Abstract
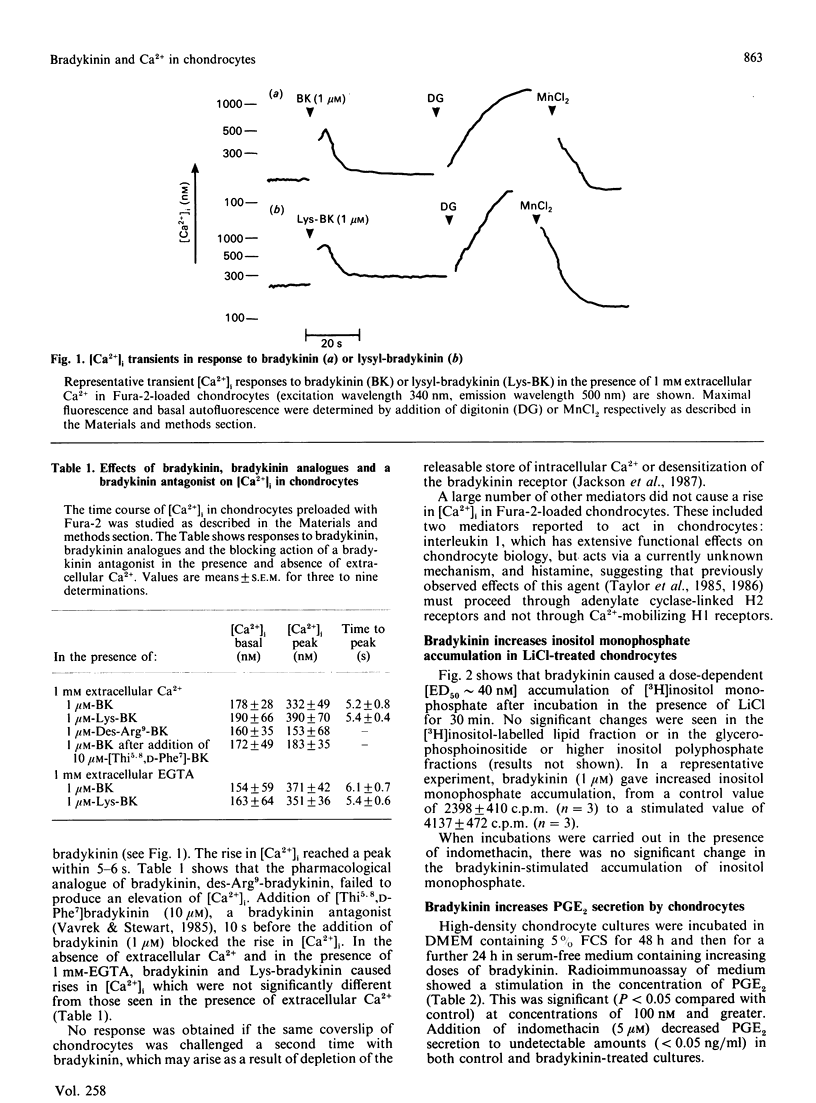
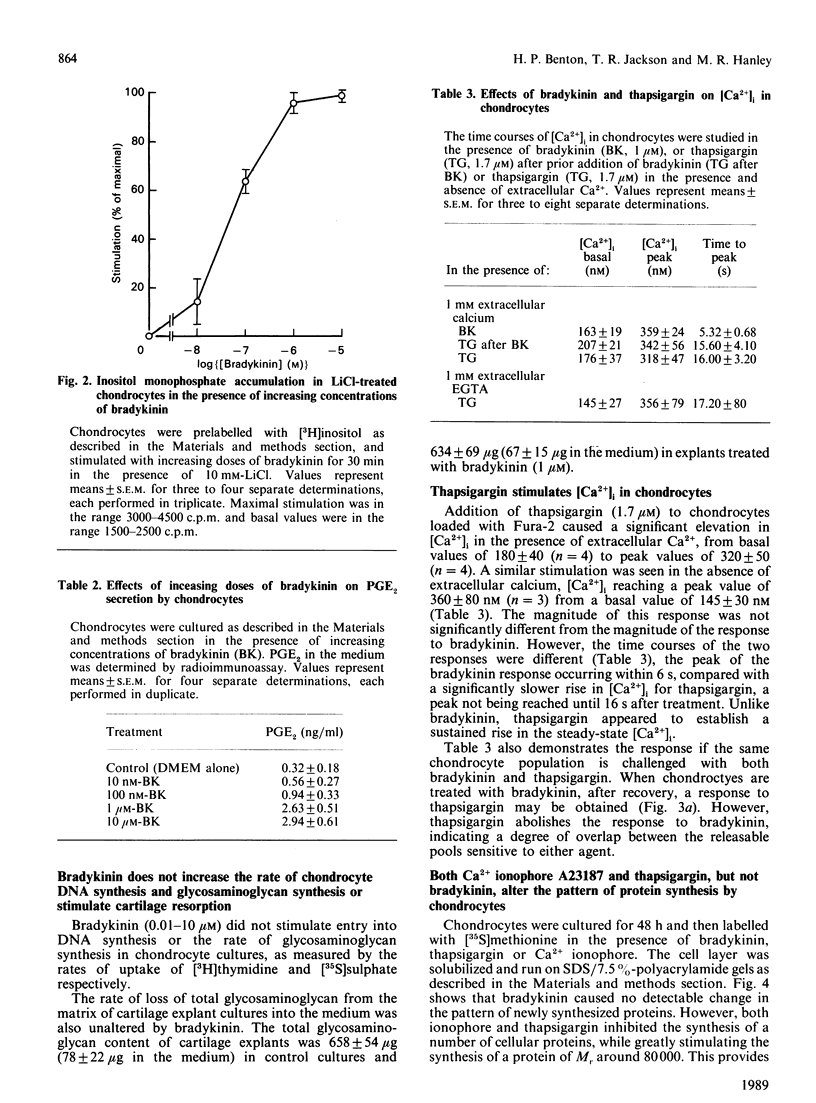
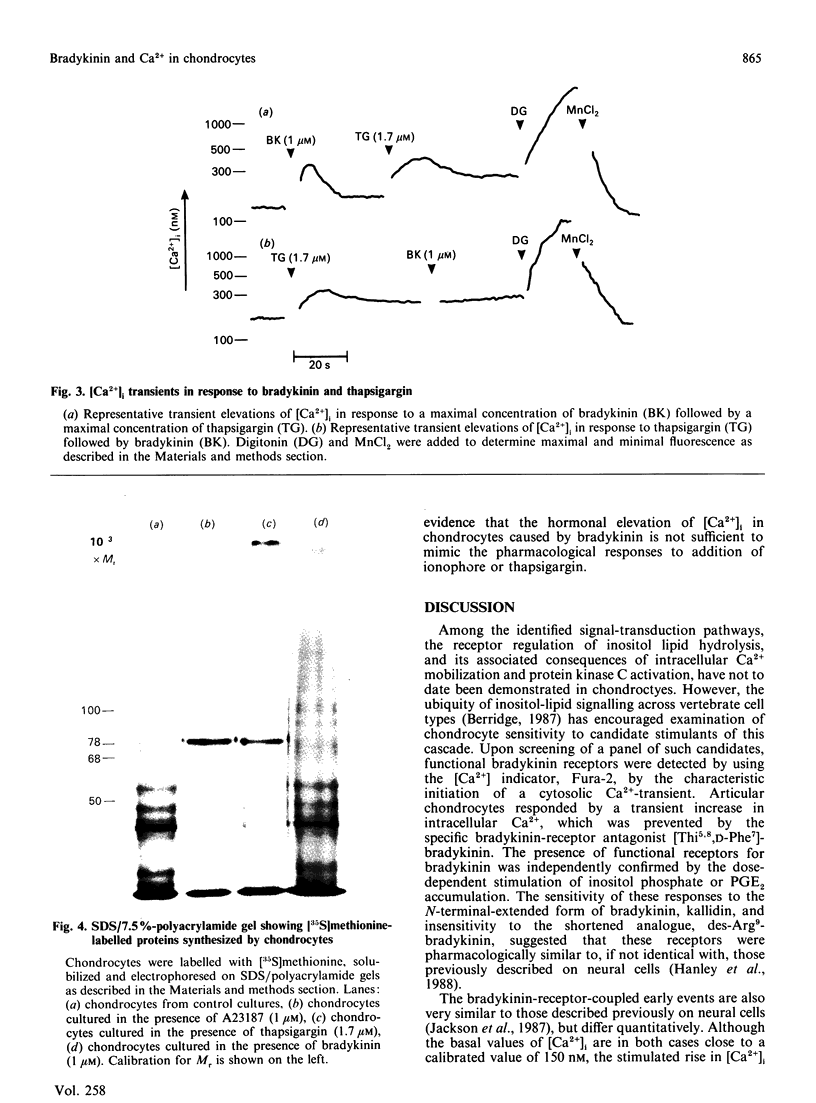
The inflammatory peptide bradykinin stimulated a rapid and transient increase in cytoplasmic [Ca2+] in primary pig chondrocytes, as measured by the fluorescent indicator dye Fura-2. This increase occurred in the absence of extracellular Ca2+, indicating a mobilization from intracellular stores. The elevation in intracellular [Ca2+] was mediated by authentic bradykinin receptors, since it was blocked by the specific bradykinin antagonist [beta-(2-thienyl)-L-Ala5,8,D-Phe7]bradykinin. Activation of chondrocytes by bradykinin induced a concentration-dependent [ED50 (dose for half-maximal response) approximately 40 nM] accumulation of inositol monophosphate in the presence of LiCl and a concentration-dependent increase in production of prostaglandin E2. The generation of the secondary mediator prostaglandin E2 was a biologically relevant output response induced by bradykinin, but chondrocyte responses, such as the rate of entry into DNA synthesis, the rate and pattern of new protein synthesis and the rate of synthesis and resorption of cartilage proteoglycan, were unaltered by bradykinin treatment. Chondrocytes were also shown to be activated by two pharmacological mediators of cytosolic [Ca2+] elevation, i.e. the ionophore A23187 and thapsigargin, which both produced alterations in protein synthesis which were mimicked by bradykinin. Thus Ca2+-sensitive pathways exist which are not functionally responsive to a Ca2+-mobilizing and inositol phosphate-generating hormone, potentially indicating other routes of regulation. These results call attention to bradykinin and related peptides as another class of inflammatory mediators which may regulate physiological and pathological chondrocyte metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton H. P., Tyler J. A. Inhibition of cartilage proteoglycan synthesis by interleukin I. Biochem Biophys Res Commun. 1988 Jul 15;154(1):421–428. doi: 10.1016/0006-291x(88)90703-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Beit-Or A., Nevo Z. Decrease in cytosolic free Ca2+ and enhanced proteoglycan synthesis induced by cartilage derived growth factors in cultured chondrocytes. Biochem Biophys Res Commun. 1985 Oct 30;132(2):770–779. doi: 10.1016/0006-291x(85)91199-4. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. R., Vallejo M., Patterson S. I., Thastrup O., Lightman S., Rogers J., Henderson G., Pini A. Neural function: metabolism and actions of inositol metabolites in mammalian brain. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):381–398. doi: 10.1098/rstb.1988.0083. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Lee C. M., Michell R. H., Jones L. M. Similar effects of substance P and related peptides on salivation and on phosphatidylinositol turnover in rat salivary glands. Mol Pharmacol. 1980 Jul;18(1):78–83. [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R., Higgs G. A. Mediators of rheumatoid arthritis. Br Med Bull. 1987 Apr;43(2):415–428. doi: 10.1093/oxfordjournals.bmb.a072191. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Jackson T. R., Hallam T. J., Downes C. P., Hanley M. R. Receptor coupled events in bradykinin action: rapid production of inositol phosphates and regulation of cytosolic free Ca2+ in a neural cell line. EMBO J. 1987 Jan;6(1):49–54. doi: 10.1002/j.1460-2075.1987.tb04717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G. Platelet-activating factor stimulates the phosphatidylinositol cycle. Appearance of phosphatidic acid is associated with the release of serotonin in horse platelets. J Biol Chem. 1982 Jul 10;257(13):7314–7317. [PubMed] [Google Scholar]
- Lerner U. H., Jones I. L., Gustafson G. T. Bradykinin, a new potential mediator of inflammation-induced bone resorption. Studies of the effects on mouse calvarial bones and articular cartilage in vitro. Arthritis Rheum. 1987 May;30(5):530–540. doi: 10.1002/art.1780300507. [DOI] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Marceau F., Lussier A., Regoli D., Giroud J. P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen Pharmacol. 1983;14(2):209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Martin M., Resch K. Interleukin 1: more than a mediator between leukocytes. Trends Pharmacol Sci. 1988 May;9(5):171–177. doi: 10.1016/0165-6147(88)90033-8. [DOI] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Lys-bradykinin stimulates Na+ influx and DNA synthesis in cultured human fibroblasts. Cell. 1983 Mar;32(3):979–985. doi: 10.1016/0092-8674(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Yoffe J. R., Brown D. M., Woolley D. E. Histamine H2 receptors on chondrocytes derived from human, canine and bovine articular cartilage. Biochem J. 1985 Jan 15;225(2):315–319. doi: 10.1042/bj2250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. J., Yoffe J. R., Brown D. M., Woolley D. E. Histamine stimulates prostaglandin E production by rheumatoid synovial cells and human articular chondrocytes in culture. Arthritis Rheum. 1986 Feb;29(2):160–165. doi: 10.1002/art.1780290202. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., van Paridon P. A., Verlaan I., Wirtz K. W., de Laat S. W., Moolenaar W. H. Inositol phosphate metabolism in bradykinin-stimulated human A431 carcinoma cells. Relationship to calcium signalling. Biochem J. 1987 May 15;244(1):129–135. doi: 10.1042/bj2440129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A., Benton H. P. Synthesis of type II collagen is decreased in cartilage cultured with interleukin 1 while the rate of intracellular degradation remains unchanged. Coll Relat Res. 1988 Sep;8(5):393–405. doi: 10.1016/s0174-173x(88)80013-x. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrek R. J., Stewart J. M. Competitive antagonists of bradykinin. Peptides. 1985 Mar-Apr;6(2):161–164. doi: 10.1016/0196-9781(85)90033-6. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]