Wei J, Liu MY, Zhou HP, Li QF, Shi X, Lv X. 4-octyl itaconate ameliorates alveolar macrophage pyroptosis against ARDS via rescuing mitochondrial dysfunction and suppressing the cGAS/STING pathway. Int Immunopharmacol. 2023;118:110104. doi: 10.1016/j.intimp.2023.110104. [DOI] [PubMed] [Google Scholar]
- 69.Su C, Cheng T, Huang J, Zhang T, Yin H. 4-Octyl itaconate restricts STING activation by blocking its palmitoylation. Cell Rep. 2023;42:113040. doi: 10.1016/j.celrep.2023.113040. [DOI] [PubMed] [Google Scholar]
- 70.Bambouskova M, Potuckova L, Paulenda T, Kerndl M, Mogilenko DA, Lizotte K, Swain A, Hayes S, Sheldon RD, Kim H, Kapadnis U, Ellis AE, Isaguirre C, Burdess S, Laha A, Amarasinghe GK, Chubukov V, Roddy TP, Diamond MS, Jones RG, Simons DM, Artyomov MN. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep. 2021;34:108756. doi: 10.1016/j.celrep.2021.108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xin L, Zhou F, Zhang C, Zhong W, Xu S, Jing X, Wang D, Wang S, Chen T, Song J. Four-Octyl itaconate ameliorates periodontal destruction via Nrf2-dependent antioxidant system. Int J Oral Sci. 2022;14:27. doi: 10.1038/s41368-022-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaiswal AK, Yadav J, Makhija S, Mazumder S, Mitra AK, Suryawanshi A, Sandey M, Mishra A. Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation. Mucosal Immunol. 2022;15:301–313. doi: 10.1038/s41385-021-00462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang YM, Gong FC, Qi X, Zheng YJ, Zheng XT, Chen Y, Yang ZT, Qing-Ye, Mao EQ, Chen EZ. Mucin 1 Inhibits ferroptosis and sensitizes vitamin E to alleviate sepsis-induced acute lung injury through GSK3β/Keap1-Nrf2-GPX4 pathway. Oxid Med Cell Longev. 2020;2022:2405943. doi: 10.1155/2022/2405943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Zhang J, Xie W. The role of ferroptosis in acute lung injury. Mol Cell Biochem. 2022;477:1453–1461. doi: 10.1007/s11010-021-04327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu P, Feng Y, Li H, Chen X, Wang G, Xu S, Li Y, Zhao L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett. 2020;25:10. doi: 10.1186/s11658-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, St Croix CM, Mikulska-Ruminska K, Liu B, Shrivastava IH, Tyurin VA, Ting HC, Wu YL, Gao Y, Shurin GV, Artyukhova MA, Ponomareva LA, Timashev PS, Domingues RM, Stoyanovsky DA, Greenberger JS, Mallampalli RK, Bahar I, Gabrilovich DI, Bayır H, Kagan VE. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–290. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi J, Cai C. Research progress on the mechanism of itaconate regulating macrophage immunometabolism. Front Immunol. 2022;13:937247. doi: 10.3389/fimmu.2022.937247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qu C, Dai E, Lai T, Cao G, Liu J, Kang R, Han L, Tang D, Zhou D. Itaconic acid induces ferroptosis by activating ferritinophagy. Biochem Biophys Res Commun. 2021;583:56–62. doi: 10.1016/j.bbrc.2021.10.054. [DOI] [PubMed] [Google Scholar]
- 80.Zhao Y, Liu Z, Liu G, Zhang Y, Liu S, Gan D, Chang W, Peng X, Sung ES, Gilbert K, Zhu Y, Wang X, Zeng Z, Baldwin H, Ren G, Weaver J, Huron A, Mayberry T, Wang Q, Wang Y, Diaz-Rubio ME, Su X, Stack MS, Zhang S, Lu X, Sheldon RD, Li J, Zhang C, Wan J, Lu X. Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1. Cell Metab. 2023;35:1688–1703. e10. doi: 10.1016/j.cmet.2023.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cordes T, Lucas A, Divakaruni AS, Murphy AN, Cabrales P, Metallo CM. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol Metab. 2020;32:122–135. doi: 10.1016/j.molmet.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maassen S, Coenen B, Ioannidis M, Harber K, Grijpstra P, Van den Bossche J, van den Bogaart G. Itaconate promotes a wound resolving phenotype in pro-inflammatory macrophages. Redox Biol. 2022;59:102591. doi: 10.1016/j.redox.2022.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang S, Zhang X, Zhang H, Lin X, Chen X, Zhang Y, Lin X, Huang L, Zhuge Q. Dimethyl itaconate inhibits LPS-induced microglia inflammation and inflammasome-mediated pyroptosis via inducing autophagy and regulating the Nrf-2/HO-1 signaling pathway. Mol Med Rep. 2021;24:672. doi: 10.3892/mmr.2021.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Runtsch MC, Angiari S, Hooftman A, Wadhwa R, Zhang Y, Zheng Y, Spina JS, Ruzek MC, Argiriadi MA, McGettrick AF, Mendez RS, Zotta A, Peace CG, Walsh A, Chirillo R, Hams E, Fallon PG, Jayamaran R, Dua K, Brown AC, Kim RY, Horvat JC, Hansbro PM, Wang C, O’Neill LAJ. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 2022;34:487–501. e8. doi: 10.1016/j.cmet.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Blanco LP, Patino-Martinez E, Nakabo S, Zhang M, Pedersen HL, Wang X, Carmona-Rivera C, Claybaugh D, Yu ZX, Desta E, Kaplan MJ. Modulation of the itaconate pathway attenuates murine lupus. Arthritis Rheumatol. 2022;74:1971–1983. doi: 10.1002/art.42284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crossley JL, Ostashevskaya-Gohstand S, Comazzetto S, Hook JS, Guo L, Vishlaghi N, Juan C, Xu L, Horswill AR, Hoxhaj G, Moreland JG, Tower RJ, Levi B. Itaconate-producing neutrophils regulate local and systemic inflammation following trauma. JCI Insight. 2023;8:e169208. doi: 10.1172/jci.insight.169208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomlinson KL, Riquelme SA, Baskota SU, Drikic M, Monk IR, Stinear TP, Lewis IA, Prince AS. Staphylococcus aureus stimulates neutrophil itaconate production that suppresses the oxidative burst. Cell Rep. 2023;42:112064. doi: 10.1016/j.celrep.2023.112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burczyk G, Cichon I, Kolaczkowska E. Itaconate suppresses formation of neutrophil extracellular traps (NETs): involvement of hypoxia-inducible factor 1α (Hif-1α) and heme oxygenase (HO-1) Front Immunol. 2022;13:864638. doi: 10.3389/fimmu.2022.864638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cull AH, Snetsinger B, Buckstein R, Wells RA, Rauh MJ. Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol. 2017;55:56–70. e13. doi: 10.1016/j.exphem.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Cong B, Zhang Q, Cao X. The function and regulation of TET2 in innate immunity and inflammation. Protein Cell. 2021;12:165–173. doi: 10.1007/s13238-020-00796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ichiyama K, Chen T, Wang X, Yan X, Kim BS, Tanaka S, Ndiaye-Lobry D, Deng Y, Zou Y, Zheng P, Tian Q, Aifantis I, Wei L, Dong C. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity. 2015;42:613–626. doi: 10.1016/j.immuni.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W, Zhang X, Lu X, You L, Song Y, Luo Z, Zhang J, Nie J, Zheng W, Xu D, Wang Y, Dong Y, Yu S, Hong J, Shi J, Hao H, Luo F, Hua L, Wang P, Qian X, Yuan F, Wei L, Cui M, Zhang T, Liao Q, Dai M, Liu Z, Chen G, Meckel K, Adhikari S, Jia G, Bissonnette MB, Zhang X, Zhao Y, Zhang W, He C, Liu J. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017;27:1243–1257. doi: 10.1038/cr.2017.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu YP, Lv L, Liu Y, Smith MD, Li WC, Tan XM, Cheng M, Li Z, Bovino M, Aubé J, Xiong Y. Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy. J Clin Invest. 2019;129:4316–4331. doi: 10.1172/JCI129317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aso K, Kono M, Kanda M, Kudo Y, Sakiyama K, Hisada R, Karino K, Ueda Y, Nakazawa D, Fujieda Y, Kato M, Amengual O, Atsumi T. Itaconate ameliorates autoimmunity by modulating T cell imbalance via metabolic and epigenetic reprogramming. Nat Commun. 2023;14:984. doi: 10.1038/s41467-023-36594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Domínguez-Andrés J, Novakovic B, Li Y, Scicluna BP, Gresnigt MS, Arts RJW, Oosting M, Moorlag SJCFM, Groh LA, Zwaag J, Koch RM, Ter Horst R, Joosten LAB, Wijmenga C, Michelucci A, van der Poll T, Kox M, Pickkers P, Kumar V, Stunnenberg H, Netea MG. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab. 2019;29:211–220. e5. doi: 10.1016/j.cmet.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Ali MM, Mahmoud AM, Le Master E, Levitan I, Phillips SA. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2019;316:H647–H663. doi: 10.1152/ajpheart.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kovacs-Kasa A, Kovacs L, Cherian-Shaw M, Patel V, Meadows ML, Fulton DJ, Su Y, Verin AD. Inhibition of Class IIa HDACs improves endothelial barrier function in endotoxin-induced acute lung injury. J Cell Physiol. 2021;236:2893–2905. doi: 10.1002/jcp.30053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lei I, Huang W, Noly PE, Naik S, Ghali M, Liu L, Pagani FD, Abou El Ela A, Pober JS, Pitt B, Platt JL, Cascalho M, Wang Z, Chen YE, Mortensen RM, Tang PC. Metabolic reprogramming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci Transl Med. 2023;15:eade3782. doi: 10.1126/scitranslmed.ade3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhong WJ, Yang HH, Guan XX, Xiong JB, Sun CC, Zhang CY, Luo XQ, Zhang YF, Zhang J, Duan JX, Zhou Y, Guan CX. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J Cell Physiol. 2019;234:4641–4654. doi: 10.1002/jcp.27261. [DOI] [PubMed] [Google Scholar]
- 100.Liao ST, Han C, Xu DQ, Fu XW, Wang JS, Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun. 2019;10:5091. doi: 10.1038/s41467-019-13078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu Z, Umehara T, Tsujita N, Kawai T, Goto M, Cheng B, Zeng W, Shimada M. Itaconate regulates the glycolysis/pentose phosphate pathway transition to maintain boar sperm linear motility by regulating redox homeostasis. Free Radic Biol Med. 2020;159:44–53. doi: 10.1016/j.freeradbiomed.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Liu G, Wu Y, Jin S, Sun J, Wan BB, Zhang J, Wang Y, Gao ZQ, Chen D, Li S, Pang Q, Wang Z. Itaconate ameliorates methicillin-resistant Staphylococcus aureus-induced acute lung injury through the Nrf2/ARE pathway. Ann Transl Med. 2021;9:712. doi: 10.21037/atm-21-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao T, Fang R, Ding J, Liu Y, Cheng M, Zhou F, Liu F, Li W, Li S, Jiang K, Shi X, Liu M, Xu B, Zou X, Zhu H, Zhou L. Melatonin ameliorates multiorgan injuries induced by severe acute pancreatitis in mice by regulating the Nrf2 signaling pathway. Eur J Pharmacol. 2024;975:176646. doi: 10.1016/j.ejphar.2024.176646. [DOI] [PubMed] [Google Scholar]
- 104.Xu L, Cai J, Li C, Yang M, Duan T, Zhao Q, Xi Y, Sun L, He L, Tang C, Sun L. 4-Octyl itaconate attenuates LPS-induced acute kidney injury by activating Nrf2 and inhibiting STAT3 signaling. Mol Med. 2023;29:58. doi: 10.1186/s10020-023-00631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S, Lin F, Zhang C, Gao D, Qi Z, Wu S, Wang W, Li X, Pan L, Xu Y, Tan B, Yang A. Xuanbai Chengqi Decoction alleviates acute lung injury by inhibiting NLRP3 inflammasome. J Ethnopharmacol. 2024;319:117227. doi: 10.1016/j.jep.2023.117227. [DOI] [PubMed] [Google Scholar]
- 106.Wu X, Yi X, Zhao B, Zhi Y, Xu Z, Cao Y, Cao X, Pang J, Yung KKL, Zhang S, Liu S, Zhou P. The volume regulated anion channel VRAC regulates NLRP3 inflammasome by modulating itaconate efflux and mitochondria function. Pharmacol Res. 2023;198:107016. doi: 10.1016/j.phrs.2023.107016. [DOI] [PubMed] [Google Scholar]
- 107.Wan J, Lin S, Huang X, Li Q, Zeng L, Du S. ZJ01, a small molecule inhibitor of the Kelch-Like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2 (Keap1-Nrf2) protein-protein interaction, reduces hyperoxic acute lung injury in a mouse model. Med Sci Monit. 2020;26:e920467. doi: 10.12659/MSM.920467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Auger JP, Zimmermann M, Faas M, Stifel U, Chambers D, Krishnacoumar B, Taudte RV, Grund C, Erdmann G, Scholtysek C, Uderhardt S, Ben Brahim O, Pascual Maté M, Stoll C, Böttcher M, Palumbo-Zerr K, Mangan MSJ, Dzamukova M, Kieler M, Hofmann M, Blüml S, Schabbauer G, Mougiakakos D, Sonnewald U, Hartmann F, Simon D, Kleyer A, Grüneboom A, Finotto S, Latz E, Hofmann J, Schett G, Tuckermann J, Krönke G. Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids. Nature. 2024;629:184–192. doi: 10.1038/s41586-024-07282-7. [DOI] [PubMed] [Google Scholar]
- 109.Wang Q, Li XL, Mei Y, Ye JC, Fan W, Cheng GH, Zeng MS, Feng GK. The anti-inflammatory drug dimethyl itaconate protects against colitis-associated colorectal cancer. J Mol Med (Berl) 2020;98:1457–1466. doi: 10.1007/s00109-020-01963-2. [DOI] [PubMed] [Google Scholar]
- 110.Gautam AK, Kumar P, Raj R, Kumar D, Bhattacharya B, Rajinikanth PS, Chidambaram K, Mahata T, Maity B, Saha S. Preclinical evaluation of dimethyl itaconate against hepatocellular carcinoma via activation of the e/iNOS-mediated NF-κB-dependent apoptotic pathway. Front Pharmacol. 2022;12:823285. doi: 10.3389/fphar.2021.823285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weiss JM, Davies LC, Karwan M, Ileva L, Ozaki MK, Cheng RY, Ridnour LA, Annunziata CM, Wink DA, McVicar DW. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. J Clin Invest. 2018;128:3794–3805. doi: 10.1172/JCI99169. [DOI] [PMC free article] [PubMed] [Google Scholar]