Abstract
Objective: Patients with Asherman’s Syndrome (AS) and an endometrial thickness (EMT) less than 7 mm are infertile women with suboptimal endometrium due to uterine scarring or endometrial atrophy. This study aimed to examine the effect of intrauterine injections of adipose-derived mesenchymal stem cells (ADMSC) from the Stromal Vascular Fraction (SVF) of adipose tissue on EMT and in vitro fertilization (IVF) outcomes: which are improvements in EMT and pregnancy rates. Methods: This double-arm retrospective study included 41 AS patients with hysteroscopic adhesiolysis. Twenty-one patients with AS refractory endometrium (Group 2) were given ADMSC to improve EMT, and 20 non-treated, age-matched patients served as controls (Group 1). For Group 2, SVF was isolated from 15 ml of adipose tissue and transmyometrial injected into the patient’s uterine cavity. For all patients, EMT was examined using ultrasound before embryo transfer. Results: In Group 2, after ADMSC treatment, EMT significantly improved (3.2 ± 1.8 mm, P<0.001). Afterward, three patients spontaneously became pregnant, and eighteen underwent frozen embryo transfer. A significant increase in implantation (66.7% vs. 4.8%, P = 0.002) and live birth rates (0.0% vs. 47.6%, P = 0.001) were recorded. No significant difference was observed in EMT, cycle implantation, or clinical pregnancy between the two groups, but the live birth rate in Group 2 after ADMSC treatment was higher than in Group 1. Conclusion: The results demonstrate that autologous intrauterine ADMSC injection can improve EMT, implantation, and pregnancy rates in AS patients with refractory endometrium. This research underscores the life-changing potential of autologous ADMSC treatment for patients with refractory endometrium, providing a promising avenue for future treatments.
Keywords: Endometrial receptivity, frozen embryo transfer, stromal vascular fraction, Asherman’s syndrome, recurrent implantation failure, refractory thin endometrium
Introduction
Endometrium cycling between proliferative and secretory phases with each menstrual cycle has a significant role in assisted reproduction technology (ART) [1]. Embryo implantation and growth depend on the coordinated crosstalk between intrauterine factors and the embryo [2]. An optimal endometrium for embryo transfer is trilaminar with a minimum thickness of 7 mm [3-5]. Therefore, the endometrium health is a key factor for successful pregnancies.
Affecting four in every 10,000 women, Asherman Syndrome (AS) is considered a rare disease caused by endometrial trauma leaving intrauterine adhesions [6]. Atrophy produces a thin and incapable endometrium (less than 5 mm) [7], leading to a lack of endometrial responsiveness and infertility. Like other tissues, the uterus possesses a stem cell niche with resident adult stem cells called endometrial mesenchymal stem cells (EnMSC) [8]. Endometrial regeneration occurs in patients with AS if EnMSC can replace the functional endometrial layer [9]. To mimic this effect when the endometrial stem cell niche is damaged or diminished, the therapeutical use of MSC is a promising approach to treating female infertility [9-12], improving endometrial quality for achieving pregnancy [9,13].
MSC can be obtained from different tissue sources. Autologous adipose-derived stem cells are easily obtained and avoid graft rejection after transplantation. This easy-to-access tissue yields significant quantities of MSC with a minimum invasive technique encompassing a low risk of morbidity, minimal patient discomfort, and little chance of other possible complications [11]. Adipose-derived mesenchymal stem cells (ADMSC) have prolonged self-renewal ability, the capability to differentiate into various mature somatic lineages [14], and possess neovascularization, immune-modulating, and anti-inflammatory properties [15]. The Stromal Vascular Fraction (SVF) is a minimum manipulated heterogeneous cell population isolated from adipose tissue with comparable regenerative potential to cultured ADMSC. SVF contains ADMSC, endothelial precursors, T-regulatory and smooth muscle cells, macrophages, pericytes, and preadipocytes [16]. Thus, the SVF could be an optimal source of MSC for improving endometrial quality.
Here, we explore the efficiency of intrauterine implantation of autologous ADMSC from the SVF to rehabilitate endometrial tissue and to improve reproductive outcomes in AS patients with refractory endometrium subjected to ART.
Materials and methods
Study design and participants
Charts were reviewed for patients with AS undergoing IVF who attended the Ingenes Institute in México City between July 2020 and June 2022. AS severity was assessed using the American Fertility Society 1988 classification [17]. This double-arm retrospective study included a non-treated historical control cohort matching for age and BMI with hysteroscopic adhesiolysis performed during the same period (Group 1). Another group consisted of patients with refractory endometrium (EMT <7 mm at Day 10 of endometrial preparation for embryo transfer) and a failed cycle after hysteroscopic adhesiolysis that underwent ADMSC treatment (Group 2). To be considered for the treatment, a case-by-case evaluation was performed at the weekly multidisciplinary review meeting held by specialists in Reproductive Endocrinology and Infertility, an embryologist, and research staff. For the decision-making process, factors considered were findings during the hysteroscopic correction procedure, age, embryo availability, and potential medical and surgical comorbidities. Failure of previous conservative management options (hysteroscopic synechiae resection, high dose of vaginal estradiol valerate) was validated. After reaching a consensus, a board-certified plastic surgeon evaluated and cleared the patient for micro-liposuction.
The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [18]. The inclusion criteria were: 1) between 30 to 49 years at the time of enrollment; 2) have AS and hysteroscopic adhesiolysis; 3) had a history of at least one failed IVF cycle at our center (embryos transferred but no pregnancy); and 4) had good quality embryos (at least BC grade) available for embryo transfer (from donated ova if the patient was >39 years). The patients were excluded if it was documented or suspected that 1) they suffered from an auto-immune disease, thrombophilia, hematologic disorders, uncontrolled endocrine or other medical conditions; 2) the patient suffered congenital and untreated acquired uterine abnormalities; 3) couples with genetic and chromosomal abnormalities; or 4) only produced poor-quality embryos and were not willing to use gamete donation. Patients were contacted, and written informed consent was obtained from those who agreed, following the Declaration of Helsinki.
The primary outcome was to evaluate the maximal EMT reached during endometrial preparation. The secondary outcomes assessed were the implantation and the clinical pregnancy rates. The Ethics Committee of the Ingenes Institute approved this study (ISF190220).
In vitro fertilization
Patients underwent controlled ovarian stimulation for 10-14 days with gonadotrophin-releasing hormone agonists and antagonists 0.25 mg/day Cetrotide (Merck) or 0.25 mg/day Ganirelix acetate (Orgalutran MSD) in the luteal phase after menses (variable doses with a minimal daily dose of 300 IU, adjusted for the patient’s age, ovarian responsiveness, and serum estradiol levels). The ovarian response was assessed by measuring serum estradiol levels, and follicular development was evaluated by ultrasound examination every 2-3 days. Oocyte retrieval was conducted 36 hours after the human chorionic gonadotropin (hCG) administration (Choriomon 5000 U, IBSA). At the same time, the partner’s semen was prepared by density gradient centrifugation. The oocytes were inseminated by intracytoplasmic sperm injection, and fertilization was judged by forming two pronuclei 19 hours after insemination. Embryos were cultured in Global Total for Fertilization media (Cat#LGGT-30, Life Global) and incubated at 37°C in 8% CO2, 5% O2, and 87% N2. For patients only producing low-quality embryos in previous cycles, embryos were obtained using donated oocytes under the patient’s approval. An embryologist monitored and recorded all information about the embryo’s development.
Hysteroscopic adhesiolysis
Hysteroscopic adhesiolysis was performed under sedation in all patients. The cervix was initially dilated using Hegar’s dilators. An 8-mm 12° rigid telescope (Olympus) was introduced into the uterine cavity. A standard saline solution (A81324B, Baxter) was used as a distending medium through an automated hysteroscopic distension pump. Adhesiolysis was performed with sharp scissors. To guide the extent of adhesiolysis and prevent uterine perforation, concomitant transabdominal ultrasonography was performed in patients with extensive and dense adhesions and was diagnosed using office hysteroscopy. Cefazolin sodium (1 g) was administered to all patients during the procedure.
Isolation and characterization of ADMSCs in SVF
Adipose tissue was obtained by micro-liposuction from the patient’s abdominal periumbilical fat. Tumescent local anesthesia was induced by applying 20 ml of Klein’s solution in the subdermal compartment with a 2.4×20 mm infiltration cannula (Infiltrator Cannula, Tulip Medical Products). Fifteen minutes later, 20 ml of adipose tissue was aspirated with a 2.4×20 mm liposuction cannula (Sforza Cannula, Tulip Medical Products). Adipose tissue was immediately processed. Lipoaspirate was washed with PBS (Cat#10010023, Gibco), mechanically disaggregated, and treated with 1% collagenase type I (Cat#C0130, Sigma-Aldrich) to isolate SVF. Pelleted SVF was filtered through a 100 μm sterile nylon mesh to remove any remaining tissue fragments. Isolated cells were washed and counted. Their viability was assessed with trypan blue staining (Cat#T8154, Sigma-Aldrich) before trans-myometrial injection.
The autologous MSC from the SVF from adipose was assessed according to the International Society for Cell and Gene Therapy’s minimal criteria for defining multipotent MSC [14]. Cells were cultured in high glucose Dulbecco’s Modified Eagle’s Medium (Cat#D6429, Sigma-Aldrich) supplemented with 10% Fetal Bovine Serum (Cat#A5955, Gibco, Grand Island, USA) and 1% Antibiotic Antimycotic Solution (Cat#A5955, Sigma-Aldrich, Steinheim, Germany; final concentration of 100 U penicillin, 0.1 mg streptomycin, and 0.25 μg amphotericin B/mL). MSC presented the ability to attach to the plastic surface in culture, fibroblast shape, and the formation of fibroblast-like colonies. Culture media was replaced every 4-5 days, and cells were passaged when 80% confluence was achieved. MSC phenotype was assessed by flow cytometry at the Laboratorio Nacional de Citometría de Flujo at Universidad Nacional Autónoma de México. MSCs were stained with antibodies against human CD73, CD90, CD105, CD44, CD45, CD34 (Cat#ab9375, ab133582, ab254022, Abcam), and viability marker VivaFIX (Cat#1351112, Bio-Rad). Data were acquired using the Attune NxT Flow Cytometer (ThermoFisher) and calibrated with Attune’s tracking beads (Cat#4449754, ThermoFisher). No staining and single staining controls were used for voltage calibration in each channel in the negative region and spectral superposition correction. Fluorescence-negative controls were applied to distinguish negative zones from positive target zones. MCS’s plasticity was confirmed by differentiation towards chondrogenic lineage. MSCs were cultured for 21 days in the presence of lineage-specific induction factors (MesenCult-ACF Chondrogenic Differentiation Kit, Cat#05455, STEMCELL Technologies). High-density micromass pellets were stained with Alcian Blue (Cat#B8438, Sigma-Aldrich) to confirm differentiation. Cells were observed with a VWR® VistaVision inverted brightfield microscope using a 10/0.25 objective.
A hypersensitivity test was performed before the transmyometrial injection. 0.1 ml of the cell suspension was subcutaneously injected into the patient’s right forearm to detect any possible undesirable reaction attributed to the cellular product or any trace remaining reagent used for SVF isolation. The test was considered negative if no response was observed at the application site within 15-20 minutes. If any hives, redness, or itchiness were reported, the test was considered positive, and the procedure was canceled.
Autologous ADMSC implantation
After successfully isolating the adipose tissue’s SVF and having a negative result from the hypersensitivity test, patients were taken to the operating room for stem cell implantation, according to Sudoma et al., with slight modifications [11]. The procedure was performed under intravenous anesthesia and prophylactic antibiotic treatment with the patient in a lithotomy position. Asepsis and antisepsis of the external genitalia were performed with surgical soap, in which a medium-sized vaginal mirror was placed for vaginal double cleansing. Under transvaginal ultrasound guidance, the uterus, ovaries, and adnexal structures were located, and the endometrium was identified. Doppler ultrasound was performed to identify the most vascularized myometrial zone to inject the autologous SVF using an ovum-pick 35 cm 17G single-lumen needle (Cat#G55490, Cook Medical). Under the transversal uterine plane, autologous SVF was implanted transmyometrial by injecting 3 ml of the autologous cell suspension at the uterine cavity’s fundus, anterior and posterior regions at the myometrium layer. Vascular integrity and absence of free pelvic fluid were assessed. Afterward, the patient was taken to the recovery room and discharged 60-90 minutes later with antibiotics and oral analgesic therapy. Uterine cavity status was assessed one week after the procedure. If no complications were observed, endometrial quality was evaluated by ultrasound monthly for at least three months before embryo transfer was considered.
Embryo transfer
Patients were transferred with frozen-thawed embryos during an estrogen-primed cycle. With the patient’s approval, the physician determined which cycle and the number of embryos to transfer. The uterine transfer occurred during a controlled endometrial development cycle free of gonadotropins. Endometrial preparation was performed with estrogen valerate starting on menstrual cycle Day 2 or 3 in a 10-day increasing dose scheme, beginning with 2 mg/daily until a maximum of 8 mg/daily, assessing EMT every other day via ultrasound. Then 800 mg/day of micronized progesterone for luteal phase support was administered for five days. Day 5 or Day 6 blastocysts were transferred. Embryo implantation was confirmed on Day 14 by serum β-hCG concentrations >10 mUI/ml (Cat#105310, Roche) and clinical pregnancy by the presence of a fetal heartbeat using ultrasound at 6-8 weeks. All the patients’ demographics, IVF cycle information, implantation rate, EMT, and IVF outcomes (pregnancies and miscarriages) were recorded by the physician.
Sample size
For the pre and post-ADMSC treatment comparison, the sample size was calculated using the SCCS package [19,20]. The inputs were based on the results from similar studies for the pre- [11,21] and post-treatment groups [22,23], in which the clinical pregnancy rate for the control (<6 mm) and the treated group was 11.1% and 72.2%, respectively. Therefore, with an alpha = 0.05 and power = 0.80, the sample size was calculated to be 11 subjects.
Statistical analysis
P-values <0.05 (two-tailed) were considered statistically significant. Data are the mean ± standard deviation unless otherwise indicated. Analyses were performed with the GraphPad Prism Software version 5.00. The chi-squared or Fisher’s Exact tests were performed to compare the two groups for categorical data. T-tests were used to compare the characteristics of study participants (independent) and pre-and post-treatment EMT (paired). One-way ANOVA, followed by a post hoc Tuckey test, was used to compare the net EMT differences between groups when stratified by the obstetric outcome. The two-way Repeated Measures ANOVA determined differences between pre-and post-treatment EMT by implantation success or pregnancy success, whereas the McNemar test analyzed implantation, clinical pregnancy, and live birth rates.
Results
Identification of study participants
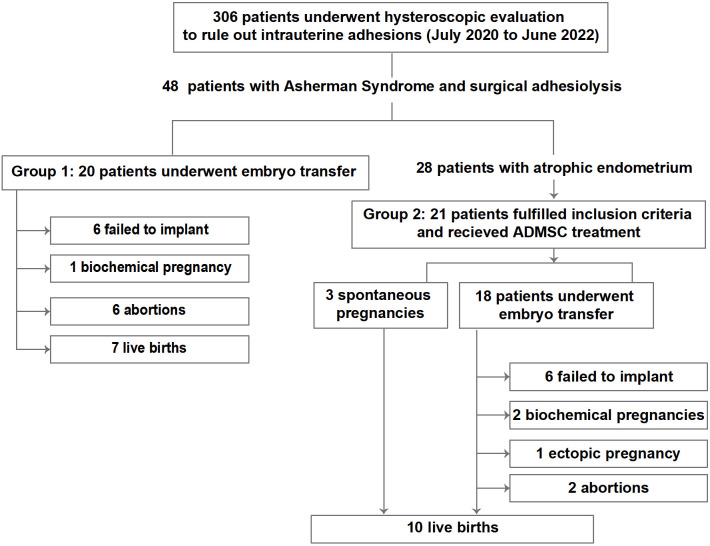
During the two years, 306 patients were subjected to standard hysteroscopic evaluation to rule out uterine adhesions. Forty-eight patients were diagnosed with AS, and as a standard procedure, hysteroscopic adhesiolysis was performed. Of 28 patients, only 21 fulfilled the inclusion criteria (Group 2) and underwent ADMSC treatment. Twenty age-matched patients (Group 1) with hysteroscopic adhesiolysis but no ADMSC treatment were included (Figure 1). The mean age was 40.0 ± 4.7 years for the ADMSC treatment group and 37.9 ± 5.9 for the non-treatment group. The groups were well balanced across AS severity (P = 0.8386, Table 1).
Figure 1.
The study flow chart follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Table 1.
Characteristics of study participants
| Category | Group 1 | Group 2 | p-value | 95% CI |
|---|---|---|---|---|
| Hysteroscopic adhesiolysis | Yes | Yes | - | - |
| ADMSC treatment | No | Yes | - | - |
| Sample size (n) | 20 | 21 | - | - |
| Age (years) | 37.9 ± 5.9 | 40.0 ± 4.7 | 0.2160 | -1.277 to 5.477 |
| BMI (kg/m2) | 26.2 ± 3.2 | 24.8 ± 2.4 | 0.1135 | -3.175 to 0.3520 |
| Infertility duration (years) | 4.6 ± 3.2 | 3.5 ± 3.2 | 0.2500 | -3.152 to 0.8448 |
| Previous IVF cycles with implantation failure (n)a | 0.6 ± 0.8 | 0.8 ± 0.9 | 0.4494 | -0.3451 to 0.7642 |
| No. of patients with canceled ET | 11 | 9 | - | - |
| No. of patients with at least one failed cycle | 9 | 12 | - | - |
| AS classification at the time of enrollment | ||||
| Removed IUA at the time of ADMSC application | Not applicable | 33.3% (7/21) | ||
| Stage I, mild | 55.0% (11/20) | 42.9% (9/21) | ||
| Stage II, moderate | 30.0% (6/20) | 14.3% (3/21) | ||
| Stage III, severe | 15.0% (3/20) | 9.5% (2/21) | ||
Abbreviations: 95% CI: 95% confidence interval; ADMSC: adipose-derived mesenchymal stem cells; AS: Asherman’s syndrome; BMI: Body-Mass Index; IVF: in vitro fertilization; ET: embryo transfer; IUA: intrauterine adhesions. Values are percentage (frequency) or average ± standard deviation. P-value was calculated using an unpaired t-test.
The mean of previous IVF cycles with implantation failure is below one as for some patients with poor quality endometrium; their embryo transfer was canceled.
AS classification according to the American Fertility Society 1988 at the time of enrollment [17].
ADMSC treatment
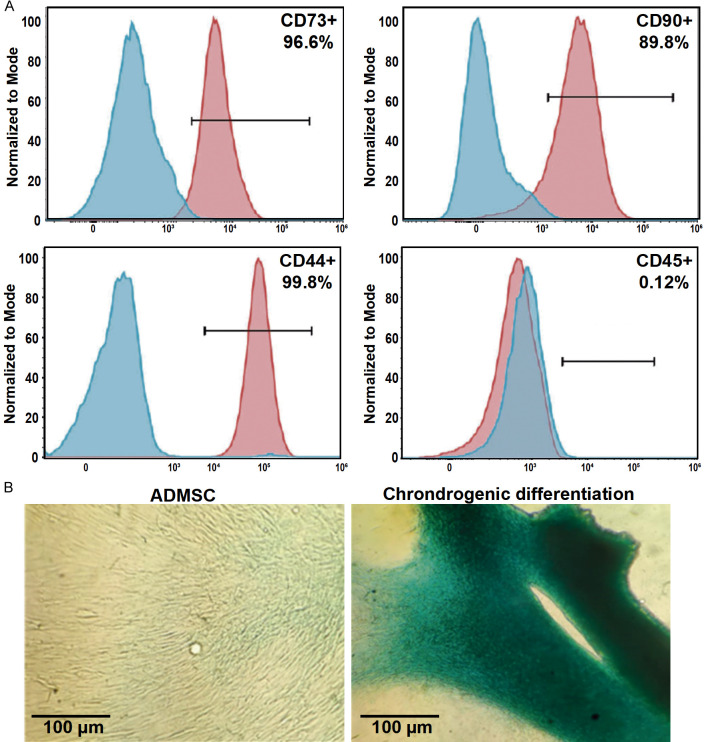
The presence of autologous ADMSC from the SVF was validated with the expression of MSC surface markers, with >90% of the population being positive for CD44, CD73, and CD90, while <2% expressing CD45 (Figure 2). Also, the ability to differentiate into chondrocytes was confirmed. Bruises were the most reported complication, disappearing within five days at the liposuction site (18 patients). No adverse effects related to the ADMSC injection were reported.
Figure 2.
Characterization of cultured human adipose-derived mesenchymal stem cells (ADMSC) contained in the stromal vascular fraction of the adipose tissue. A. Representative flow cytometry results of MSC markers: CD44, CD45, CD73, and CD90. B. Representative images of ADMSC differentiated into chondrocytes: ADMSC cultured under normal (left) and micro mass conditions (right) stained with Alcian Blue.
Of the 21 patients in Group 2, 18 underwent embryo transfer, and three became pregnant naturally. From the 18 IVF cycles, embryo implantation was achieved in 12, resulting in an implantation rate of 66.7% (Figure 1; Table 2). Two biochemical pregnancies, one ectopic pregnancy, and two abortions were recorded. Spontaneous pregnancies and implanted embryos resulted in 10 live births without complications (clinical pregnancy rate = 47.61%, Table 2).
Table 2.
Comparison of pregnancy outcomes in Group 2 before and after ADMSC treatment
| Category | Group 2, before ADMSC | Group 2, after ADMSC | p-value |
|---|---|---|---|
| No. of embryos transferred per cycle | 2.3 ± 0.7 | 2.6 ± 0.8 | 0.1177a |
| Cycle implantation rate | 4.8% (1/21) | 66.7% (12/18) | 0.002*,b |
| Clinical pregnancy rate | 0.0% (0/21) | 57.1% (12/21) | 0.001*,b |
| Live birth rate | 0.0% (0/21) | 47.6% (10/21) | 0.001*,b |
Abbreviations: ADMSC: adipose-derived mesenchymal stem cells. Values are either percentage (frequency) or average ± standard deviation.
p-value was calculated using an unpaired t-test. A significant difference is indicated with an *.
p-value was calculated using the McNemar test. A significant difference is indicated with an *.
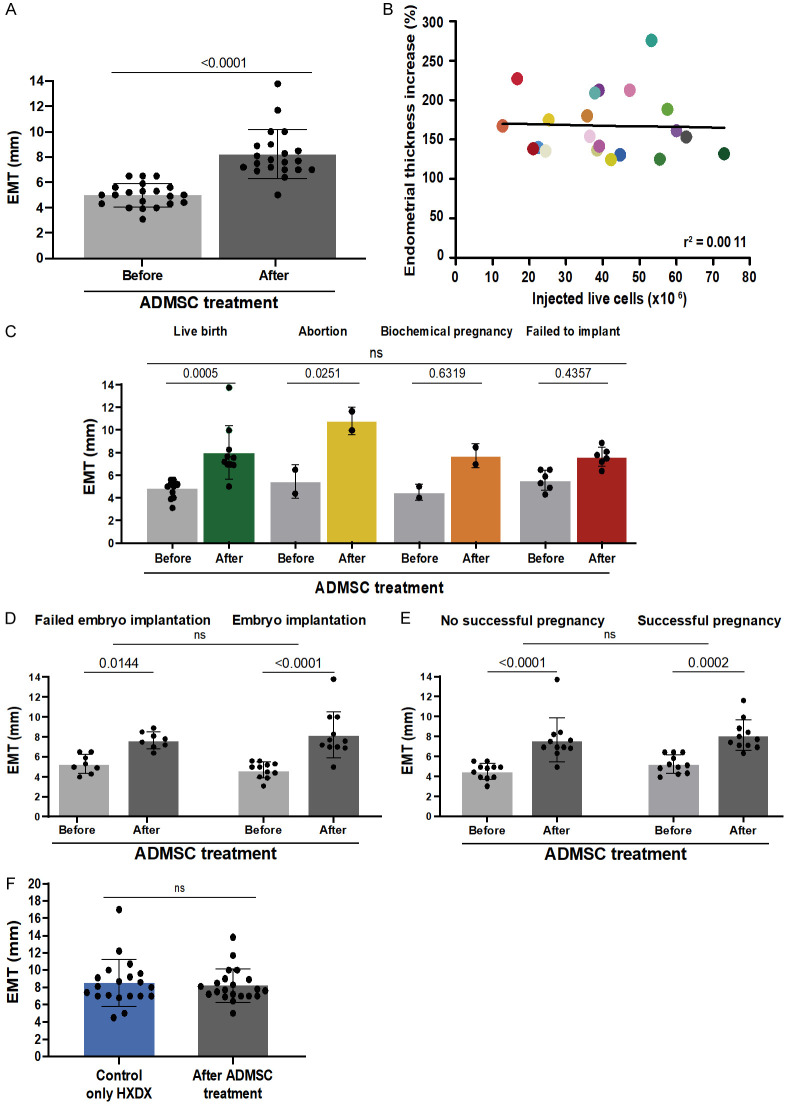
In Group 2, the average EMT at Day 10 of endometrial preparation after synechiae lysis and before ADMSC treatment was 4.9 ± 0.9 mm (Figure 3A). After the SVF injection (average = 8.2 ± 1.9 mm), a significant improvement in EMT was observed (3.23 ± 1.8 mm, P<0.0001). Only three patients (14%) did not attain the optimal EMT >7 mm. No correlation was observed between the number of injected cells and EMT (r2 = 0.0011, P = 0.8842, Figure 3B). When stratified by IVF outcome, there was no difference in the net increase in the EMT (P = 0.117, Figure 3C). There was also no significant difference in EMT when the cohort was stratified into patients who did attain embryo implantation versus those who did not (P = 0.231, Figure 3D) and with clinical pregnancy (P = 0.776, Figure 3E). However, when considering the subgroups with treatment cycles resulting in implantation and clinical pregnancy, EMTs were significantly increased (P<0.001) compared to the IVF cycle after hysteroscopic adhesiolysis and before the ADMSC treatment.
Figure 3.
Endometrial thickness comparison before and after treatment of adipose-derived mesenchymal stem cells (ADMSC). (A) Endometrial mean thickness (EMT) pre- and post-stromal vascular fraction injection for Group 2. (B) Linear regression comparing EMT and the number of injected live cells (ranging from 12.7×106 to 73×106 cells). (C) EMT for pre-and post-ADMSC injection was graphed and stratified by IVF outcome. (D) EMT comparison in the context of the embryo implantation result or (E) if the pregnancy was achieved. (F) EMT was recorded in Group 1 with only hysteroscopic adhesiolysis (HXDX) and compared with when adhesiolysis was followed by ADMSC treatment (Group 2). All reported EMTs were measured on Day 10 of estrogen-primed endometrial preparation before embryo transfer.
As a primary outcome, in Group 1, the average EMT at Day 10 of endometrial preparation after adhesiolysis was 8.5 ± 2.7 mm. There was no significant difference in EMT between Group 2 and Group 1 (Figure 3F). As for the secondary outcomes, there was no significant difference in cycle implantation, clinical pregnancy, or live birth rate between Group 2 and Group 1 (Table 3). Even though not significantly different, the live birth rate in Group 2 after ADMSC treatment was higher than in Group 1.
Table 3.
Comparison of pregnancy outcomes between no-treatment control and ADMSC treatment
| Category | Group 1 | Group 2 | p-value |
|---|---|---|---|
| Hysteroscopic adhesiolysis | Yes | Yes | - |
| ADMSC treatment | No | Yes | - |
| No. of embryos transferred per cycle | 2.5 ± 0.61 | 2.6 ± 0.77 | 0.4602a |
| Cycle implantation rate | 55.0% (11/20) | 66.7% (12/18) | 0.7475b |
| Clinical pregnancy rate | 65.0% (13/20) | 57.1% (12/21) | 0.7513b |
| Live birth rate | 35.0% (7/20) | 47.6% (10/21) | 0.5303b |
Abbreviations: ADMSC: adipose-derived mesenchymal stem cells. Values are percentage (frequency) or average ± standard deviation.
p-value was calculated using an independent t-test.
p-value was calculated using Fisher’s exact test.
Discussion
AS has been widely investigated, with surgical adhesiolysis being the standard treatment for symptomatic patients with infertility [6,24]. However, in roughly half of the cases, conservative interventions fail to restore fertility [25-27]. The precise mechanism that determines fertility in women with AS has not yet been elucidated. Recent reports suggest that intrauterine adhesions are the consequence rather than the cause of AS, as global endometrial dysfunction, altering cellular and molecular abnormalities, is present during the secretory phase of AS patients [28]. This dysfunction might even be the result of a genetic predisposition [24]. Moreover, patients with AS and thin endometrium have lower pregnancy rates than those with AS and increased endometrial thickness [27].
Here, we describe a stem-cell-based approach to improve reproductive outcomes in AS patients with endometrial atrophy after hysteroscopic adhesiolysis. In 21 infertile women with suboptimal endometrium due to endometrial atrophy secondary to AS, the ADMSC treatment improved the EMT in 21; only three did not attain the optimal EMT over 7 mm. Reproductive outcomes (implantation, clinical pregnancy, and live birth rates) improved compared to the previous cycle, matching the ranges of normal-responder patients to standard adhesiolysis.
EMT is a critical factor for embryo implantation in ART [29,30], with an EMT >7 mm considered to be the minimal optimal threshold for achieving pregnancy [31]. In infertile patients, EMT <7 mm was associated with a decreased rate of clinical pregnancy when compared to 7-9 mm EMTs [32]. After synechia removal in AS patients, EMT ≤5 mm was associated with a higher frequency of miscarriages compared to those with EMT >5 mm [33]. EMT was reported to be significantly thicker in women who had live births than in those who did not [27]. In our study, ADMSC treatment improved the EMT in 21 patients with AS who had previously failed to get pregnant after standard adhesiolysis. Moreover, pregnancy rates improved to match those of patients without endometrial atrophy after adhesiolysis, suggesting that this novel therapeutic approach could be beneficial for patients with uterine-related infertility associated with atrophic endometrium.
For patients who had failed to respond to the standard hysteroscopic adhesiolysis and estrogen-primed endometrial preparation, the therapeutic use of MSC is a promising approach when the unsolved female infertility problem has poor endometrial function [9-12]. Two mechanisms of action for MSC over endometrial tissue have been proposed. First, the endometrial niche can be colonized by autologous stem cells derived from different tissues [34]; therefore, MSC’s ability to transdifferentiate into endothelial cells will restore damaged or diminished uterine stem cell niche to allow endometrial growth in response to hormonal stimuli [11,35]. The second potential mechanism consists of modulating inflammatory and immune reactions via their paracrine effects on angiogenesis (vascular endothelial growth factor), immunomodulation (Leukemia inhibitory factor and transforming growth factor beta 1), antiapoptotic (B-cell lymphoma 2 and vascular endothelial growth factor), and antifibrotic actions (Matrix metalloproteinase) [6]. In addition, MSC-endometrial cell-cell contact and secretion of bioactive molecules can promote angiogenesis and tissue repair, thereby inhibiting scarring, modulating inflammatory and immune reactions, and activating tissue-specific progenitor cells that may restore fertility [8]. In this study, the composition of the SVF was not explicitly evaluated. As SVF is a heterogeneous cell population, white blood cell composition and viability can vary. Previous studies have demonstrated the feasibility of using MSC in patients with unsuccessful ART to improve EMT, restore endometrial function, and achieve pregnancy [9,11,36,37]. This work is the first two-arm study to describe the clinical utility of MSC via SVF application in AS patients with refractory endometrium where no other option is available.
Limitations of our study include the sample size and the use of a historical control group. The relatively small sample size is due to the limited data availability of patients with rare diseases. The incidence of AS infertile patients undergoing ART ranges from 2 to 22% for uterine adhesion [36,38] and from 0.5% to 3.1% for endometrial atrophy [29,39]. The use of non-treated historical control may introduce confounding effects due to changes in medical practice. To mitigate this effect, we only included AS patients attending the clinic in the same period as the ADMSC treated. Groups 1 and 2 were balanced concerning AS grade prevalence, age, and BMI to overcome heterogeneity and other variables that impact fertility.
Conclusions
Autologous intrauterine ADMSC injections improved EMT, implantation, and pregnancy rates in AS patients with refractory endometrium, matching the ranges of AS patients who responded to standard adhesiolysis. No known complications were associated with the collection, preparation, and administration of ADMSC. This study opens the possibility to explore the use of ADMSC in other forms of infertility relating to endometrial dysfunction.
Acknowledgements
We want to thank this study’s participants and the IVF and medical staff at Ingenes, particularly Abril Romero Jarillo, Maribel Avila, Rocio Castro, and their nursing teams for their support throughout the study. In addition, we thank Laboratorio Nacional de Citometría de Flujo at Universidad Nacional Autónoma de México (CONACYT) for the technical support in acquiring flow cytometry samples. The research was supported by the Ingenes Institute for materials. DHM received support from the Consejo Nacional de Humanidades, Ciencias y Tecnologías, México (CONAHCYT, scholarship number 790971).
Written informed consent was obtained from all subjects involved in the study.
Disclosure of conflict of interest
None.
References
- 1.Strowitzki T, Germeyer A, Popovici R, Von Wolff M. The human endometrium as a fertility-determining factor. Hum Reprod Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- 2.Bos-Mikich A, Ferreira MO, de Oliveira R, Frantz N. Platelet-rich plasma or blood-derived products to improve endometrial receptivity? J Assist Reprod Genet. 2019;36:613–620. doi: 10.1007/s10815-018-1386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouanness M, Ali-Bynom S, Jackman J, Seckin S, Merhi Z. Use of Intra-uterine Injection of Platelet-rich Plasma (PRP) for endometrial receptivity and thickness: a literature review of the mechanisms of action. Reprod Sci. 2021;28:1659–1670. doi: 10.1007/s43032-021-00579-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33:1883–1888. doi: 10.1093/humrep/dey281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranisavljevic N, Raad J, Anahory T, Grynberg M, Sonigo C. Embryo transfer strategy and therapeutic options in infertile patients with thin endometrium: a systematic review. J Assist Reprod Genet. 2019;36:2217–2231. doi: 10.1007/s10815-019-01576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreisler E, Kjer JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191–198. doi: 10.2147/IJWH.S165474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu KE, Hartman M, Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online. 2019;39:49–62. doi: 10.1016/j.rbmo.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Cousins FL, Filby CE, Gargett CE. Endometrial stem/progenitor cells-their role in endometrial repair and regeneration. Front Reprod Health. 2021;3:811537. doi: 10.3389/frph.2021.811537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell based therapy. J Hum Reprod Sci. 2014;7:93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao YX, Chen SR, Su PP, Huang FH, Shi YC, Shi QY, Lin S. Using mesenchymal stem cells to treat female infertility: an update on female reproductive diseases. Stem Cells Int. 2019;2019:9071720. doi: 10.1155/2019/9071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudoma I, Pylyp L, Kremenska Y, Goncharova Y. Application of autologous adipose-derived stem cells for thin endometrium treatment in patients with failed ART programs. J Stem Cell Ther Transplant. 2019;3:001–008. [Google Scholar]
- 12.Fan D, Wu S, Ye S, Wang W, Guo X, Liu Z. Umbilical cord mesenchyme stem cell local intramuscular injection for treatment of uterine niche: Protocol for a prospective, randomized, double-blinded, placebo-controlled clinical trial. Medicine (Baltimore) 2017;96:e8480. doi: 10.1097/MD.0000000000008480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31:1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Feisst V, Meidinger S, Locke MB. From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning. 2015;8:149–162. doi: 10.2147/SCCAA.S64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. J Plast Reconstr Aesthet Surg. 2016;69:170–179. doi: 10.1016/j.bjps.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Manchanda R, Rathore A, Carugno J, Della Corte L, Tesarik J, Torok P, Vilos GA, Vitale SG. Classification systems of Asherman’s syndrome. An old problem with new directions. Minim Invasive Ther Allied Technol. 2021;30:304–310. doi: 10.1080/13645706.2021.1893190. [DOI] [PubMed] [Google Scholar]
- 18.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 19.Farrington P. Censoring on outcome is not valid in self-controlled case series studies. J Clin Epidemiol. 2013;66:1428–1429. doi: 10.1016/j.jclinepi.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med. 2006;25:2618–2631. doi: 10.1002/sim.2477. [DOI] [PubMed] [Google Scholar]
- 21.Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31:2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Bahadur A, Mittal S, Malhotra N, Bhatt A. Predictive value of endometrial thickness, pattern and sub-endometrial blood flows on the day of hCG by 2D doppler in in-vitro fertilization cycles: a prospective clinical study from a tertiary care unit. J Hum Reprod Sci. 2011;4:29–33. doi: 10.4103/0974-1208.82357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SL, Wu FR, Luo C, Chen X, Shi XY, Zheng HY, Ni YP. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: a retrospective cohort study. Reprod Biol Endocrinol. 2010;8:30. doi: 10.1186/1477-7827-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santamaria X, Isaacson K, Simon C. Asherman’s syndrome: it may not be all our fault. Hum Reprod. 2018;33:1374–1380. doi: 10.1093/humrep/dey232. [DOI] [PubMed] [Google Scholar]
- 25.Hooker AB, de Leeuw RA, Twisk JWR, Brolmann HAM, Huirne JAF. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70–81. doi: 10.1093/humrep/deaa289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.March CM. Management of Asherman’s syndrome. Reprod Biomed Online. 2011;23:63–76. doi: 10.1016/j.rbmo.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Fouks Y, Kidron A, Lavie I, Shapira Z, Cohen Y, Levin I, Azem F, Cohen A. Reproductive outcomes and overall prognosis of women with Asherman’s syndrome undergoing IVF. J Minim Invasive Gynecol. 2022;29:1253–1259. doi: 10.1016/j.jmig.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Santamaria X, Roson B, Perez-Moraga R, Venkatesan N, Pardo-Figuerez M, Gonzalez-Fernandez J, Llera-Oyola J, Fernandez E, Moreno I, Salumets A, Vankelecom H, Vilella F, Simon C. Decoding the endometrial niche of Asherman’s syndrome at single-cell resolution. Nat Commun. 2023;14:5890. doi: 10.1038/s41467-023-41656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20:221–228. doi: 10.1097/GCO.0b013e328302143c. [DOI] [PubMed] [Google Scholar]
- 30.Kupesic S, Bekavac I, Bjelos D, Kurjak A. Assessment of endometrial receptivity by transvaginal color Doppler and three-dimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J Ultrasound Med. 2001;20:125–134. doi: 10.7863/jum.2001.20.2.125. [DOI] [PubMed] [Google Scholar]
- 31.Bashiri A, Halper KI, Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16:121. doi: 10.1186/s12958-018-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, Broekmans FJ. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:530–541. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 33.Baradwan S, Shafi D, Baradwan A, Bashir MS, Al-Jaroudi D. The effect of endometrial thickness on pregnancy outcome in patients with Asherman’s syndrome post-hysteroscopic adhesiolysis. Int J Womens Health. 2018;10:77–82. doi: 10.2147/IJWH.S151283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa R. The importance of adipose-derived stem cells and vascularized tissue regeneration in the field of tissue transplantation. Curr Stem Cell Res Ther. 2006;1:13–20. doi: 10.2174/157488806775269043. [DOI] [PubMed] [Google Scholar]
- 35.Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22:137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4:43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SY, Shin JE, Kwon H, Choi DH, Kim JH. Effect of autologous adipose-derived stromal vascular fraction transplantation on endometrial regeneration in patients of Asherman’s syndrome: a pilot study. Reprod Sci. 2020;27:561–568. doi: 10.1007/s43032-019-00055-y. [DOI] [PubMed] [Google Scholar]
- 38.Ventolini G, Zhang M, Gruber J. Hysteroscopy in the evaluation of patients with recurrent pregnancy loss: a cohort study in a primary care population. Surg Endosc. 2004;18:1782–1784. doi: 10.1007/s00464-003-8258-y. [DOI] [PubMed] [Google Scholar]
- 39.Dhakhwa R, Bhattarai R, Shah J, Shakya A, Pradhan S. Benign histopathologic findings of endometrium among perimenopausal women presenting with abnormal uterine bleeding: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2021;59:1141–1145. doi: 10.31729/jnma.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]