Abstract
Immune reconstitution inflammatory syndrome (IRIS) describes a syndrome of aberrant reconstituted immunity, often in association with HIV infection, beginning with a normalization of CD4+ T-cell counts resulting in a dysregulated immune response against an infecting opportunistic pathogen and the host. In this chapter, we discuss the unique nature of IRIS when present in the central nervous system (CNS IRIS) and the changes experienced with each host pathogen and its unique influence on the immune system. Consensus on the mechanism of action of the immune system in IRIS pathology is less clear and multiple theories have been proposed. Here we explore the early history of the term IRIS, proposed mechanisms and animal models, as well as common CNS pathogens associated with IRIS, and management strategies.
INTRODUCTION TO IRIS
With the advent of combination antiretroviral therapy (cART) in 1996, reports of atypical presentations of well-described opportunistic infections following induction of antiretroviral therapy began to appear in the medical human immunodeficiency virus (HIV) literature. In 1997 and 1998, The Lancet published two case series of patients with absolute CD4+ T-cell counts below 50 cells/μL who responded to antiretroviral therapy with a two- to threefold increase in baseline CD4 T-cell levels. These patients proceeded to develop an inflammatory response to a previously unrecognized or asymptomatic opportunistic infection within 1–24 weeks of therapy, as well as a more clinically severe opportunistic infection than was expected at their current CD4 T-cell counts (Jacobson et al., 1997; Race et al., 1998).
In 2000, the term immune reconstitution syndrome was used to describe the cART-associated inflammatory syndrome following reconstitution of CD4+ T-cell lymphopenia in HIV-seropositive patients with new or previously unrecognized opportunistic infections (Behrens et al., 2000). Interestingly, an enhanced inflammatory response against hepatitis C, which is not regarded as an opportunistic infection, was also noted in this early case series. Inclusion of any underlying infection and abnormal inflammatory response, not just opportunistic infections, was used to highlight the pathogen-specific cell-mediated immune response described in these early immune reconstitution syndrome case series.
An alternate term, immune restoration disease, was introduced nearly a year later to describe the same clinical syndrome (Price et al., 2001). Within 2 years of an initial definition, the term immune reconstitution inflammatory syndrome (IRIS) was defined at the Annual Meeting of Infectious Disease Society of America in San Francisco, by a group of researchers at Baylor University and continues to be the most widely used term to date (Shelburne et al., 2002). While the term IRIS has been associated with HIV infection, the phenomenon is not new. The classic descriptions of tuberculoid leprosy and the Jarisch–Herxheimer reaction in syphilis represent similar enhanced immune responses against the bacilli (Yang et al., 2010; Bhat et al., 2013).
The consensus regarding IRIS pathophysiology in HIV infection is that reconstituted immunity, either complete or partial, usually beginning with increasing CD4+ T-cell counts, results in a dysregulated immune response against the infecting pathogen and host. There are two types of clinical manifestations of IRIS: (1) paradoxic IRIS; and (2) unmasked IRIS. Paradoxic IRIS defines clinical scenarios where an opportunistic infection is known to be present prior to immune reconstitution, and on reversal, the patient experiences a worsening of the known opportunistic infection along with a robust inflammatory response. Unmasked IRIS defines clinical scenarios where an opportunistic infection was not known to be present, i.e., asymptomatic, but was “unmasked” during immune reconstitution whereby the immune response to the pathogen makes its symptomatic. For the clinician, these definitions reflect a convenient method to define a clinical IRIS scenario.
The incidence of IRIS changes with each host pathogen and its unique influence on the immune system – for instance, Mycobacterium tuberculosis and Cryptococcus neoformans have far higher rates of IRIS, even in the non-HIV population, than their classic counterpart HIV opportunistic infection Toxoplasma gondii. For example, to date, there is only one reported case of paradoxic IRIS involving T. gondii in the literature (Gatti-Mays et al., 2016). The theories describing interactions between host immune response and specific pathogens and their role in IRIS pathology will be further described in this chapter, but it is important to remember that both host and pathogen interaction are instrumental in the development of IRIS. Although IRIS is not limited to the central nervous system (CNS), and indeed all organ systems can be affected by IRIS, for the remainder of this discussion our focus will be on IRIS in relationship to the CNS.
Consensus on the mechanism of action of the immune system in IRIS pathology is less clear and multiple theories have been proposed. Several consensus definitions for IRIS exist, often dependent on the underlying host pathogen (Muller et al., 2010). Here we will attempt to describe some of the major key theories regarding the role of the effector CD4+ T-cell, activated CD8+ T cells, and the innate immune system in IRIS pathology and to develop an overarching consensus definition of IRIS.
A majority favor a CD4+ T-cell mediated pathway to explain the aberrant immune response in IRIS. After all, HIV-induced CD4+ T-cell depletion and reconstitution through cART case series prompted the definition of IRIS in the first place. Mechanistically, there are two camps regarding CD4+ T-cell-mediated IRIS pathophysiology: one proposes that dysregulated reconstitution of effector T-cell response directed against the host pathogen is responsible for IRIS (French, 2009) and the other asserts that IRIS results from an uncoupling of the innate and adaptive immune system that is driven by CD4+ T-cell depletion in HIV-positive patients (Barber et al., 2012). Both sides agree that CD4+ T-cell depletion and reconstitution are necessary for the development of IRIS, but the side that favors an uncoupling hypothesis points to cases of IRIS in T-cell-replete patients. Examples include IRIS in non-HIV patients receiving tumor necrosis factor-alpha (TNF-α) antagonists and IRIS against JC virus reactivation in patients previously receiving natalizumab, an α4-integrin inhibitor (Clifford et al., 2010; Rivoisy et al., 2011; Barber et al., 2012).
Proponents of a specific effector CD4+ T-cell-driven immune response against the host and pathogen point to data suggesting that TNF-α antagonists prime the T-cell differentiation towards a suppressive phenotype and inhibit T-cell proliferation, suggesting that withdrawal of TNF-α antagonists might induce a dysregulated TH1-mediated T-cell proliferation (Boks et al., 2014). Those in favor of a greater role of the innate immune system might point to the fact that natalizumab indeed increases the number of circulating peripheral CD4+ T cells, CD8+T cells, natural killer T cells, and B cells (Marousi et al., 2013). Those supporting a dysregulated CD4+ T-cell-driven response point to the fact that natalizumab decreases the CD4/CD8 T-cell ratio due to a differential expression of unbound α4 integrin on the cell surface between the two subsets of T cells (Stuve et al., 2006). Indeed, repeated exposure to natalizumab decreases the CNS CD4+:CD8+ ratio to levels similar to those of HIV-positive patients (Stuve et al., 2006; Marousi et al., 2013).
As the incidence of IRIS in HIV-positive patients dramatically increases with a rapidly rising CD4+ T-cell count after profound CD4+T-cell depletion, there is ample reason to suggest that a dysregulated CD4+ T-cell response is causal in IRIS pathology. However, cases of peripheral IRIS have rarely been reported in patients with normal (>500 × 103 cells/mL) CD4+ T-cell counts, suggesting that depletion is not necessary for the abnormal T-cell response in IRIS (Mori et al., 2009). However, despite normal CD4+ T-cell counts, these reports invariably involve patients with HIVor on immunosuppression that could be postulated to change CD4+ T-cell cascades.
Recent evidence suggests a role for CD8+ T cells in IRIS pathology (Johnson and Nath, 2014). Case series of IRIS after cART have demonstrated predominant CD8+ T-cell infiltration in the brain parenchyma, both in cases of IRIS associated with opportunistic infections and in cases of IRIS with HIV encephalitis (Rushing et al., 2008; Johnson and Nath, 2014). The cell-mediated responses may be directed against the specific microorganism causing the opportunistic infection, such as in M. tuberculosis IRIS, suggesting varying inflammatory pathways for disease pathogenesis (Espinosa et al., 2013).
Further research is needed to answer the complex questions regarding the immune and cytokine cascades that are involved in IRIS pathophysiology. However, a consensus definition for CNS IRIS that meets the criteria of the above is possible and should be generated to create a clear dialogue between clinicians.
We propose the following.
CNS IRIS
evidence of underlying reversible CD4+ T-cell dysfunction or leukopenia
rising CD4+ and/or CD8+ T-cell counts in response to initiation or withdrawal of therapy to reverseCD4+ T-cell dysregulation or leukopenia
in HIV-positive patients, a concurrent stable or decreasing HIV viral load
evidence of worsening or change in neurologic symptoms and/or examination from baseline that cannot be attributed to opportunistic infection alone
if present, evidence of inflammation on magnetic resonance imaging (MRI) or T2 fluid-attenuated inversion recovery (FLAIR) signal change adds additional evidence for CNS IRIS, but is not required for diagnosis.
PATHOPHYSIOLOGY
Animal models for IRIS
Several animal models have been created to attempt to identify the mechanisms underpinning IRIS pathophysiology, many supporting the role of dysregulated CD4+ and CD8+ T-cell cascades (Table 13.1). Newer mouse models have also looked at the role of checkpoint inhibitors, such as programmed cell death protein 1 (PD-1) in IRIS. PD-1 is expressed on both B and T cells, and is involved in establishing immune tolerance. In humans, it is upregulated in chronic viral infections, marking “immune exhaustion” of the T cells to the host pathogen. Interestingly, one mouse model found that, although PD-1 is involved in the susceptibility to opportunistic infections, its expression did not alter the development of IRIS (Mutnal et al., 2013).
Table 13.1.
Select examples of animal models of immune reconstitution inflammatory syndrome (IRIS)
| Animal model | Pathogen | Intervention | Immune response |
|---|---|---|---|
|
| |||
| T-cell-deficient T-cell receptor (TCR)α−/− mice (Barber et al., 2010) | Mycobacterium avium | Adoptive CD4 + T-cell transfer | IRIS with fewer, but more activated, donor CD4 cells |
| Anti-TNF-α-neutralizing mAb | IRIS with expansion of CD45.1 + donor CD4 T cells | ||
| Lymphocyte-deficient RAG-1−/− mice (Eschke et al., 2015) | Cryptococcus neoformans | Adaptive CD4 + T cells 32 days postinfection | Systemic proinflammatory response with cytokines IFN-γ, IL-6, and TNFα Granulomatous inflammation of the liver and increased brain microglial activation compared to controls Increased CXCL10 and donor CD4+ T cells in brain compared to controls |
| RAG2−/− mice (either IFN-Rγ or IL-4Rα deficient) (Wang et al., 2015) |
Pneumocystis jiroveci pneumonia (PcP) |
Immune reconstituted at 3 weeks postinfection with splenic BALB/c mice lymphocytes | Delayed loss of weight at 2 weeks postreconstitution compared to RAG2−/− control mice with IRIS Increased lung injury in RAG2/IFN-Rγ−/− mice compared to RAG2/IL-4Rα−/− and RAG2−/− control mice with IRIS Excessive IFN-γ and impaired T-cell response in RAG2/IFN-Rγ−/− Polarization of macrophages does not limit PcP clearance |
| Murine AIDS model (LP-BM5-infected C57BL/6 mice) (Mutnal et al., 2013) | LP-BM5 murine leukemia virus (MuLV) micturen HSV-1 strain 17syn+ | Adoptive spleen and lymph T-cell transfer from HSV-primed donor mice | Increase in PD-1 expression on CD4 and CD8 + T-cell post-LP-BM5 expression with peak at 8 weeks postinfection Susceptibility in MAIDS mice to HSV-1 was determined by PD-1 expression and T-cell exhaustion, but immune reconstitution was independent of PD-1 expression on T cells Dose-dependent mortality was observed among adoptive T-cell recipients and was associated with proinflammatory mediators IL-2, IFN-γ, iNOS, CXCL9, and CXCL10 |
AIDS, acquired immune deficiency syndrome; HSV-1, herpes simplex virus type 1; IFN, interferon; IL, interleukin; iNOS, inducible nitrate oxide synthase; mAb, monoclonal antibody; MAIDS, murine acquired immunodeficiency syndrome; PD-1, programmed cell death protein 1; TNF-α, tumor necrosis factor-α.
Pathology of CNS IRIS
The interplay between CD4+ and CD8+ T cells in IRIS is complex, and can be challenging to interpret based on the initial characteristics of the host prior to immune reconstitution, as well as nature of the infecting organism itself. Take, for example, the animal model of Pneumocystis jiroveci pneumonia-induced IRIS in T-cell-deficient RAG2 knockout mice. After immune reconstitution with wild-type lymphocytes, there was a prominent CD4+ T-cell-mediated IRIS in the lungs with a relative absence of a CD8+ T-cell response (Wang et al., 2015). The absence of a CD8+ T-cell response also reflected a reduction in the CD8+ regulatory T-cell response (CD25+FoxP3+) and in this model it was presumed that the interferon-gamma receptor modulated the CD4+ T-cell-driven IRIS by promoting the upregulation of regulatory or suppressor CD8+ T cells.
In the murine acquired immune deficiency syndrome (AIDS) model infected with neurotrophic herpes simplex virus type 1 (HSV-1), meant to mimic a scenario of HIV CNS opportunistic infection, no IRIS-like phenomena were seen in infected mice in the absence of donor T-cell immune reconstitution. Th1-driven immune responses were upregulated and major histocompatibility complex class II expression on microglia was significantly increased in animals receiving donor T-cell transfers compared to HSV-1-infected animal controls, suggesting a CD4+ T-cell-driven response (Mutnal et al., 2013). This finding is consistent in the literature and across several IRIS animal models. However, both CD4+ and CD8+ appear to play critical roles in CNS IRIS and it is likely that the immune response is influenced by the nature of the CNS pathogen itself during IRIS.
Interestingly, in this study PD-1 was found to be upregulated prior to immune reconstitution in murine AIDS (MAIDS) mice, suggesting immune exhaustion in the setting of chronic viral infection in the host mice. This is a finding consistent with chronic HIV infection. However, in MAIDS PD-1 knockout mice the animals retained their resistance to CNS HSV-1 infection. Although PD-1 corresponded to both T-cell exhaustion and susceptibility of the host to CNS opportunistic infection (OI), mice without PD-1 expression were equally susceptible to IRIS, suggesting that the alteration of PD-1 T-cell expression does not have a significant role in IRIS. More work will need to be done to elucidate the full immunopathology of IRIS – likely in the setting of the individual opportunistic infection and its influence on the host immune response.
RADIOLOGY AND CLINICAL PERSPECTIVES IN CNS IRIS
Opportunistic infections
Patterns of CNS IRIS are disease-specific – with each individual opportunistic infection in the setting of HIV and immune reconstitution there will often be a unique inflammatory pattern on imaging. Brain MRI is the imaging modality of choice in patients with suspected CNS IRIS and combination MRI sequences can be used as additional evidence of CNS IRIS. In a study of HIV-positive, post-cART population with CNS IRIS, four MRI characteristics were felt to be predictive: intrinsic T1 hyperintensity, marginal reduced diffusion, and marginal enhancement or perivascular enhancement (Narvid et al., 2016). Together, these findings yielded a sensitivity of 88%, specificity of 79%, positive predictive value of 71%, and negative predictive value of 83%. Clearly, CNS imaging alone cannot be used to make a diagnosis of CNS IRIS, but utilization of MRI patterns and clinical progression can inform the astute clinician when CNS IRIS is present, leading to informed treatment decisions.
Cryptococcal meningitis and meningoencephalitis
Although cerebral toxoplasmosis is the most common CNS opportunistic infection in resource-rich countries, cryptococcal meningitis is responsible for the majority of CNS opportunistic infections in many resource-poor countries, most notably sub-Saharan Africa, and accounts for the majority of CNS IRIS-related deaths in HIV-positive people in these countries. Cryptococcal meningitis IRIS is often characterized by contrast-enhancing lesions on MRI, although it should be noted that cryptococcal meningitis is not always evidenced on MRI. Typical findings of cryptococcal meningitis include findings such as enlargement of Virchow–Robin spaces, gelatinous pseudocysts, ischemic infarcts, or more classic meningoencephalitis. Diagnosis is established through a combination of cerebrospinal fluid (CSF) studies, MRI, and clinical presentation. Lesions of cryptococcal meningitis IRIS can be meningeal or parenchymal, and focal leptomeningitis over one or more convexities is more likely to be seen in IRIS patterns than in cryptococcal meningitis without IRIS (Katchanov et al., 2016). The diagnosis of cryptococcal meningitis IRIS that is not evident on MRI will be based on evidence of clinical worsening post-cART therapy in the setting of improved CD4+ counts and improving or stable disease.
IRIS has been reported in up to 45% of HIV-positive patients with cryptococcal meningitis started on ART and up to 8 years’ post-initial infection (Rolfes et al., 2014; Katchanov et al., 2015). Although the majority of IRIS occurs 2 weeks to 2 months’ postinitiation of ART for most CNS opportunistic infections, IRIS can present well beyond the expected initial timeline.
In the case illustrated by Figure 13.1, a 34-year-old HIV-positive man was referred to the neuroinfectious disease clinic for a history of continued headaches. He emigrated from Guyana as a child. One year ago he had been diagnosed with cryptococcal meningitis, as well as HIV infection, and his viral load at the time was 79,308 copies/mL with a CD4 count of 17 cells/mm3. He underwent induction therapy for cryptococcal meningitis with liposomal amphotericin B. Combined ART (emtricitabine/tenofovir/darunavir/cobicistat) was started 12 weeks after starting antifungal therapy. His cryptococcal antigen titers fell from 1:320 to 1:64 and he was discharged on maintenance antifungal therapy with fluconazole, as well as cART therapy. His HIV RNA viral load by this time had dropped to 530 copies/mL and his CD4 increased to 71 cells/mm3, but continued enhancement of the leptomeninges was seen on MRI. There was concern for noncompliance versus incomplete HIV treatment.
Fig. 13.1.
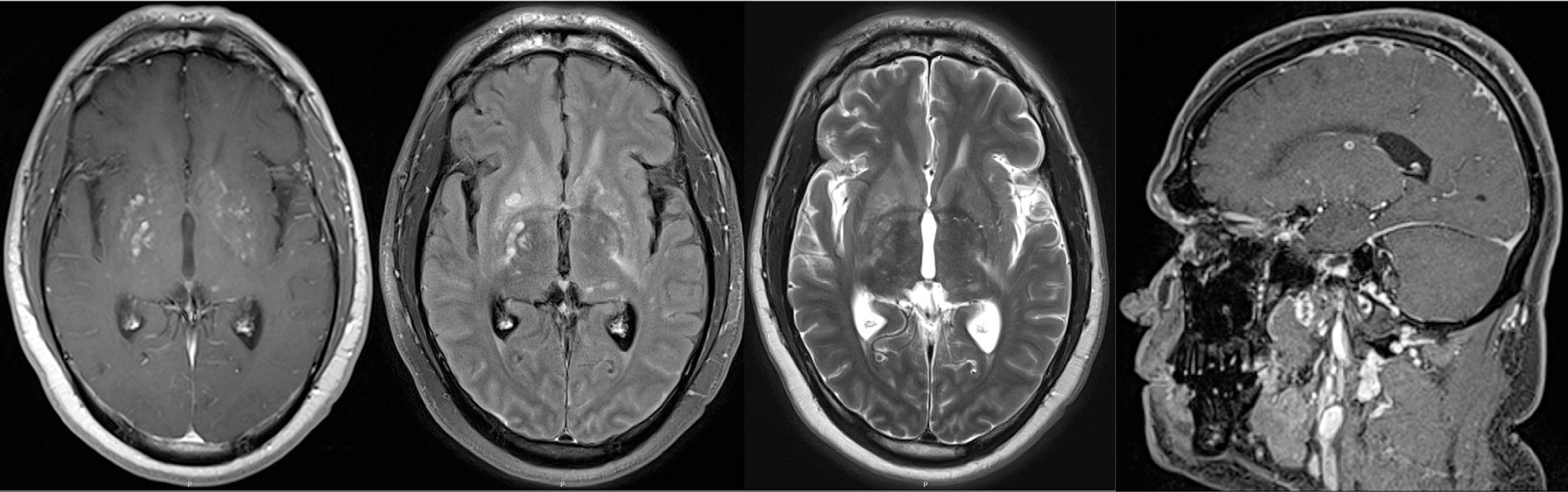
Recurrent cryptococcal meningitis immune reconstitution inflammatory syndrome. Magnetic resonance imaging (MRI) sequences from left to right: axial T1 postcontrast, postcontrast T2 fluid-attenuated inversion recovery (FLAIR), T2, and sagittal three-dimensional (3D) T1 postcontrast. MRI sequences show multiple enhancing foci in the bilateral basal ganglia and periventricular white matter. Abnormal enhancement is seen along the posterior margins of the anterior interhemispheric fissure in the sagittal 3D T1 images and abnormal postcontrast signal within the anterior falx and leptomeninges representing meningitis is seen on contrasted T2 FLAIR.
The cART was changed to abacavir/lamivudine/dolutegravir and he was continued on fluconazole. At current presentation, he had a headache, stiff neck, and blurred vision bilaterally. CSF showed a cryptococcal antigen titer of 1:5210 with elevated opening pressure of 40 mm H2O. He was treated with amphotericin and flucytosine for recurrent cryptococcal meningitis. After induction, CSF showed a rising white blood cell (WBC) count of 170 cells/mm3. MRI with and without contrast of the brain showed multiple foci of enhancement in the bilateral basal ganglia and periventricular white mater, increased from previous imaging. There were multifocal areas of leptomeningeal enhancement as well (Fig. 13.1). He was diagnosed with paradoxic IRIS and was started on oral prednisone for IRIS, along with continued cARTand maintenance fluconazole therapy. One month later, opening pressure decreased to 27 mm H2O, CSF WBC count fell to 1 cell/mm3, and cryptococcal antigen titer to 1:512.
Cerebral toxoplasmosis
Toxoplasma encephalitis is often characterized by multiple ring-enhancing lesions in the cortex and/or basal ganglia. Although these are the two most common sites for T. gondii lesions, they can be found both supra- and infratentorially. Toxoplasma encephalitis can bedifficult to distinguish from primary CNS lymphoma in HIV-positive patients, and care should be taken to establish the correct diagnosis prior to diagnosing a patient with Toxoplasma encephalitis IRIS if standard T. gondii treatment fails. Toxoplasma encephalitis IRIS is most often seen as an “unmasking” IRIS post-cART therapy, and MRI will typically show progression of contrast-enhancing lesions with perilesional edema and increased contrast enhancement over time (Atreya et al., 2014). Patients will often clinically deteriorate with worsening MRI scans in the setting of serially improving T. gondii polymerase chain reaction (PCR) parasite loads. In our experience, severe cases of Toxoplasma encephalitis IRIS can hemorrhage into lesions and have some restricted diffusion on MRI where focal edema is severe. However, it should be noted that this is rare. Toxoplasma encephalitis IRIS is rarely of “paradoxic” type, although this is reported in the literature.
Primary CNS lymphoma
HIV infection increases the risk of primary CNS lymphoma by 5000-fold (Shiels et al., 2011) and 5-year survival remains poor, at approximately 9%, which can be complicated by IRIS during treatment (Olszewski et al., 2016; Shiels et al., 2016). Diagnosis typically relies on characteristic imaging findings with infiltrative tumors invading neural parenchyma or subarachnoid spaces from perivascular cuffs (Sugita et al., 2015). On imaging, HIV-related primary CNS lymphomas are often multifocal lesions on computed tomography and MRI that exhibit necrotic foci, and contrast enhancement is commonly irregular or ring-like in up to 75% of the cases which can be mistaken for Toxoplasma encephalitis (Haldorsen et al., 2011). Concurrent IRIS can make this differential more challenging and care needs to be taken to establish the diagnosis prior to treatment. Often, an abrupt clinical deterioration and increased marginal enhancement on brain MRI following cART are supportive of CNS IRIS, especially if T. gondii PCR titers in the CSF are negative in a patient who is seronegative for T. gondii. In seropositive T. gondii patients, brain biopsy can be necessary to establish the correct diagnosis and treatment plan.
Progressive multifocal leukoencephalopathy (PML)
PML IRIS in HIV-infected patients occurs almost exclusively in those recently started on antiretroviral therapy, with both paradoxic and unmasking events most often within the first 8 weeks after initiating therapy (Tan et al., 2009). Symptoms of PML IRIS resemble those of PML with the areas affected dictating the manifestations and most commonly seen are problems with coordination, cognition, and motor weakness. Contrast enhancement on MRI with PML IRIS is more common than with PML alone (Sainz-de-la-Maza et al., 2016). The pattern of contrast enhancement may be circumferential, whereby the enhancement is around the periphery of the lesion or nodular which appears as punctate areas of enhancement within the lesion itself (Fig. 13.2). The former likely represents infiltration of predominantly macrophages at the spreading edge of the lesion while the nodules likely represent perivascular lymphocytic infiltrates composed of mostly CD8 cells (Vendrely et al., 2005).
Fig. 13.2.
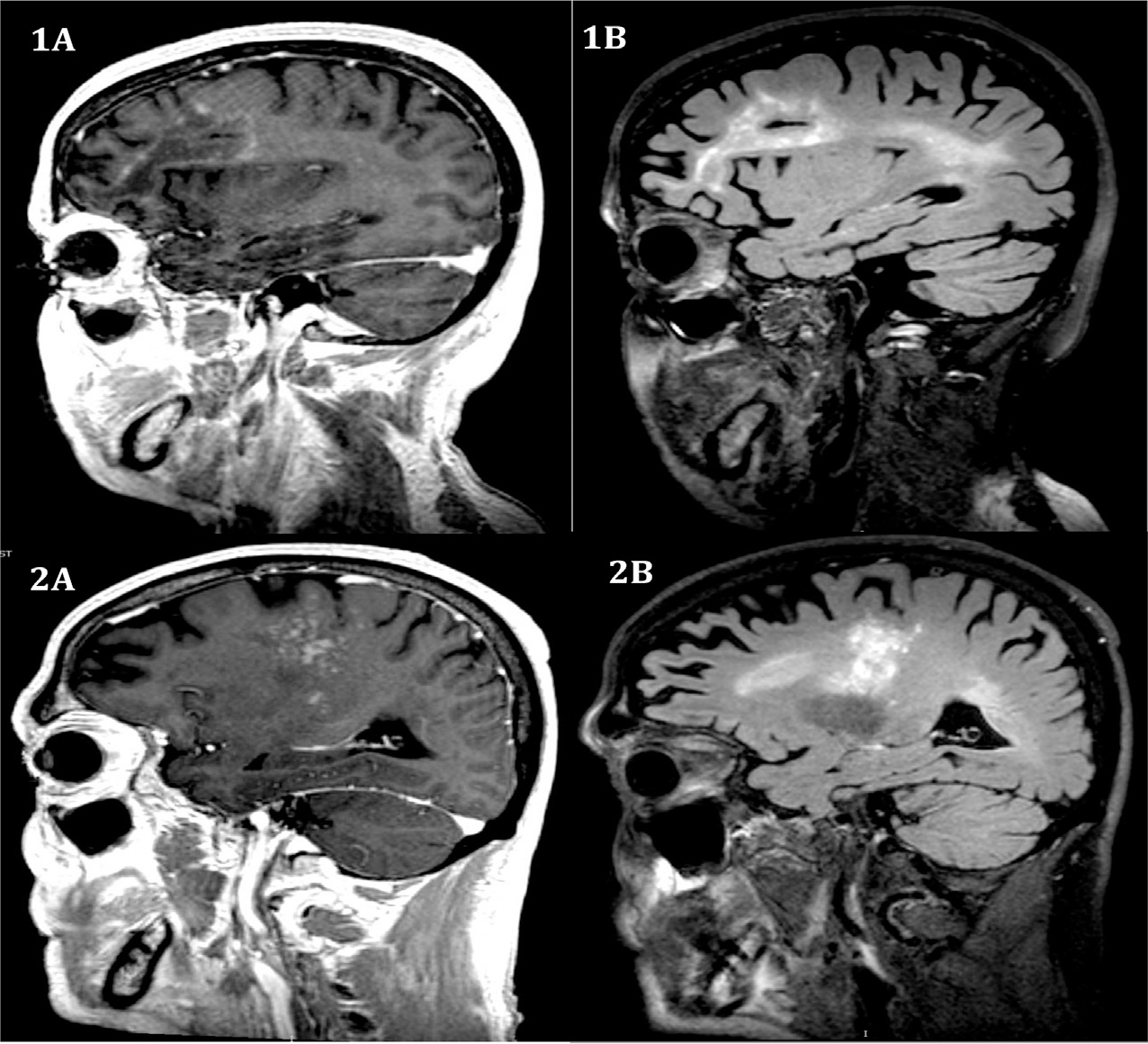
Patterns of contrast enhancement in progressive multifocal leukoencephalopathy immune reconstitution inflammatory syndrome. Magnetic resonance imaging sequences from left to right: post-GAD T1, post-GAD T2 fluid-attenuated inversion recovery (FLAIR). (1A) Circumferential enhancement with a left frontal-lobe lesion with incomplete linear enhancement at the leading edge. (1B) Large left frontal and confluent left parietal lobe lesion with extension to the left temporal lobe. Patchy contrast enhancement is again seen at the leading edges of the frontal lobe lesion. (2A) Nodular enhancement in the right posterior frontal lobe extending into the periventricular deep white-matter structures and right thalamus. (2B) Diffuse T2/FLAIR signal hyperintensity within the right thalamus extending into the right frontal lobe, and right periventricular deep white matter.
The CSF usually remains without significant pleocytosis despite the inflammatory response with WBC counts often below 25 cells/mm3 (Sainz-de-la-Maza et al., 2016). Pathologically, PML IRIS is markedly distinct from PML, with lesions demonstrating a robust CD-8 lymphocytic response with a paucity of JC virus infiltration (Martin-Blondel et al., 2013). The clinical management of PML IRIS in the setting of antiretroviral therapy is uncertain; however, early corticosteroids that are tapered off slowly may provide improved prognosis (Tan et al., 2009).
In the case illustrated in Figure 13.3, a 59-year-old man with HIV infection presented with new imbalance over the course of a week. He could no longer ride a bicycle and had several falls to the left while walking. When first diagnosed with HIV 5 months earlier, his CD4 count was 16 cells/mm3, his plasma viral load was 2 million copies/mL, and he had Pneumocystis jiroveci pneumonia. After antibiotic treatment for the pneumonia, he began treatment with a combination of elvitegravir, cobicistat, emtricitabine, and tenofovir 1 month later. Nine weeks later, he noted new left hemiataxia that progressed over 1 week. He had left arm and leg dysdiadochokinesis and dysmetria as well as trunk ataxia. MRI brain with contrast showed a largely confluent lesion extending from the right subcortical white matter through the thalamus and brainstem into the contralateral cerebellum. Multifocal enhancement with gadolinium was in a punctate pattern. The CD4 count at the time of presentation was 201 cells/mm3 and HIV viral load in plasma was 65 RNA copies/mL. CSF showed 1 WBC/mm3, glucose of 53 mg/dL, and elevated protein of 146 mg/dL. JC virus DNA was detectable by PCR in CSF at 11 copies/mL and testing for other infectious and neoplastic etiologies was unremarkable, including PCR testing for cytomegalovirus, Epstein–Barr virus, varicella-zoster virus, HSV, human herpesvirus-6 and 7, and Toxoplasma gondii.
Fig. 13.3.
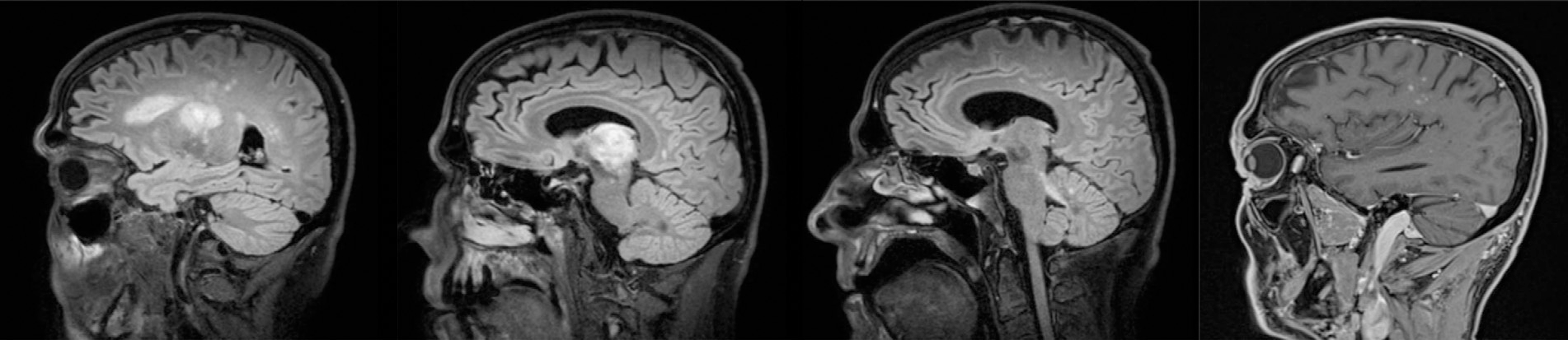
Progressive multifocal leukoencephalopathy immune reconstitution inflammatory syndrome. Magnetic resonance imaging sequences from left to right show extensive T2 fluid-attenuated inversion recovery hyperintensity extending from the right subcortical white matter through the right thalamus into the left cerebellar peduncle. T1 postcontrast imaging in the fourth panel shows punctate enhancement.
The patient was continued on antiretroviral therapy and corticosteroids were initiated at a dose of 60 mg prednisone daily. His ataxia worsened within the first 2 weeks of hospitalization and then steadily improved. Oral prednisone was tapered slowly over 3 months, and 4months after symptom onset, the patient had no neurologic abnormalities on examination. Serial testing of the JC virus DNA in CSF throughout the course of infection showed a peak of 505 copies/mL 8 weeks after PML IRIS presentation, followed by a decline to less than the limit of detection after another 8 weeks. Serial brain MRIs showed no extension of T2 FLAIR lesions; the punctate contrast enhancement resolved 5 months after presentation.
HIV encephalitis
IRIS can occur in HIV-infected patients in the absence of an opportunistic infection in the context of cART. When it affects the brain it results in a subacute encephalopathy which manifests as a change in mentation, decreased cognitive abilities, alteration in consciousness, and even seizures. The clinical presentation is quite dramatic and occurs over days to weeks. MRI of the brain may be unrevealing or show large patchy areas of increased intensity in the white matter on FLAIR sequences. CSF may be normal or show a mild pleocytosis with increased protein. In some patients the viral load in the CSF may be greater than that found in the blood (Venkataramana et al., 2006). This has been termed, “CNS escape.” In others HIV may not be detectable in blood or CSF, but immunohistologic analysis of the brain shows that the HIV-Tat protein is still expressed in the infiltrating macrophages, even though the core antigen p24 cannot be detected. This suggests that, in the absence of complete viral replication, the early viral proteins are still expressed. Importantly, there is a prominent infiltration of CD8+ T cells in the brain parenchyma resulting in encephalitis (Johnson et al., 2013). When the amount of HIV antigens expressed in the brain exceeds the amount present in the periphery, it leads to an antigenic gradient, resulting in T-cell infiltration in the brain (Gray et al., 2005). Rarely, IRIS can result in encephalitis with demyelination. These patients have a more fulminant course (Langford et al., 2002; Lindzen et al., 2008).
IRIS MANAGEMENT
The mainstay of treatment is to control the inflammation since inflammation can cause bystander damage to the brain (Fig. 13.4). Since IRIS occurs in the context of an underlying infection, the flip side of controlling the inflammation is that the infection may become unchecked and thus cause harm. Hence several scenarios need to be considered before anti-inflammatory therapy is used.
Fig. 13.4.
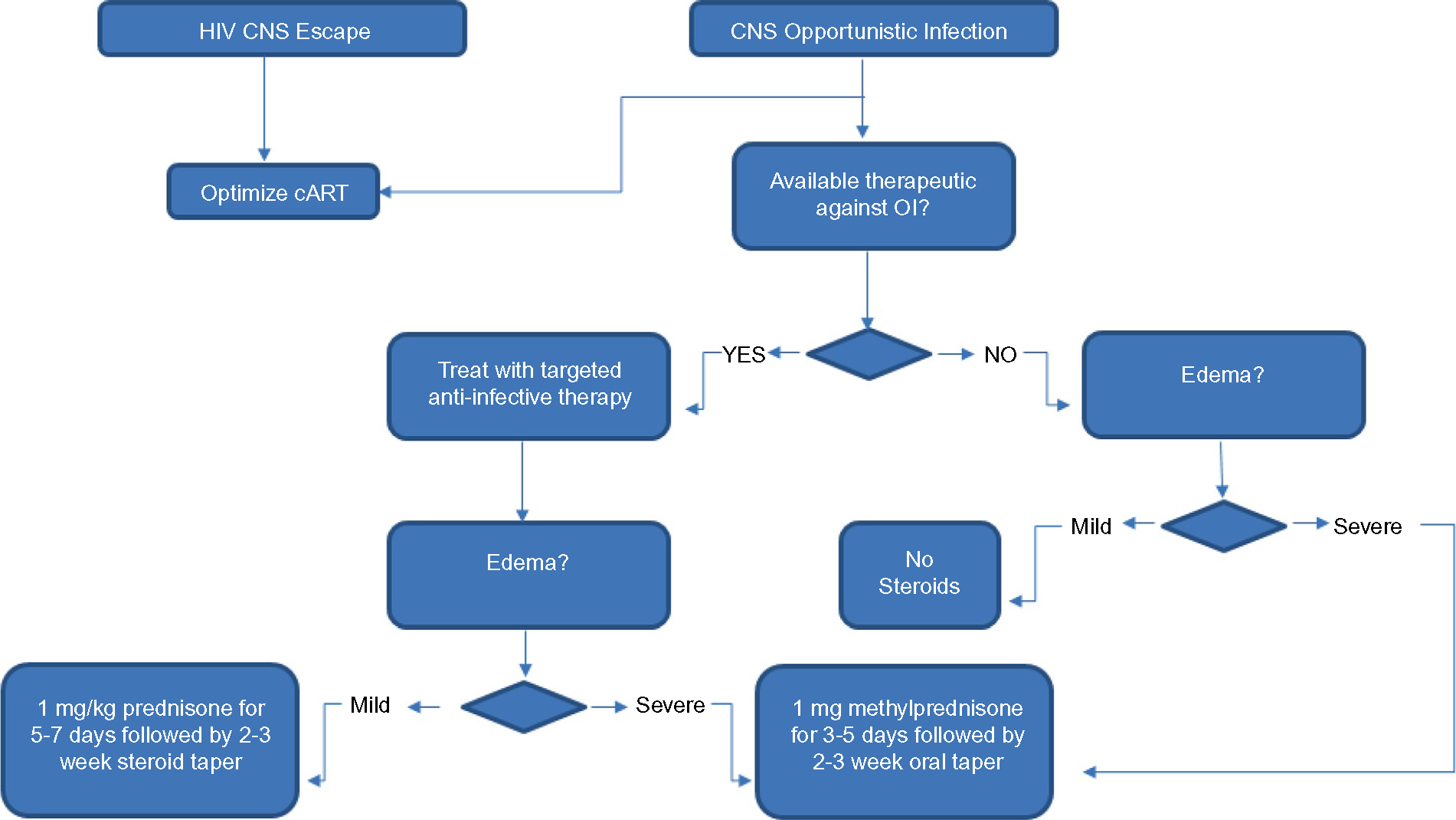
Schematic of immune reconstitution inflammatory syndrome IRIS management. cART, combination antiretroviral therapy; CNS, central nervous system; HIV, human immunodeficiency virus; OI, opportunistic infection.
When an effective antimicrobial agent is available for treating the underlying infection: infections such as cerebral toxoplasmosis can be effectively treated with antibiotics, hence the inflammation can be treated with corticosteroids once the antibiotics have been initiated. The same is true of cryptococcal meningitis. In HIV-infected patients without opportunistic infections but with HIV CNS escape, the cART needs to be optimized to use drugs with increased CNS penetration.
When antimicrobials are not available for treating the underlying infection: for patients with PML, currently there is no effective antiviral agent available, hence the mainstay of defense is the immune system. In these patients, some caution is necessary when anti-inflammatory drugs are used. If the inflammation is substantial and pressing on vital structures and has substantial mass effect, a course of corticosteroids may be beneficial. Here the goal is not to totally obliterate the inflammation but rather to temper the response, since the cytotoxic T cells are needed to control the viral infection.
Choice of drugs for treating IRIS: corticosteroids are the mainstay of therapy since they restore the blood–brain barrier, decrease T-cell activation, and prevent influx of inflammatory cells. While their broad effects on the immune system are desirable, their inability to distinguish between the cells that are nonspecifically activated and those that are targeted against the organism is a drawback. Until such drugs can be developed that can distinguish between such cell types, corticosteroids are the drug of choice. In the absence of any clinical trials, the use of these medications, their dosage, and duration are based on clinical experience. In patients who have massive edema or impending herniation, a loading dose of 1 gram methylprednisolone a day for 3–5 days followed by a tapering dose over 2–3 weeks is recommended. In others the recommended dosage is 1 mg/kg of prednisone for 5–7 days followed by a tapering dose over another 2–3 weeks. Methylprednisolone, prednisone, and dexamethasone can be used interchangeably
FUTURE DIRECTIONS
Specific immune modulators
Ideally we need drugs that would preserve the immune responses directed against the microbial pathogen but downregulate the bystander effects on the surrounding brain tissue. This can only be accomplished if we develop a better understanding of the underlying pathophysiologic mechanisms that mediate these immune responses. However, for some infections such as cryptococcal infection we need to develop better mechanisms to clear the antigen from the CNS, since current fungicidal agents may kill the pathogen but the antigen may remain in the CNS and intrathecal space for months, thus driving the immune response. In HIV-infected patients, where the inflammation is triggered by the Tat protein despite the use of adequate cART, agents need to be developed that will block the production of Tat or antagonize its effects on the HIV long terminal repeat and thus drive it along protein degradation pathways.
REFERENCES
- Atreya AR, Arora S, Gadiraju VT et al. (2014). Toxoplasma encephalitis in an HIV-infected patient on highly active antiretroviral therapy despite sustained immune response. Int J STD AIDS 25: 383–386. [DOI] [PubMed] [Google Scholar]
- Barber DL, Mayer-Barber KD, Antonelli LRV et al.(2010).Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood 116: 3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Andrade BB, Sereti I et al. (2012). Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol 10: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens GM, Meyer D, Stoll M et al. (2000). Immune reconstitution syndromes in human immuno-deficiency virus infection following effective antiretroviral therapy. Immunobiology 202: 186–193. [DOI] [PubMed] [Google Scholar]
- Bhat R, Pinto M, Dandakeri S et al. (2013). Ulcerating type 1 lepra reaction mimicking lazarine leprosy: an unusual presentation of immune reconstitution inflammatory syndrome in an HIV-infected patient. Int J STD AIDS 24: 992–994. [DOI] [PubMed] [Google Scholar]
- Boks MA, Kager-Groenland JR, Mousset CM et al. (2014). Inhibition of TNF receptor signaling by anti-TNFalpha biologicals primes naive CD4(+) T cells towards IL-10(+) T cells with a regulatory phenotype and function. Clin Immunol 151: 136–145. [DOI] [PubMed] [Google Scholar]
- Clifford DB, De Luca A, Simpson DM et al. (2010). Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 9: 438–446. [DOI] [PubMed] [Google Scholar]
- Eschke M, Piehler D, Schulze B et al. (2015). A novel experimental model of Cryptococcus neoformans-related immune reconstitution inflammatory syndrome (IRIS) provides insights into pathogenesis. Eur J Immunol 45:3339–3350. [DOI] [PubMed] [Google Scholar]
- Espinosa E, Romero-Rodriguez DP, Cantoral-Diaz MT et al. (2013). Transient expansion of activated CD8(+) T cells characterizes tuberculosis-associated immune reconstitution inflammatory syndrome in patients with HIV: a case control study. J Inflamm (Lond) 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MA (2009). HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 48: 101–107. [DOI] [PubMed] [Google Scholar]
- Gatti-Mays ME, Manion M, Bowen LN et al. (2016). Toxoplasmosis encephalitis with immune-reconstitution inflammatory syndrome in an allogeneic stem cell transplant patient: a case report. Bone Marrow Transplantation 51: 1622–1624. [DOI] [PubMed] [Google Scholar]
- Gray F, Bazille C, Adle-Biassette H et al. (2005). Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. Journal of Neurovirology 11 (Suppl 3): 16–22. [DOI] [PubMed] [Google Scholar]
- Haldorsen IS, Espeland A, Larsson EM (2011). Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 32: 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MA, Zegans M, Pavan PR et al. (1997). Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet 349: 1443–1445. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Nath A (2014). New insights into immune reconstitution inflammatory syndrome of the central nervous system. Curr Opin HIV AIDS 9: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR et al. (2013). Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proceedings of the National Academy of Sciences of the United States of America 110: 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchanov J, Blechschmidt C, Nielsen K et al. (2015). Cryptococcal meningoencephalitis relapse after an eight-year delay: an interplay of infection and immune reconstitution. Int J STD AIDS 26: 912–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchanov J, Branding G, Jefferys L et al. (2016). Neuroimaging of HIV-associated cryptococcal meningitis: comparison of magnetic resonance imaging findings in patients with and without immune reconstitution. Int J STD AIDS 27: 110–117. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD et al. (2002). Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. Aids 16: 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzen E, Jewells V, Bouldin T et al. (2008). Progressive tumefactive inflammatory central nervous system demyelinating disease in an acquired immunodeficiency syndrome patient treated with highly active antiretroviral therapy. Journal of Neurovirology 14: 569–573. [DOI] [PubMed] [Google Scholar]
- Marousi S, Karkanis I, Kalamatas T et al. (2013). Immune cells after prolonged Natalizumab therapy: implications for effectiveness and safety. Acta Neurol Scand 128: e1–5. [DOI] [PubMed] [Google Scholar]
- Martin-Blondel G, Bauer J, Cuvinciuc V et al. (2013). In situ evidence of JC virus control by CD8+ T cells in PML-IRIS during HIV infection. Neurology 81: 964–970. [DOI] [PubMed] [Google Scholar]
- Mori S, Polatino S, Estrada YMRM (2009). Pneumocystis-associated organizing pneumonia as a manifestation of immune reconstitution inflammatory syndrome in an HIV-infected individual with a normal CD4+ T-cell count following antiretroviral therapy. Int J STD AIDS 20:662–665. [DOI] [PubMed] [Google Scholar]
- Muller M, Wandel S, Colebunders R et al. (2010). Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 10:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutnal MB, Schachtele SJ, Hu S et al. (2013). T-cell reconstitution during murine acquired immunodeficiency syndrome (MAIDS) produces neuroinflammation and mortality in animals harboring opportunistic viral brain infection. Journal of Neuroinflammation 10: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvid J, Rehani B, Talbott JF (2016). Diagnostic performance of brain MRI in immune reconstitution inflammatory syndrome. J Neuroimaging 26: 303–308. [DOI] [PubMed] [Google Scholar]
- Olszewski AJ, Fallah J, Castillo JJ (2016). Human immunodeficiency virus-associated lymphomas in the antiretroviral therapy era: analysis of the National Cancer Data Base. Cancer. 10.1002/cncr.30112. [DOI] [PubMed] [Google Scholar]
- Price P, Mathiot N, Krueger R et al. (2001). Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy. J Clin Virol 22:279–287. [DOI] [PubMed] [Google Scholar]
- Race EM, Adelson-Mitty J, Kriegel GR et al. (1998). Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet 351: 252–255. [DOI] [PubMed] [Google Scholar]
- Rivoisy C, Amrouche L, Carcelain G et al. (2011). Paradoxical exacerbation of tuberculosis after TNFalpha antagonist discontinuation: beware of immune reconstitution inflammatory syndrome. Joint Bone Spine 78: 312–315. [DOI] [PubMed] [Google Scholar]
- Rolfes MA, Hullsiek KH, Rhein J et al. (2014). The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 59: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing EJ, Liappis A, Smirniotopoulos JD et al. (2008). Immune reconstitution inflammatory syndrome of the brain: case illustrations of a challenging entity. J Neuropathol Exp Neurol 67: 819–827. [DOI] [PubMed] [Google Scholar]
- Sainz-de-la-Maza S, Casado JL, Perez-Elias MJ et al. (2016). Incidence and prognosis of immune reconstitution inflammatory syndrome in HIV-associated progressive multifocal leucoencephalopathy. Eur J Neurol 23: 919–925. [DOI] [PubMed] [Google Scholar]
- Shelburne SA 3, Hamill RJ, Rodriguez-Barradas MC et al. (2002). Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 81: 213–227. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Hall HI et al. (2011). Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA 305: 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Besson C et al. (2016). Trends in primary central nervous system lymphoma incidence and survival in the U.S. British Journal of Haematology 174:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O, Marra CM, Bar-Or A et al. (2006). Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 63:1383–1387. [DOI] [PubMed] [Google Scholar]
- Sugita Y, Muta H, Ohshima K et al. (2015). Primary central nervous system lymphomas and related diseases: Pathological characteristics and discussion of the differential diagnosis. Neuropathology. 10.1111/neup.12276. [DOI] [PubMed] [Google Scholar]
- Tan K, Roda R, Ostrow L et al. (2009). PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 72: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrely A, Bienvenu B, Gasnault J et al. (2005). Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathologica 109: 449–455. [DOI] [PubMed] [Google Scholar]
- Venkataramana A, Pardo CA, McArthur JC et al. (2006). Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67: 383–388. [DOI] [PubMed] [Google Scholar]
- Wang DD, Zheng MQ, Zhang N et al. (2015). Investigation of Pneumocystis jirovecii colonization in patients with chronic pulmonary diseases in the People’s Republic of China. Int J Chron Obstruct Pulmon Dis 10: 2079–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CJ, Lee NY, Lin YH et al. (2010). Jarisch-Herxheimer reaction after penicillin therapy among patients with syphilis in the era of the HIV infection epidemic: incidence and risk factors. Clin Infect Dis 51: 976–979. [DOI] [PubMed] [Google Scholar]


