Abstract
Objective: To explore the clinical value of assessing early postoperative blood lipid metabolism levels in predicting anastomotic leakage (AL) after esophageal cancer (EC) surgery. Methods: The clinical data of EC patients who underwent surgery at the Northern Jiangsu People’s Hospital from May 2021 to May 2023 were retrospectively studied. Totally, 28 patients who developed AL were included in the AL group, while 110 patients who did not develop AL were included in the non-AL group. Outcomes compared between the two groups included clinical baseline data, total cholesterol (TC), triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels. Logistic regression analysis was performed to identify independent risk factors for postoperative AL. The predictive value of early postoperative blood lipid metabolism levels for AL was evaluated using Receiver Operating Characteristic (ROC) curves. Results: The AL group exhibited significantly elevated levels of TC and LDL-C but significantly reduced HDL-C levels compared to the non-AL group (all P<0.05). However, there was no significant difference in triglyceride levels between the two groups (P>0.05). Logistic regression analysis revealed that low BMI (P=0.012; OR: 4.409; 95% CI: 1.391-13.976), comorbid hypertension (P=0.011; OR: 5.891; 95% CI: 1.492-23.259), comorbid diabetes (P=0.022; OR: 4.522; 95% CI: 1.238-16.521), low HDL-C (P=0.007; OR: 19.965; 95% CI: 2.293-173.809), and high LDL-C (P=0.012; OR: 4.321; 95% CI: 1.388-13.449) were independent risk factors for developing AL after EC surgery. The combined prediction model using TC, HDL-C, and LDL-C yielded an area under the curve (AUC) of 0.876, with a sensitivity of 79.09%, specificity of 85.71%, and overall accuracy of 80.44%, significantly outperforming individual lipid measurements. Conclusion: The combined assessment of TC, HDL-C, and LDL-C can effectively predict the occurrence of AL after EC surgery. For EC patients with relatively low BMI, hypertension, diabetes, relatively low HDL-C, and relatively high LDL-C, prioritizing weight management, hypertension and diabetes control, and lipid management can significantly reduce the risk of AL post-surgery.
Keywords: Lipid metabolism, esophageal cancer, postoperative anastomotic leakage, risk factors, predictive value
Introduction
Esophageal cancer (EC) is a prevalent malignancy of the digestive system in China, and surgery is the main treatment modality [1]. However, EC surgery is associated with long operation time, intricate techniques, considerable patient trauma, and a high risk of postoperative complications. Anastomotic leakage (AL) is one of the serious complications after EC surgery [2]. AL often leads to systemic toxic symptoms in patients, exacerbating the already poor nutritional status of those with EC, thereby substantially increasing the risk of circulatory and respiratory failure [3]. Therefore, it is of particular importance to identify high-risk factors for AL after EC surgery for timely and effective intervention.
Currently, predicting the occurrence of AL after EC surgery remains challenging. In clinical practice, prediction is often based on preoperative assessment (age, gender, body mass index), analysis of intraoperative factors (degree of surgical trauma, operation time, intraoperative blood loss), and postoperative monitoring [4]. However, given the complex nature of EC surgery and the multifactorial nature of its associated complications, there is no universally accepted standardized approach for predicting AL [5]. Research in other surgical fields has shown that dyslipidemia may be associated with an increased risk of postoperative complications [6-8]. Blood lipid metabolism levels, such as high cholesterol, high triglycerides, and abnormal lipoprotein profiles, may be related to inflammation, immune function suppression, and impaired tissue repair capacity, which could affect postoperative recovery and prognosis [9]. However, there is currently limited research on the association between early postoperative blood lipid metabolism levels and AL after EC surgery.
Accordingly, this study explored the clinical value of early postoperative blood lipid metabolism in predicting the occurrence of AL after EC surgery, aiming to provide a more comprehensive evaluation of postoperative risk and help develop individualized management strategies for EC patients. This approach provides a novel perspective and methodology by using blood lipid metabolism indices for anticipating AL post-surgery, potentially enhancing patient outcome through more precise and proactive intervention.
Materials and methods
Sample source
With the approval from the Medical Ethics Committee of the Northern Jiangsu People’s Hospital (LW2023260), the clinical data of EC patients who underwent surgery at our hospital from May 2021 to May 2023 were retrospectively studied.
Sample size determination
We anticipated a 20% effect size difference and employed a two-tailed t-test for hypothesis testing, with a significance level (α) set at 0.05 and a statistical power (1-β) of 0.80. We utilized the sample size calculation formula to determine the minimum required sample size: n = (Zα/2 + Zβ)^2 * (2 * σ^2)/Δ^2. Based on estimates of the standard deviation from similar research, we assumed a population standard deviation of 10. Finally, we obtained that at least 138 patients were required to achieve sufficient statistical power.
Patient screening and grouping
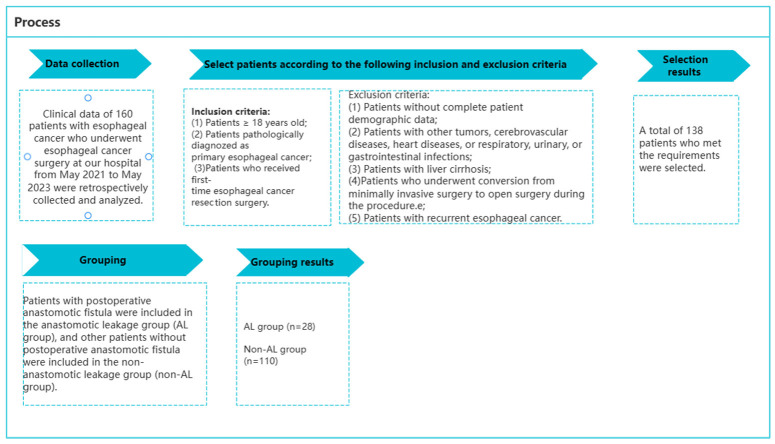
Based on the following criteria, 138 eligible patients were selected from the initial pool of 160 patients. Inclusion criteria: (1) patients with age ≥18 years; (2) patients pathologically diagnosed with primary EC [1]; (3) patients who underwent first-time EC resection surgery. Exclusion criteria: (1) patients with incomplete basic patient information; (2) patients with other tumors, including cerebrovascular diseases, heart diseases, respiratory, urinary, or gastrointestinal infections; (3) patients with liver cirrhosis; (4) patients who required intraoperative conversion to open abdominal or thoracic surgery; (5) patients with recurrent EC. Based on the occurrence of postoperative AL, 28 patients who developed AL were included in the AL group, while the other 110 patients who did not develop AL were included in the non-AL group, as shown in Figure 1.
Figure 1.
Patient screening and grouping process.
Data collection
Clinical and laboratory data were meticulously gathered from patient outpatient and medical records, including age, gender, body mass index (BMI), history of alcohol consumption, history of smoking, comorbidities, place of residence, postoperative hemoglobin levels, postoperative white blood cell count, intraoperative blood loss, and early postoperative blood lipid metabolism indices.
Outcome measures
(1) The clinical baseline data of the two groups were compared. (2) The total cholesterol (TC), triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were compared between the two groups. For this purpose, fasting venous blood (5 mL) was collected from each patient in the early morning and analyzed using a fully automated biochemical analyzer. (3) Logistic regression analysis was performed to identify independent risk factors for postoperative AL. (4) The predictive value of early postoperative blood lipid metabolism for AL was evaluated using Receiver Operating Characteristic (ROC) curves.
Statistical analyses
Statistical analysis was performed using SPSS 20.0, and graphical representation was realized by using GraphPad Prism 7. Counted data were expressed as [n (%)] and compared using χ2 test. Measured data were expressed as mean ± standard deviation, and the inter-group comparison was conducted using t-test. Logistic regression analysis was conducted to identify the risk factors influencing the occurrence of AL after EC surgery. Receiver Operating Characteristic (ROC) curve analysis was used to determine the predictive value of early postoperative blood lipid metabolism levels for the occurrence of AL. P<0.05 was indicative of a significant difference.
Results
Comparison of clinical baseline data
The clinical data of the AL group and non-AL group were analyzed and compared (Table 1). There were no significant differences in gender, postoperative white blood cell count, intraoperative blood loss, drinking history, smoking history or place of residence between the two groups (all P>0.05). However, significant differences were noted in age, BMI, postoperative hemoglobin, diabetes and hypertension (all P<0.05).
Table 1.
Univariate analysis of factors influencing the occurrence of anastomotic leakage after radical resection for EC
| Factor | AL group (n=28) | Non-AL group (n=110) | P value | |
|---|---|---|---|---|
| Age | 65.9±7.0 | 60.6±5.2 | <0.0001 | |
| Sex | Male/female | 20/8 | 74/36 | 0.6524 |
| BMI (kg/m2) | 18.58±2.21 | 20.56±3.02 | 0.0014 | |
| Hemoglobin after operation (g/L) | 100.08±18.88 | 109.80±20.0 | 0.0221 | |
| Postoperative white blood cell count (109/L) | 12.66±2.87 | 12.19±3.10 | 0.4729 | |
| Intraoperative blood loss (mL) | 98.17±10.70 | 97.01±13.26 | 0.6669 | |
| History of drinking | Yes/no | 6/22 | 22/88 | 0.8667 |
| History of smoking | Yes/no | 8/20 | 32/78 | 0.9569 |
| Comorbid diabetics | Yes/no | 10/18 | 20/90 | 0.0446 |
| Comorbid hypertension | Yes/no | 9/19 | 17/93 | 0.0438 |
| Place of residence | Rural/urban areas | 15/13 | 71/39 | 0.2847 |
Note: BMI: Body mass index.
Comparison of early postoperative blood lipid metabolism levels between the two groups
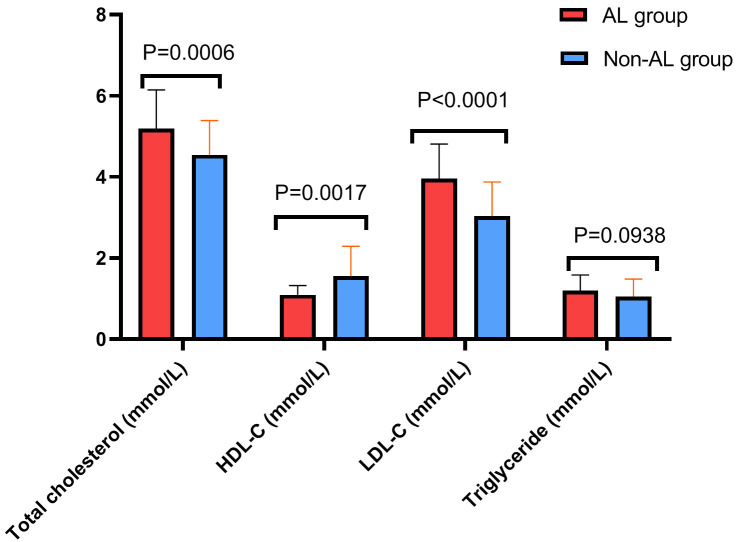
The lipid profiles, specifically TC, triglycerides, HDL-C, and LDL-C, were compared early postoperatively between the two groups. The results revealed that the AL group exhibited significantly higher levels of TC and LDL-C compared to the non-AL group (all P<0.05) and significantly lower HDL-C level than the non-AL group (P<0.05). No significant difference was found between the two groups in triglyceride levels (P>0.05, Figure 2).
Figure 2.
Comparison of blood lipid metabolism in early postoperative period between the two groups. Notes: TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol.
Factors influencing the occurrence of AL after radical resection of EC
Assignment was made to the notably different indexes in clinical data comparison and TC, HDL-C and LDL-C (Table 2) for a subsequent multivariate analysis. Logistic regression analysis identified relatively low BMI, comorbid hypertension, comorbid diabetes, relatively low HDL-C, and relatively high LDL-C as independent risk factors influencing the occurrence of AL after EC surgery (Table 3).
Table 2.
Variable assignment
| Factor | Assignment |
|---|---|
| Age | ≥61=1, <61=0 |
| BMI | <20=1, ≥20=0 |
| Hemoglobin after operation (g/L) | <107=1, ≥107=0 |
| Comorbid diabetics | Yes =1, None =0 |
| Comorbid hypertension | Yes =1, None =0 |
| Total cholesterol (mmol/L) | ≥4.6=1, <4.6=0 |
| HDL-C (mmol/L) | <1.5=1, ≥1.5=0 |
| LDL-C (mmol/L) | ≥3.2=1, <3.2=0 |
| Postoperative anastomotic leakage | Yes =1, None =0 |
Notes: BMI: Body mass index; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol.
Table 3.
Multivariate logistic regression analysis
| Factors | B | S.E. | Wals | df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| Age | 1.219 | 0.626 | 3.792 | 1 | 0.051 | 3.383 | 0.992 | 11.537 |
| BMI | 1.484 | 0.589 | 6.351 | 1 | 0.012 | 4.409 | 1.391 | 13.976 |
| Hemoglobin after operation (g/L) | 0.457 | 0.564 | 0.656 | 1 | 0.418 | 1.579 | 0.523 | 4.768 |
| Comorbid diabetics | 1.509 | 0.661 | 5.211 | 1 | 0.022 | 4.522 | 1.238 | 16.521 |
| Comorbid hypertension | 1.773 | 0.701 | 6.405 | 1 | 0.011 | 5.891 | 1.492 | 23.259 |
| Total cholesterol (mmol/L) | 0.438 | 0.605 | 0.524 | 1 | 0.469 | 1.549 | 0.474 | 5.069 |
| HDL-C (mmol/L) | 2.994 | 1.104 | 7.353 | 1 | 0.007 | 19.965 | 2.293 | 173.809 |
| LDL-C (mmol/L) | 1.463 | 0.579 | 6.380 | 1 | 0.012 | 4.321 | 1.388 | 13.449 |
Notes: BMI: Body mass index; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol.
Prediction efficacy of blood lipid metabolism levels for the occurrence of AL after radical resection of EC
Finally, the predictive power of TC, HDL-C, LDL-C and their combination in predicting AL after radical resection of EC was analyzed. ROC curve analysis revealed that the joint prediction using TC, HDL-C, and LDL-C for the occurrence of AL after EC surgery had an AUC of 0.876, which was significantly higher than the individual predictions (P<0.05). Furthermore, the joint prediction had higher accuracy than individual predictions (Figure 3 and Table 4).
Figure 3.
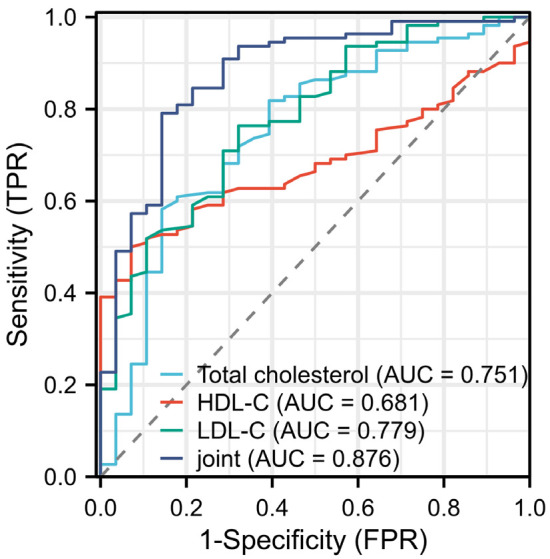
ROC curve analysis of lipid metabolism levels for predicting anastomotic leakage after radical resection of EC. Notes: TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol.
Table 4.
ROC curve values of lipid metabolism level in predicting anastomotic leakage after radical operation for esophageal cancer
| Total cholesterol | High density lipoprotein cholesterol | Low density lipoprotein cholesterol | Combination | |
|---|---|---|---|---|
| AUC | 0.751 | 0.681 | 0.779 | 0.876 |
| Sensitivity | 58.18% | 50.00% | 76.63% | 79.09% |
| Specificity | 85.71% | 92.86% | 67.86% | 85.71% |
| Accuracy | 63.77% | 58.70% | 74.64% | 80.44% |
Note: AUC: Area under the curve.
Discussion
Anastomotic leakage (AL) is one of the most serious complications following EC resection, with high morbidity and mortality rates [10]. This severe complication not only prolongs the postoperative hospital stay, but also significantly reduces the quality of life, and may delay treatment and promote tumor recurrence [11,12]. Therefore, achieving real-time monitoring and early warning is crucial in reducing the negative impact of AL on patients.
This study investigated the clinical value of assessing early postoperative TC, HDL-C, and LDL-C levels in predicting the occurrence of AL after EC surgery. The results demonstrated that the combined prediction using these three factors exhibited significantly higher predictive efficacy compared to the individual predictions.
Some studies suggest that blood lipid metabolism markers can serve as prognostic indicators for cancer patients [13,14]. Clinically, serum lipid levels are used to assess body lipid metabolism. The manifests of abnormal blood lipid levels include increased TC, triglycerides, increased LDL-C concentration, and decreased HDL-C concentration. In recent years, studies have found that blood lipid metabolism levels can affect the development of various types of cancer [15,16]. In this study, patients who developed AL after EC surgery had notably higher levels of TC and LDL-C compared to those without AL. On the other hand, HDL-C levels were notably lower in the AL group. These findings align with the aforementioned viewpoint. He et al. [17] identified several risk factors for AL after colon cancer surgery, including male gender, BMI, obesity, comorbid lung disease, anesthesia, ASA score, emergency surgery, open surgery, and resection method. This study also found that low BMI was a risk factor for AL after EC surgery. A lower BMI may indicate inadequate nutritional and energetic reserves necessary for effective wound healing and immune function [18-20]. In the study by van Kooten et al. [21], hypertension was identified as a prognostic factor influencing AL. Kentaro Maejima et al. [22] identified diabetes as an independent risk factor for esophagojejunal AL and pointed out that diabetes affected wound healing, not just at surgical incision sites but also at intestinal anastomoses. Consistent with these studies, this research also confirmed that both hypertension and diabetes are independent risk factors for AL after EC surgery. The combination of hypertension and diabetes leads to inadequate blood supply, hinders tissue repair, and increases risk of infection. Hence, it is crucial to emphasize the importance of preoperative and postoperative blood pressure control as well as glucose management in order to mitigate the risk of AL. Furthermore, low HDL-C and high LDL-C levels are associated with increased inflammation, decreased immune function, and abnormal vascular function, all of which may interfere with the wound healing process and increase the risk of AL [23,24]. Aligning with these reports, this study, through logistic regression analysis, found that low HDL-C and high LDL-C were independent risk factors influencing the occurrence of AL after EC surgery.
Due to the lack of significant differences in triglyceride levels between the two groups, the final analysis of this study focused only on the value of TC, HDL-C, LDL-C, and their joint prediction for the occurrence of AL after EC surgery. The joint prediction yielded an AUC of 0.876 and an accuracy of 86.96%, which significantly surpassed the predictive performance of the individual factors.
The study confirms that a combined approach, utilizing TC, HDL-C, and LDL-C, can effectively predict the occurrence of AL following EC surgery. For EC patients with relatively low BMI, hypertension, diabetes, relatively low HDL-C, and relatively high LDL-C, it is recommended to optimize weight management, control hypertension and diabetes, and manage lipid levels to reduce the occurrence of AL. However, it is important to note that this study’s retrospective case-control design inherently limits the scope of its findings. The selection of patients who underwent EC surgery was based on the results obtained from medical records. The inclusion of patients and the available data on risk factors were limited, which may have affected the conclusions drawn from the study.
Disclosure of conflict of interest
None.
References
- 1.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210–215. doi: 10.1016/j.asjsur.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Schizas D, Vailas M, Sotiropoulou M, Ziogas IA, Mylonas KS, Katsaros I, Kapelouzou A, Liakakos T. Surgery for metachronous oligometastatic esophageal cancer: is there enough evidence? Cir Esp (Engl Ed) 2021;99:490–499. doi: 10.1016/j.cireng.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. 2022;40:101708. doi: 10.1016/j.suronc.2022.101708. [DOI] [PubMed] [Google Scholar]
- 4.Bolívar-Rodríguez MA, Magaña-Olivas F, Cázarez-Aguilar MA, Pamanes-Lozano A, Osuna-Wong BA, Peraza-Garay FJ. Risk factors associated to intestinal anastomotic leakage in elective surgery. Cir Cir. 2022;90:84–89. doi: 10.24875/CIRU.20001324. [DOI] [PubMed] [Google Scholar]
- 5.Ishimaru T, Shinjo D, Fujiogi M, Michihata N, Morita K, Hayashi K, Tachimori H, Kawashima H, Fujishiro J, Yasunaga H. Risk factors for postoperative anastomotic leakage after repair of esophageal atresia: a retrospective nationwide database study. Surg Today. 2023;53:1269–1274. doi: 10.1007/s00595-023-02682-0. [DOI] [PubMed] [Google Scholar]
- 6.Song R, Hu M, Qin X, Qiu L, Wang P, Zhang X, Liu R, Wang X. The roles of lipid metabolism in the pathogenesis of chronic diseases in the elderly. Nutrients. 2023;15:3433. doi: 10.3390/nu15153433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian H, Zhao X, Zhang Y, Xia Z. Abnormalities of glucose and lipid metabolism in myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2023;163:114827. doi: 10.1016/j.biopha.2023.114827. [DOI] [PubMed] [Google Scholar]
- 8.Ai XM, Ho LC, Han LL, Lu JJ, Yue X, Yang NY. The role of splenectomy in lipid metabolism and atherosclerosis (AS) Lipids Health Dis. 2018;17:186. doi: 10.1186/s12944-018-0841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Yi H, Guan J, Zou J, Xu H, Liu Y. Effect of smoking on glucose, lipid metabolism and sleep structure in postoperative patients with obstructive sleep apnea. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;35:146–151. doi: 10.13201/j.issn.2096-7993.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liesenfeld LF, Sauer P, Diener MK, Hinz U, Schmidt T, Müller-Stich BP, Hackert T, Büchler MW, Schaible A. Prognostic value of inflammatory markers for detecting anastomotic leakage after esophageal resection. BMC Surg. 2020;20:324. doi: 10.1186/s12893-020-00995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H, Song X, Li J, Zhao G. Application of mediastinal drainage tube in intrathoracic esophageal anastomotic leakage for early diagnosis and effective treatment: a retrospective study. J Cardiothorac Surg. 2021;16:52. doi: 10.1186/s13019-021-01435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu WQ, Gao HJ, Shi GD, Tang JY, Wang HF, Hu SY, Wei YC. Development and validation of a nomogram to predict anastomotic leakage after esophagectomy for esophageal carcinoma. J Thorac Dis. 2021;13:3549–3565. doi: 10.21037/jtd-21-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Z, Ye F, Liang Y, Xu C, Zhang Z, Ou Y, Chen X, Dai X, Mou Z, Li W, Chen Y, Zhou Q, Zou L, Mao S, Jiang H. Blood lipids, lipid-regulatory medications, and risk of bladder cancer: a mendelian randomization study. Front Nutr. 2023;10:992608. doi: 10.3389/fnut.2023.992608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pope ED 3rd, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA 3rd, Mody K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets. 2019;23:473–483. doi: 10.1080/14728222.2019.1615883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Xu Y, Zeng X, Tan J, Wang Y, Wu H, Li M, Yi C. Etiological relationship between lipid metabolism and endometrial carcinoma. Lipids Health Dis. 2023;22:116. doi: 10.1186/s12944-023-01868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi A, Li Y, Yan S, Sun H, Zhao M, Chen Y. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg. 2021;10:1470–1477. doi: 10.21037/gs-21-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, He M, Tang JH, Wang XH. Anastomotic leak risk factors following colon cancer resection: a systematic review and meta-analysis. Langenbecks Arch Surg. 2023;408:252. doi: 10.1007/s00423-023-02989-z. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Kim E, Beltran C, Cho S. Corticosterone-mediated body weight loss is an important catabolic process for poststroke immunity and survival. Stroke. 2019;50:2539–2546. doi: 10.1161/STROKEAHA.119.026053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal V, Subitha L, Medha R, Deepanjali S. Impact of nutrition status and body mass index on mortality in hospitalized general medical patients: a prospective observational study. Nutr Clin Pract. 2022;37:1316–1325. doi: 10.1002/ncp.10896. [DOI] [PubMed] [Google Scholar]
- 20.Murphy N, Newton CC, Song M, Papadimitriou N, Hoffmeister M, Phipps AI, Harrison TA, Newcomb PA, Aglago EK, Berndt SI, Brenner H, Buchanan DD, Cao Y, Chan AT, Chen X, Cheng I, Chang-Claude J, Dimou N, Drew D, Farris AB, French AJ, Gallinger S, Georgeson P, Giannakis M, Giles GG, Gruber SB, Harlid S, Hsu L, Huang WY, Jenkins MA, Laskar RS, Le Marchand L, Limburg P, Lin Y, Mandic M, Nowak JA, Obón-Santacana M, Ogino S, Qu C, Sakoda LC, Schoen RE, Southey MC, Stadler ZK, Steinfelder RS, Sun W, Thibodeau SN, Toland AE, Trinh QM, Tsilidis KK, Ugai T, Van Guelpen B, Wang X, Woods MO, Zaidi SH, Gunter MJ, Peters U, Campbell PT. Body mass index and molecular subtypes of colorectal cancer. J Natl Cancer Inst. 2023;115:165–173. doi: 10.1093/jnci/djac215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Kooten RT, Voeten DM, Steyerberg EW, Hartgrink HH, van Berge Henegouwen MI, van Hillegersberg R, Tollenaar RAEM, Wouters MWJM. Patient-related prognostic factors for anastomotic leakage, major complications, and short-term mortality following esophagectomy for cancer: a systematic review and meta-analyses. Ann Surg Oncol. 2022;29:1358–1373. doi: 10.1245/s10434-021-10734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maejima K, Taniai N, Yoshida H. Risk factors for esophagojejunal anastomotic leakage in gastric cancer patients after total gastrectomy. J Nippon Med Sch. 2023;90:64–68. doi: 10.1272/jnms.JNMS.2023_90-111. [DOI] [PubMed] [Google Scholar]
- 23.Du W, Wang Z, Dong Y, Hu H, Zhou H, He X, Hu J, Li Y. Electroacupuncture promotes skin wound repair by improving lipid metabolism and inhibiting ferroptosis. J Cell Mol Med. 2023;27:2308–2320. doi: 10.1111/jcmm.17811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pils V, Terlecki-Zaniewicz L, Schosserer M, Grillari J, Lämmermann I. The role of lipid-based signalling in wound healing and senescence. Mech Ageing Dev. 2021;198:111527. doi: 10.1016/j.mad.2021.111527. [DOI] [PubMed] [Google Scholar]