Abstract
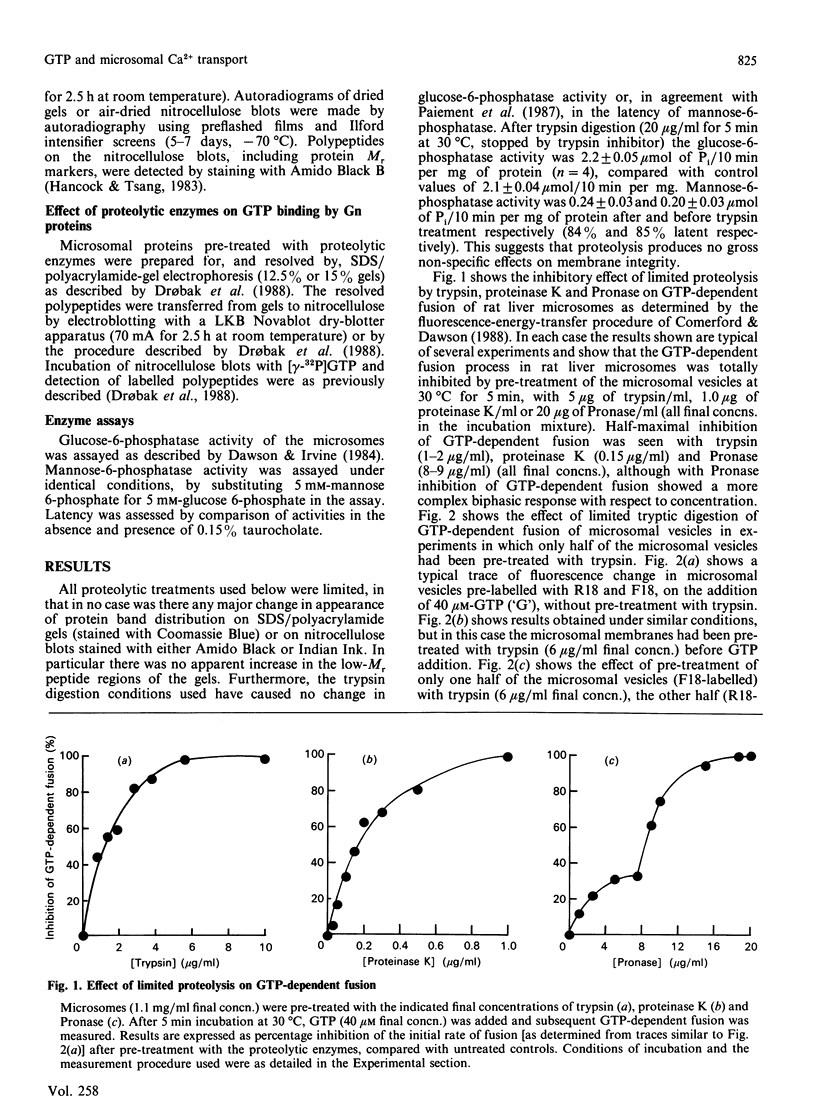
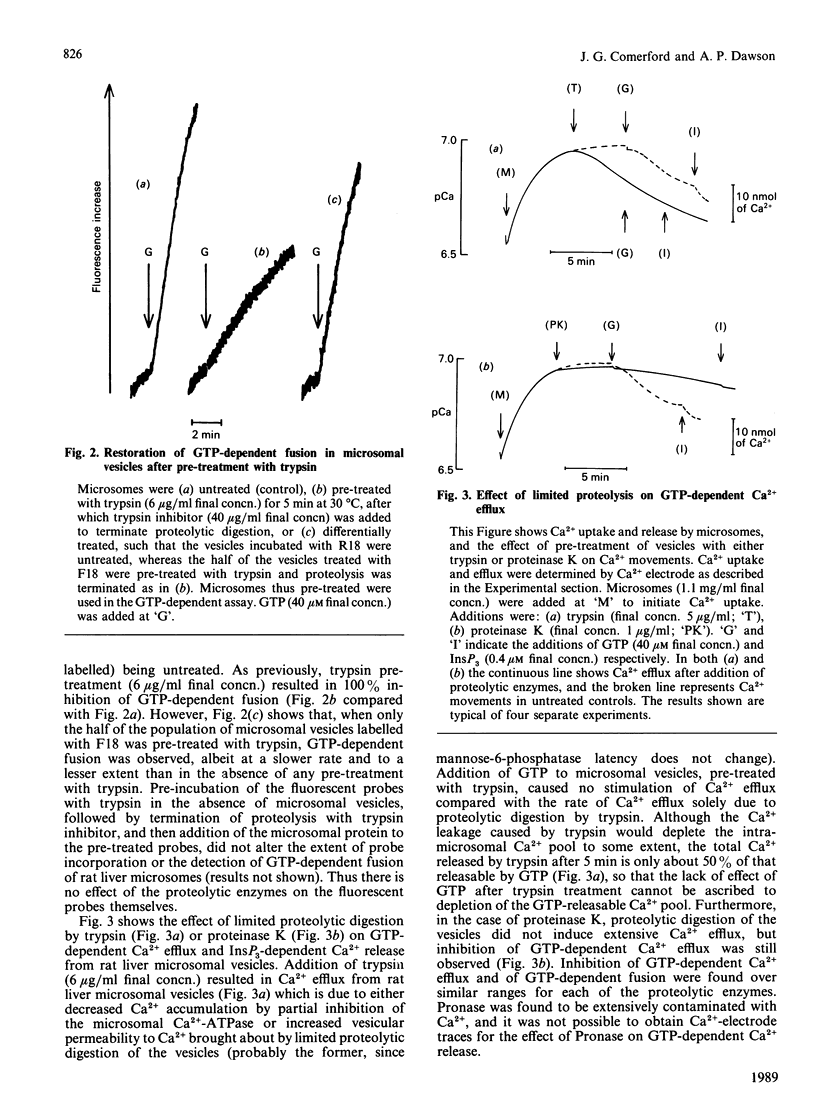
1. Limited proteolytic digestion of rat liver microsomes (microsomal fractions) with trypsin (5 micrograms/ml), proteinase K (1.0 microgram/ml) and Pronase (20 micrograms/ml final concns.) resulted in abolition of GTP-dependent vesicle fusion. 2. Vesicle fusion could be partially restored to microsomes which had undergone limited tryptic digestion, by the addition of untreated microsomal vesicles. 3. GTP-dependent Ca2+ efflux from rat liver microsomes was also observed to be inhibited by limited proteolysis with trypsin and proteinase K. 4. Limited proteolysis of rat liver microsomes had no effect on subsequent GTP-dependent phosphorylation of polypeptides of Mr 17,000 and 38,000, and thus it is unlikely that the phosphorylation of these proteins is involved in GTP-dependent Ca2+ efflux and GTP-dependent vesicle fusion. 5. GTP binding by Gn proteins [proteins which bind GTP after transfer to nitrocellulose, as defined by Bhullar & Haslam (1986) Biochem. J. 245, 617-620] was inhibited by pre-treatment of microsomes with trypsin, proteinase K and Pronase at concentrations similar to those which abolished GTP-dependent Ca2+ efflux and vesicle fusion. 6. We suggest that one or more of the Gn proteins may be involved in the molecular mechanisms of GTP-dependent vesicle fusion and Ca2+ efflux in rat liver microsomes and that limited proteolytic digestion may be a useful tool in further investigation of these processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E., Dawson A., Drøbak B., Roberts K. GTP causes calcium release from a plant microsomal fraction. Cell Signal. 1989;1(1):23–29. doi: 10.1016/0898-6568(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Bhullar R. P., Haslam R. J. Detection of 23-27 kDa GTP-binding proteins in platelets and other cells. Biochem J. 1987 Jul 15;245(2):617–620. doi: 10.1042/bj2450617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Chueh S. H., Mullaney J. M., Ghosh T. K., Zachary A. L., Gill D. L. GTP- and inositol 1,4,5-trisphosphate-activated intracellular calcium movements in neuronal and smooth muscle cell lines. J Biol Chem. 1987 Oct 5;262(28):13857–13864. [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Measurement of vesicle fusion by fluorescence energy transfer. Biochem J. 1988 Jan 1;249(1):89–93. doi: 10.1042/bj2490089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Comerford J. G., Fulton D. V. The effect of GTP on inositol 1,4,5-trisphosphate-stimulated Ca2+ efflux from a rat liver microsomal fraction. Is a GTP-dependent protein phosphorylation involved? Biochem J. 1986 Mar 1;234(2):311–315. doi: 10.1042/bj2340311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P. GTP enhances inositol trisphosphate-stimulated Ca2+ release from rat liver microsomes. FEBS Lett. 1985 Jun 3;185(1):147–150. doi: 10.1016/0014-5793(85)80759-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Hills G., Comerford J. G. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Biochem J. 1987 May 15;244(1):87–92. doi: 10.1042/bj2440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Drobak B. K., Allan E. F., Comerford J. G., Roberts K., Dawson A. P. Presence of guanine nucleotide-binding proteins in a plant hypocotyl microsomal fraction. Biochem Biophys Res Commun. 1988 Feb 15;150(3):899–903. doi: 10.1016/0006-291x(88)90713-9. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Ueda T., Chueh S. H., Noel M. W. Ca2+ release from endoplasmic reticulum is mediated by a guanine nucleotide regulatory mechanism. Nature. 1986 Apr 3;320(6061):461–464. doi: 10.1038/320461a0. [DOI] [PubMed] [Google Scholar]
- Godelaine D., Beaufay H. The membrane of the rough endoplasmic reticulum contains cytoplasmically exposed high affinity GTP-binding sites. Biochem Biophys Res Commun. 1987 Oct 14;148(1):478–484. doi: 10.1016/0006-291x(87)91136-3. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Hamachi T., Hirata M., Kimura Y., Ikebe T., Ishimatsu T., Yamaguchi K., Koga T. Effect of guanosine triphosphate on the release and uptake of Ca2+ in saponin-permeabilized macrophages and the skeletal-muscle sarcoplasmic reticulum. Biochem J. 1987 Feb 15;242(1):253–260. doi: 10.1042/bj2420253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Henne V., Söling H. D. Guanosine 5'-triphosphate releases calcium from rat liver and guinea pig parotid gland endoplasmic reticulum independently of inositol 1,4,5-trisphosphate. FEBS Lett. 1986 Jul 7;202(2):267–273. doi: 10.1016/0014-5793(86)80699-8. [DOI] [PubMed] [Google Scholar]
- Kiesel L., Lukács G. L., Eberhardt I., Runnebaum B., Spät A. Effect of inositol 1,4,5-trisphosphate and GTP on calcium release from pituitary microsomes. FEBS Lett. 1987 Jun 8;217(1):85–88. doi: 10.1016/0014-5793(87)81248-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukács G. L., Hajnóczky G., Hunyady L., Spät A. The effect of inositol 1,4,5-trisphosphate and GTP on calcium release from rat liver microsomes. Biochim Biophys Acta. 1987 Nov 12;931(2):251–254. doi: 10.1016/0167-4889(87)90213-8. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Mullaney J. M., Chueh S. H., Ghosh T. K., Gill D. L. Intracellular calcium uptake activated by GTP. Evidence for a possible guanine nucleotide-induced transmembrane conveyance of intracellular calcium. J Biol Chem. 1987 Oct 5;262(28):13865–13872. [PubMed] [Google Scholar]
- Mullaney J. M., Yu M., Ghosh T. K., Gill D. L. Calcium entry into the inositol 1,4,5-trisphosphate-releasable calcium pool is mediated by a GTP-regulatory mechanism. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2499–2503. doi: 10.1073/pnas.85.8.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Joseph S. K., Williamson J. R. GTP-mediated Ca2+ release in rough endoplasmic reticulum. Correlation with a GTP-sensitive increase in membrane permeability. Biochem J. 1987 Dec 15;248(3):741–747. doi: 10.1042/bj2480741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiement J., Rindress D., Smith C. E., Poliquin L., Bergeron J. J. Properties of a GTP sensitive microdomain in rough microsomes. Biochim Biophys Acta. 1987 Mar 26;898(1):6–22. doi: 10.1016/0005-2736(87)90105-2. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Puzicha M., Gallwitz D. Study of a temperature-sensitive mutant of the ras-related YPT1 gene product in yeast suggests a role in the regulation of intracellular calcium. Cell. 1988 May 20;53(4):635–647. doi: 10.1016/0092-8674(88)90579-x. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Thomas A. P. Enhancement of the inositol 1,4,5-trisphosphate-releasable Ca2+ pool by GTP in permeabilized hepatocytes. J Biol Chem. 1988 Feb 25;263(6):2704–2711. [PubMed] [Google Scholar]
- Ueda T., Chueh S. H., Noel M. W., Gill D. L. Influence of inositol 1,4,5-trisphosphate and guanine nucleotides on intracellular calcium release within the N1E-115 neuronal cell line. J Biol Chem. 1986 Mar 5;261(7):3184–3192. [PubMed] [Google Scholar]
- Wolf B. A., Florholmen J., Colca J. R., McDaniel M. L. GTP mobilization of Ca2+ from the endoplasmic reticulum of islets. Comparison with myo-inositol 1,4,5-trisphosphate. Biochem J. 1987 Feb 15;242(1):137–141. doi: 10.1042/bj2420137. [DOI] [PMC free article] [PubMed] [Google Scholar]