Abstract
We studied the subcellular localization of dystrophin in rabbit skeletal muscle. In Western-blot analysis of membrane preparations, dystrophin was associated with the sarcolemmal fraction, as indicated by cholesterol content and co-purification with ouabain-binding activity and beta-adrenergic receptor. Dystrophin was also found with junctional T-tubules, but not with 'free' T-tubules, longitudinal portions or terminal cisternae of the sarcoplasmic reticulum. Dystrophin was not solubilized by high salt solutions, but it was solubilized by low concentrations of detergents (Triton X-100 and deoxycholate), suggesting that it is a peripheral membrane protein.
Full text
PDF


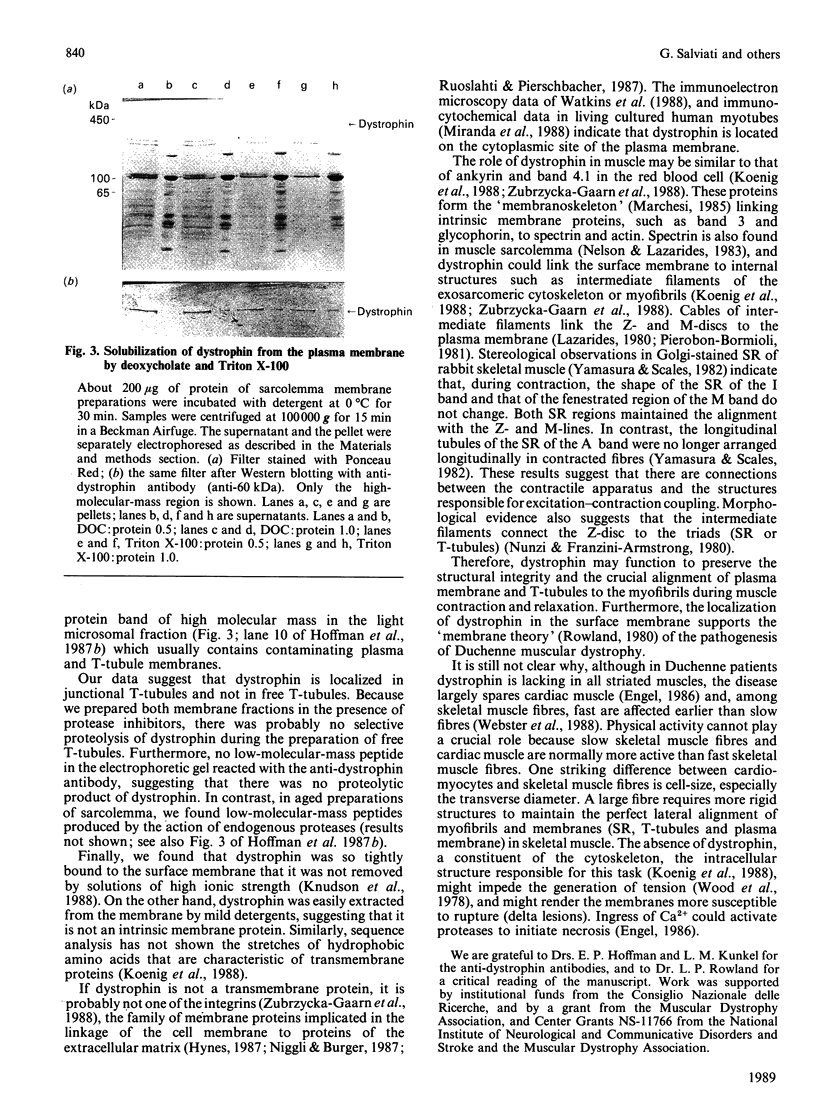

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Biral D., Damiani E., Margreth A., Scarpini E. Myosin subunit composition in human developing muscle. Biochem J. 1984 Dec 15;224(3):923–931. doi: 10.1042/bj2240923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Baker S. P., Boyd H., Potter L. T., Garcia M. beta-adrenergic receptor and adenylate cyclase in transverse tubules of skeletal muscle. J Biol Chem. 1978 May 10;253(9):3049–3054. [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Hammonds R. G., Jr Protein sequence of DMD gene is related to actin-binding domain of alpha-actinin. Cell. 1987 Oct 9;51(1):1–1. doi: 10.1016/0092-8674(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Knudson C. M., Campbell K. P., Kunkel L. M. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987 Dec 24;330(6150):754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Monaco A. P., Feener C. C., Kunkel L. M. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987 Oct 16;238(4825):347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- Horgan D. J., Kuypers R. Isolation of transverse tubules by fractionation of sarcoplasmic reticulum preparations in ion-free sucrose density gradients. Arch Biochem Biophys. 1987 Mar;253(2):377–387. doi: 10.1016/0003-9861(87)90191-3. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Jones L. R., Maddock S. W., Besch H. R., Jr Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J Biol Chem. 1980 Oct 25;255(20):9971–9980. [PubMed] [Google Scholar]
- Knudson C. M., Hoffman E. P., Kahl S. D., Kunkel L. M., Campbell K. P. Evidence for the association of dystrophin with the transverse tubular system in skeletal muscle. J Biol Chem. 1988 Jun 15;263(17):8480–8484. [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Leung A. T., Imagawa T., Block B., Franzini-Armstrong C., Campbell K. P. Biochemical and ultrastructural characterization of the 1,4-dihydropyridine receptor from rabbit skeletal muscle. Evidence for a 52,000 Da subunit. J Biol Chem. 1988 Jan 15;263(2):994–1001. [PubMed] [Google Scholar]
- Lev A. A., Feener C. C., Kunkel L. M., Brown R. H., Jr Expression of the Duchenne's muscular dystrophy gene in cultured muscle cells. J Biol Chem. 1987 Nov 25;262(33):15817–15820. [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Bonilla E., Martucci G., Moraes C. T., Hays A. P., Dimauro S. Immunocytochemical study of dystrophin in muscle cultures from patients with Duchenne muscular dystrophy and unaffected control patients. Am J Pathol. 1988 Sep;132(3):410–416. [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. D., Palade P., Fleischer S. Purification of morphologically intact triad structures from skeletal muscle. J Cell Biol. 1983 Apr;96(4):1008–1016. doi: 10.1083/jcb.96.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Expression of the beta subunit of spectrin in nonerythroid cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):363–367. doi: 10.1073/pnas.80.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V., Burger M. M. Interaction of the cytoskeleton with the plasma membrane. J Membr Biol. 1987;100(2):97–121. doi: 10.1007/BF02209144. [DOI] [PubMed] [Google Scholar]
- Nudel U., Robzyk K., Yaffe D. Expression of the putative Duchenne muscular dystrophy gene in differentiated myogenic cell cultures and in the brain. Nature. 1988 Feb 18;331(6157):635–638. doi: 10.1038/331635a0. [DOI] [PubMed] [Google Scholar]
- Nunzi M. G., Franzini-Armstrong C. Trabecular network in adult skeletal muscle. J Ultrastruct Res. 1980 Oct;73(1):21–26. doi: 10.1016/0022-5320(80)90112-4. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Volpe P., Salvatori S., Betto R., Damiani E., Margreth A., Pasquali-Ronchetti I. Biochemical heterogeneity of skeletal-muscle microsomal membranes. Membrane origin, membrane specificity and fibre types. Biochem J. 1982 Feb 15;202(2):289–301. doi: 10.1042/bj2020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler S., Fleischer S. Isolation of plasma membrane vesicles from rabbit skeletal muscle and their use in ion transport studies. J Biol Chem. 1982 Nov 25;257(22):13862–13871. [PubMed] [Google Scholar]
- Somlyo A. V. Bridging structures spanning the junctioning gap at the triad of skeletal muscle. J Cell Biol. 1979 Mar;80(3):743–750. doi: 10.1083/jcb.80.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Wood D. S., Sorenson M. M., Eastwood A. B., Charash W. E., Reuben J. P. Duchenne dystrophy: abnormal generation of tension and Ca++ regulation in single skinned fibers. Neurology. 1978 May;28(5):447–457. doi: 10.1212/wnl.28.5.447. [DOI] [PubMed] [Google Scholar]
- Yasumura T., Scales D. J. II. The three-dimensional rearrangement of mammalian sarcoplasmic reticulum after contraction. J Ultrastruct Res. 1982 Oct;81(1):27–36. doi: 10.1016/s0022-5320(82)90038-7. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]