Abstract
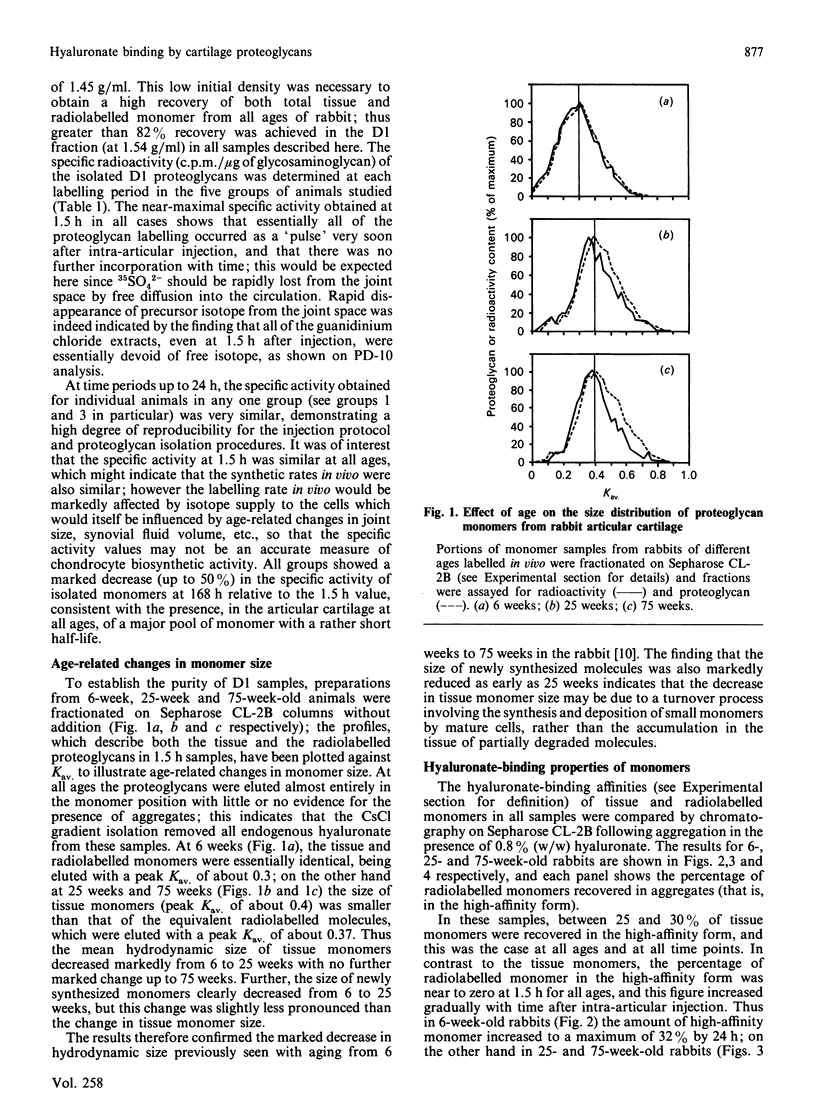
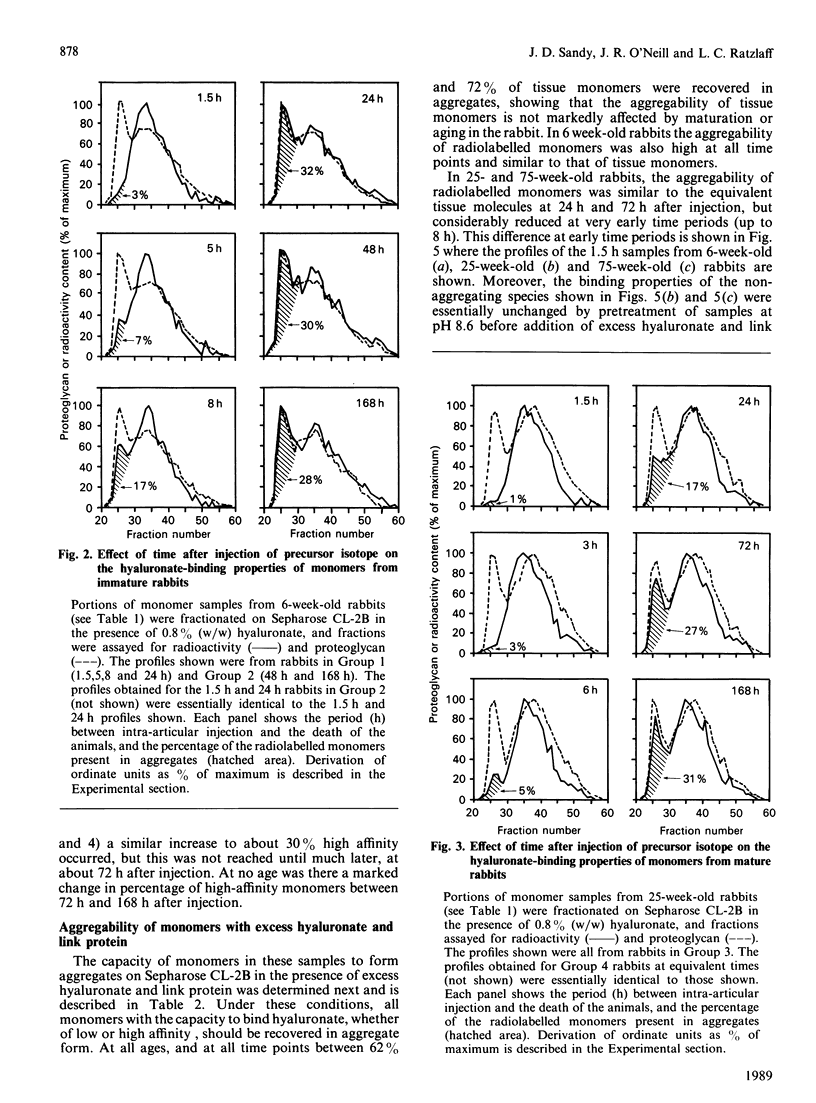
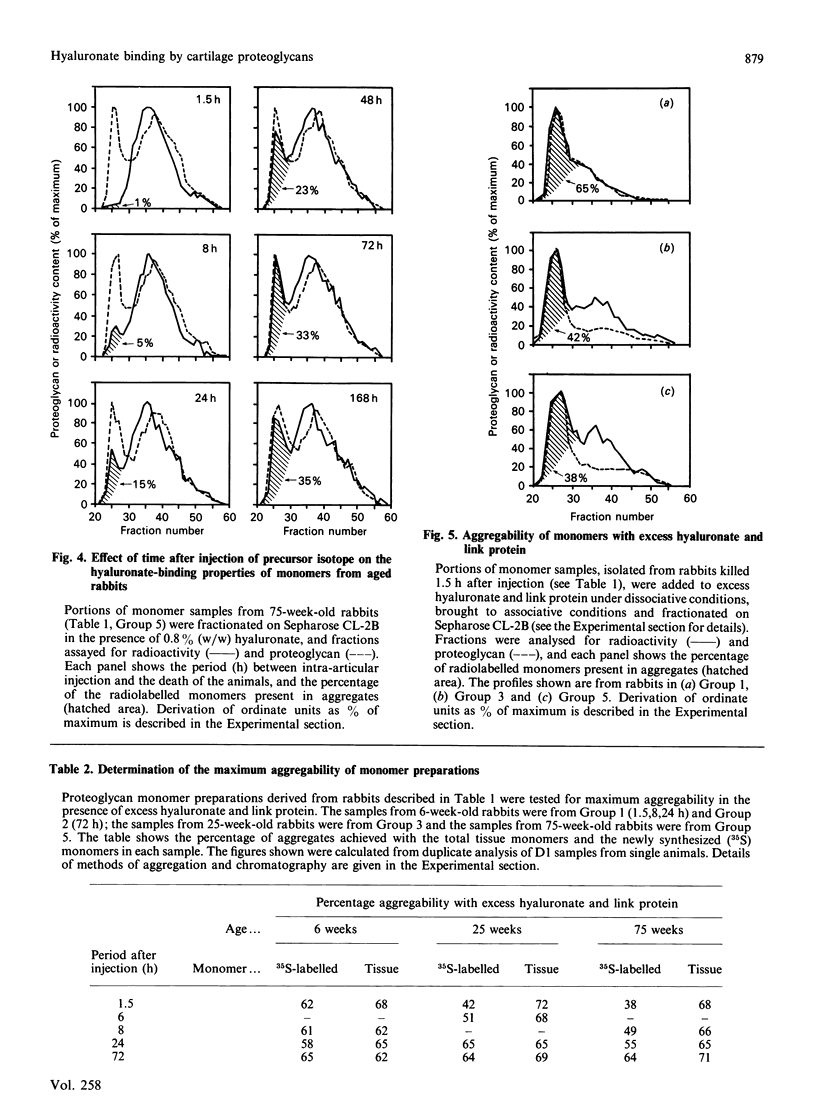
We have studied the hyaluronate-binding properties of aggregating cartilage proteoglycans synthesized in vivo by immature (6-week), mature (25-week) and aged (75-week) rabbits. Precursor isotope (35SO4) was given by intra-articular injection and articular cartilage was removed from rabbits after periods ranging from 1.5 h to 168 h. Proteoglycans were extracted with 4 M-guanidinium/HCl and monomers were isolated by CsCl gradient centrifugation under dissociative conditions. The percentages of both radiolabelled and total tissue monomers with a high affinity for hyaluronate [that is, capable of forming aggregates on Sepharose CL-2B in the presence of 0.8% (w/w) hyaluronate] were then determined. For all samples about 30% of the tissue monomers were high-affinity; however, less than 5% of the radiolabelled monomers were high-affinity at 1.5 h after injection, and this figure increased gradually with time in vivo. The increase was rapid in immature rabbits, such that after 24 h, about 30% of the radiolabelled monomers were high-affinity; on the other hand for mature and aged rabbits the increase was markedly slower such that 30% high-affinity was attained only after about 72 h. The results show that aggregating cartilage proteoglycans are secreted in vivo in a 'precursor' form with a low affinity for hyaluronate, and suggest that conversion of these monomers to a form with a higher binding affinity occurs with a half-time of about 12 h in immature cartilages but greater than 24 h in mature cartilages. The possible relationship of these findings to the process of proteoglycan aggregation in vivo is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Ridgway G. D., Ali S. Y. Delayed aggregation of proteoglycans in adult human articular cartilage. Biosci Rep. 1984 Oct;4(10):827–833. doi: 10.1007/BF01138164. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ridgway G. D., Ali S. Y. Differences in the rates of aggregation of proteoglycans from human articular cartilage and chondrosarcoma. Biochem J. 1983 Dec 1;215(3):705–708. doi: 10.1042/bj2150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C. Assembly of newly synthesized proteoglycan and link protein into aggregates in cultures of chondrosarcoma chondrocytes. J Biol Chem. 1980 Aug 10;255(15):7134–7143. [PubMed] [Google Scholar]
- Kimura J. H., Shinomura T., Thonar E. J. Biosynthesis of cartilage proteoglycan and link protein. Methods Enzymol. 1987;144:372–393. doi: 10.1016/0076-6879(87)44187-6. [DOI] [PubMed] [Google Scholar]
- Melching L. I., Roughley P. J. The role of link protein in mediating the interaction between hyaluronic acid and newly secreted proteoglycan subunits from adult human articular cartilage. J Biol Chem. 1985 Dec 25;260(30):16279–16285. [PubMed] [Google Scholar]
- Oegema T. R., Jr Delayed formation of proteoglycan aggregate structures in human articular cartilage disease states. Nature. 1980 Dec 11;288(5791):583–585. doi: 10.1038/288583a0. [DOI] [PubMed] [Google Scholar]
- Plaas A. H., Sandy J. D., Kimura J. H. Biosynthesis of cartilage proteoglycan and link protein by articular chondrocytes from immature and mature rabbits. J Biol Chem. 1988 Jun 5;263(16):7560–7566. [PubMed] [Google Scholar]
- Plaas A. H., Sandy J. D. The affinity of newly synthesized proteoglycan for hyaluronic acid can be enhanced by exposure to mild alkali. Biochem J. 1986 Feb 15;234(1):221–223. doi: 10.1042/bj2340221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Plaas A. H. Age-related changes in the kinetics of release of proteoglycans from normal rabbit cartilage explants. J Orthop Res. 1986;4(3):263–272. doi: 10.1002/jor.1100040302. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]


