Abstract
Background
Molecular and transcription factor subtyping were recently introduced to identify patients with unique clinical features in small cell lung cancer (SCLC). However, its prognostic relevance is yet to be established. This study aims to investigate the clinical implications and prognostic significance of transcription factor subtyping in SCLC using immunohistochemistry.
Methods
One hundred and ninety consecutive SCLC patients treated with platinum-based chemotherapy at a single institution were retrospectively reviewed. Expression of ASCL1, NeuroD1, POU2F3, and YAP1 was assessed by immunohistochemical staining and applied to determine the transcription factor subtype of each case.
Results
The association among transcription factors was not entirely mutually exclusive. YAP1 expression was the most significant prognostic indicator compared with other transcription factors or their related subtypes. Among patients with limited-stage disease (LD), complete response (CR) rates were 46.2% and 22.4% in the YAP1-positive and YAP1-negative groups, respectively. The median duration of response among patients who achieved CR was 64.8 and 36.4 months in the YAP1-positive and YAP1-negative groups, respectively (P=0.06). Median overall survival (OS) in LD was 35.6 and 16.9 months in the YAP1-positive and YAP1-negative groups, respectively (P=0.03). In extensive-stage disease (ED), the median OS was 11.3 months for the YAP1-positive group and 11 months for the YAP1-negative group (P=0.03).
Conclusions
Positive expression of YAP1 can be associated with durable CR and favorable survival outcomes in patients with SCLC, especially in LD.
Keywords: Small cell lung cancer (SCLC), transcription factors, molecular subtyping, immunohistochemistry (IHC), YAP1 protein
Highlight box.
Key findings
• YAP1-positive small cell lung cancer (SCLC) was associated with better progression-free survival and overall survival in limited-stage disease (LD), and improved overall survival in extensive-stage disease.
• YAP1 positivity correlated with higher rates of durable complete response, especially in LD.
What is known and what is new?
• The prognostic role of YAP1 expression in SCLC is unclear, with conflicting reports of both favorable and unfavorable outcomes.
• This study suggests YAP1 expression has potential as a favorable prognostic and predictive biomarker in SCLC, particularly for LD. It highlights the complex interplay and co-expression of neuroendocrine and non-neuroendocrine markers in SCLC subtypes.
What is the implication, and what should change now?
• The prognostic value of YAP1 expression in SCLC warrants further validation in larger, prospective studies.
• Functional studies are needed to elucidate the mechanism linking YAP1 expression to immune activity in the SCLC tumor microenvironment.
• Investigating the clinical role of YAP1 in patients with SCLC receiving immunotherapy will help to assess its relevance in the current treatment landscape.
Introduction
Small cell lung cancer (SCLC) is a highly aggressive disease, representing approximately 13% of primary lung cancer (1). Despite the initial response to anticancer therapy, the prognosis of patients with SCLC is poor, with little improvement in survival in the past two decades (2). Although the introduction of immune checkpoint inhibitors has provided a new therapeutic paradigm (3,4), novel biomarkers and stratification systems are needed to identify patients who benefit from immunochemotherapy in SCLC.
Recently, molecular subtypes, including SCLC-A, SCLC-N, SCLC-P, and SCLC-Y, were defined by the expression of the key transcription regulators ASCL1, NeuroD1, POU2F3, and YAP1, respectively (5). The appropriateness of the nomenclature for the SCLC-Y subtype is debatable, some suggesting that a triple or quadruple-negative subtype may be more accurate (6,7). However, its clinical relevance remains to be determined. The SCLC-I subtype, characterized by an inflamed microenvironment and low expression of ASCL1, NeuroD1, and POU2F3, derived favorable outcomes from the addition of an immune checkpoint inhibitor (8). A previous study indicated that the YAP1-dominant subtype was associated with better prognosis in patients with stage I to III SCLC (9). In contrast, others suggested high YAP1 expression as a negative prognostic factor in patients undergoing curative resection for stage IA to IIIB SCLC (6) and those with stage I to III SCLC (10). Furthermore, another study suggested SCLC-P as a poor prognostic factor for resectable SCLC (11). Given this uncertainty, we planned to investigate further the clinical implications of immunohistochemistry (IHC)-based transcription factor subtyping in SCLC. We present this article in accordance with the REMARK reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-317/rc).
Methods
Patients and study design
All patients histologically confirmed with SCLC between July 2006 and March 2020 at Gyeongsang National University Hospital were retrospectively identified and reviewed in this study. The following inclusion criteria were applied: (I) treatment with platinum-based chemotherapy was the first-line treatment for SCLC. (II) Availability of archival tumor tissue or recent biopsy samples suitable for IHC and transcription factor subtyping. (III) Adequate clinical and follow-up data to determine key endpoints, such as overall survival (OS) and progression-free survival (PFS). Patients were excluded from the study if they had a coexisting malignancy or a history of another malignancy within the last 5 years, experienced histological transformation from non-small cell lung cancer, or had a combined SCLC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Gyeongsang National University Hospital (GNUH 2023-12-002) and individual consent for this retrospective analysis was waived.
Evaluation of IHC for SCLC subtyping
IHC was performed on the 4-µm-thick sections from formalin-fixed paraffin-embedded specimens (biopsy or cellblock). The tissue sections were attached to glass slides, deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide for 10 min to block the endogenous peroxidase activity. The slides were subsequently heated for 20 min in 10 mmol/L sodium citrate buffer (pH 6.0, homemade; for NeuroD1, POU2F3, YAP1) or tris-EDTA (pH 9.0, ab93684, Abcam, Cambridge, MA, USA; for ASCL1) in a microwave oven (700 W) and incubated with Ultra V Block (Lab Vision; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 7 min at room temperature (20–25 ℃) to block background staining. Then, the slides were incubated with each primary antibody according to the manufacturer’s protocols (Table S1). Ultravision LP Detection System HRP DAB (Thermo Fisher Scientific, Kalamazoo, MI, USA) was used to visualize antigens, and the sections were counterstained using Mayer’s hematoxylin.
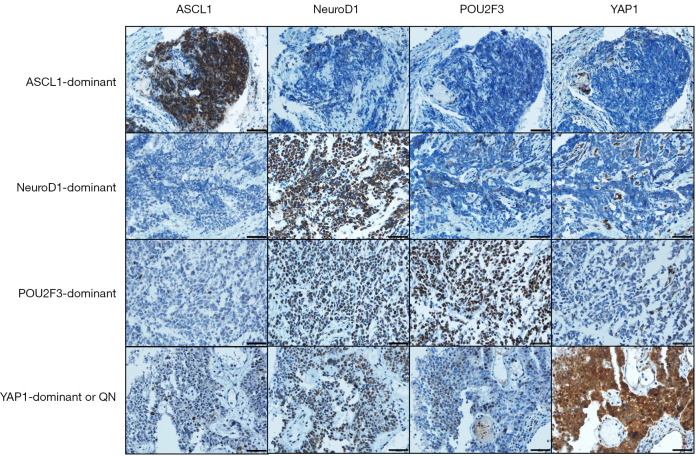
Expression of each protein was blindly examined to each IHC result. ASCL1, NeuroD1, and POU2F3 were expressed in the nuclei, and YAP1 was expressed in the nuclei and cytoplasm. Due to the small number of cases (only two) with YAP1 nuclear staining, we performed the analysis based on cytoplasmic intensity of YAP1 staining only. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The proportional score of stained tumor cells was scored as a percentage (0–100%) of positive cells for the transcription regulators. We evaluated the IHC results with an H-score. The H-score was calculated by multiplying the intensity score by the proportional score (0–300). An H-score above 50 was classified as high expression, while scores ranging from 1 to 50 indicated low expression. For binary classification, an H-score of 0 was considered a negative result, and any H-score of 1 or higher was deemed positive. In determining the dominant subtype for a given case, the subtype with the highest H-score was selected when compared to the H-scores of other subtypes within that same case. Only one case was negative in all four proteins and categorized as ‘YAP1-dominant or quadruple-negative’ subtype (Figure 1).
Figure 1.
Representative images of immunohistochemical staining for transcription factors across their subtypes. Each image includes a scale bar representing 50 µm. QN, quadruple-negative.
Endpoints and assessments
The primary objective of the study was to evaluate the prognostic implication of transcription factor and dominant subtypes on survival outcomes, treatment response, and duration of response (DoR) in SCLC patients. First, preliminary analyses were performed based on the expression of each transcription factor and its dominant subtype. Then, further analyses were planned to focus on the transcription factor or dominant subtype, which showed significantly discriminating prognostic factors for survival in the preliminary analyses. Baseline characteristics, including age at diagnosis, Eastern Cooperative Oncology Group performance status (ECOG PS), stage [limited-stage disease (LD) or extensive-stage disease (ED) according to the Veterans Administration Lung Study Group], tumor-node-metastasis (TNM) stage by the American Joint Committee on Cancer 8th edition, and metastatic lesions at presentation, were collected from electronic medical records. Furthermore, data on treatment modalities, chemotherapy regimens, number of cycles, dose intensities, use of concurrent chemoradiotherapy, and prophylactic cranial irradiation were also recorded. Follow-up assessments were conducted every 6–12 weeks or as clinically indicated, and included imaging studies and other clinical evaluations for disease progression, treatment response, and toxicities. Treatment response was assessed using RECIST 1.1. The DoR was defined as the time from the initial documentation of a tumor response [partial response (PR) or complete response (CR)] to progression of the disease or death due to any cause. Early treatment discontinuation was defined as the premature cessation of treatment for reasons other than cancer progression, occurring before the completion of the planned cycle of platinum-based chemotherapy. Treatment-related mortality was defined as death occurring within 30 days following the last dose of first-line chemotherapy and which is not a result of cancer progression. Relative dose intensity was defined as the actual dose of a drug administered per cycle, divided by the standard dose of the drug per cycle, expressed as a percentage. In this study, the relative dose intensities were calculated only for etoposide or irinotecan.
Statistical analysis
Descriptive statistics with the Chi-square test, Fisher’s exact test, the Mann-Whitney U test, and Cuzick’s test for trend were used to summarize the baseline and treatment-related characteristics of the study population. Kaplan-Meier survival analysis with log-rank tests was conducted to evaluate PFS and OS. Variables with a P value less than 0.1 in univariate analyses were included in the multivariate Cox proportional hazards models to assess the prognostic significance of the molecular markers and other covariates. A two-sided P value of <0.05 was considered statistically significant for all tests. All statistical analyses were conducted using STATA version 16.1 (StataCorp LLC, College Station, Texas, USA) and R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Expression of each transcription factor by dominant subtype
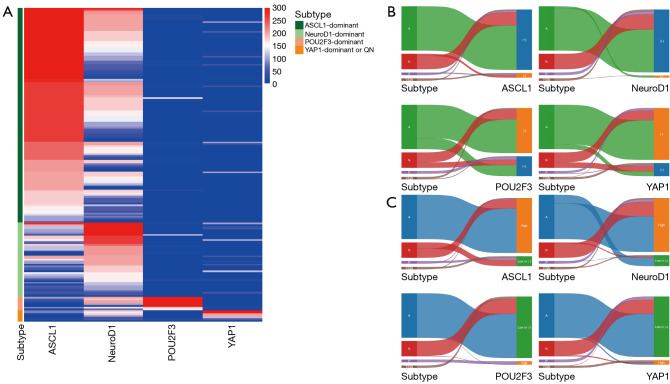
A total of 190 patients were included in the study, with an assessment of transcription factor expression conducted across four dominant subtypes (Figure 2 and Table S2). In the ASCL1-dominant subtype, which included 68.4% of our patient cohort (n=130), high ASCL1 expression was expectedly predominant. Simultaneously, high NeuroD1 expression was observed in 80.8% of these patients. Among patients with an H-score greater than 50, 82.0% of those tested for ASCL1 (132 of 161) and 65.8% of those tested for NeuroD1 (104 of 158) also had an H-score greater than 150. This coexpression aligns with the expected profile of neuroendocrine markers. However, the non-neuroendocrine markers POU2F3 and YAP1 were also positively expressed in this neuroendocrine-dominant group. Specifically, in the ASCL1-dominant subtype, POU2F3 showed positive expression in 24.6% of cases (32 of 130 patients), and YAP1 was positive in 10.8% (14 of 130 patients). For example, one ASCL1-dominant SCLC case showed high POU2F3 expression, which exhibited tissue necrosis and some cytoplasmic staining in viable tumor cells, likely due to the leakage of nuclear proteins into the cytoplasm caused by cellular damage (Figure S1). Similarly, in the subtype dominant in NeuroD1, representing 23.7% of the patient population (n=45), high expression of NeuroD1 was observed in conjunction with moderate expression of ASCL1, reflecting the cooccurrence of neuroendocrine markers. Again, challenging the expected exclusivity, both POU2F3 and YAP1 were positively expressed in 35.6% of cases (16 of 45 patients).
Figure 2.
Transcription factor expression and categorization across subtypes. (A) Heatmap visualization showing expression levels of transcription factors across different subtypes. H-scores (0–300) indicate the expression level, with color intensity corresponding to H-score values. (B,C) Sankey diagrams categorize expression levels of transcription factors as (B) high vs. low or negative and (C) positive vs. negative, showing their distribution across subtypes. A, ASCL1; N, NeuroD1; P, POU2F3; Y, YAP1; QN, quadruple-negative; (+), positive; (−), negative.
The neuroendocrine markers ASCL1 and NeuroD1 expression in non-neuroendocrine-dominant subtypes also presented similar findings. In the predominant subtype of POU2F3, which comprised 4.2% of the population of patients (n=8), positive expression of ASCL1 was observed in 62.5% (5 of 8 patients). NeuroD1 was positively expressed in all cases of this subtype, with 87.5% (7 of 8 patients) showing high expression. In the YAP1-dominant or quadruple-negative subtype, which represents 3.7% of the cohort (n=7), ASCL1 and NeuroD1 were positively expressed in 42.9% (3 of 7 patients) and 85.7% (6 of 7 patients), respectively.
Preliminary analysis to identify the key transcription factor and subtype
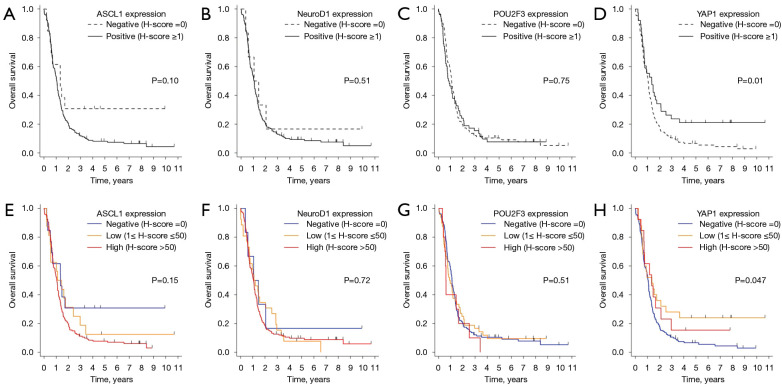
Since the association between transcription factors was not entirely mutually exclusive (Figure 2B,2C), we performed preliminary survival analyses by dominant subtype and by expression of transcription factors to identify key prognostic indicators. When patients were categorized by dominant subtype, the YAP1-dominant or quadruple-negative subtype group exhibited the most favorable prognosis, with a median OS of 23.7 months [95% confidence interval (CI): 2.56 to not reached]. However, this observation did not reach statistical significance (P=0.19), potentially because of the small sample size of the YAP1-dominant or quadruple-negative subtype group (n=7) (Figure S2). When analyzing the Kaplan-Meier survival curves for four transcription factors, considering their expressions, it was observed that ASCL1, NeuroD1, and POU2F3 did not show statistically significant differences in survival based on their expressions (Figure 3A-3C). In contrast, YAP1 expression had significant implications for OS (P=0.01 for positive vs. negative; Figure 3D). This trend maintained when we analyzed the data across three tiers of expression: negative, low, and high (Figure 3E-3H). Consequently, our subsequent analyses were primarily focused on YAP1 binary expression (negative vs. positive) as a potential prognostic indicator.
Figure 3.
Overall survival is based on expression levels of transcription factors. (A-D) Overall survival based on a binary expression classification (negative or positive) of transcription factors. (E-H) Overall survival according to transcription factors’ negative, low, and high expression categories.
Patient and treatment-related characteristics by YAP1 expression
Table 1 presents the baseline characteristics of the 190 patients analyzed on YAP1 expression. In the YAP1-negative group (n=152) and YAP1-positive group (n=38), while the YAP1-positive group tended to be younger, predominantly male, and had a better performance status, these differences did not reach statistical significance. Although the proportion of strong intensity for IHC markers supporting SCLC diagnosis was generally higher in the YAP1-negative group, only synaptophysin showed a statistically significant difference between the YAP1-negative and YAP1-positive groups (P=0.045). Both groups showed similar smoking status, disease stage distribution, metastatic lesions, and lactate dehydrogenase levels, further emphasizing the lack of significant differences between these groups in baseline characteristics.
Table 1. Baseline characteristics by YAP1 expression.
| Characteristics | YAP1-negative (n=152) | YAP1-positive (n=38) | P |
|---|---|---|---|
| Age (years) | 0.09 | ||
| <70 | 85 (55.9) | 27 (71.1) | |
| ≥70 | 67 (44.1) | 11 (28.9) | |
| Median [IQR] | 69 [62.5–75] | 64.5 [60–75] | 0.07 |
| Sex | 0.06 | ||
| Male | 126 (82.9) | 36 (94.7) | |
| Female | 26 (17.1) | 2 (5.3) | |
| ECOG PS | 0.06 | ||
| 0–1 | 110 (72.4) | 33 (86.8) | |
| 2–3 | 42 (27.6) | 5 (13.2) | |
| Smoking | 0.20 | ||
| Never-smoker | 9 (5.9) | 0 | |
| Current/former smoker | 143 (94.1) | 38 (100.0) | |
| VA Lung Study Group classification | 0.65 | ||
| LD | 58 (38.2) | 13 (34.2) | |
| ED | 94 (61.8) | 25 (65.8) | |
| TNM stage by AJCC 8th edition | 0.82 | ||
| I–II | 4 (2.6) | 4 (10.5) | |
| III | 56 (36.8) | 9 (23.7) | |
| IV | 92 (60.5) | 25 (65.8) | |
| Metastatic lesions (n=119 with ED) | |||
| Central nervous system | 23 (24.5) | 10 (40.0) | 0.12 |
| Liver | 25 (26.6) | 8 (32.0) | 0.59 |
| Bone | 41 (43.6) | 9 (36.0) | 0.49 |
| Pleural effusion | 39 (41.5) | 12 (48.0) | 0.55 |
| Lactate dehydrogenase (n=148) | 0.86 | ||
| Normal | 43 (36.1) | 10 (34.5) | |
| Elevated | 76 (63.9) | 19 (65.5) | |
| CD56 | 0.09 | ||
| Negative or weak | 3 (2.0) | 3 (7.9) | |
| Strong | 149 (98.0) | 35 (92.1) | |
| Synaptophysin (n=165) | 0.045 | ||
| Negative or weak | 18 (13.5) | 9 (28.1) | |
| Strong | 115 (86.5) | 23 (71.9) | |
| Chromogranin (n=102) | 0.056 | ||
| Negative or weak | 49 (57.6) | 14 (82.4) | |
| Strong | 36 (42.4) | 3 (17.6) | |
The variables are presented as number (%) if not otherwise specified. IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group performance status; VA, Veterans Administration; LD, limited-stage disease; ED, extensive-stage disease; TNM, tumor-node-metastasis; AJCC, American Joint Committee on Cancer.
The treatment modalities and parameters were reviewed to assess the possible biases influencing treatment response and prognosis between the YAP1-positive and YAP1-negative groups (Table S3). Both groups showed similar distributions in chemotherapy regimens, with etoposide/cisplatin being the most prevalent. An immunotherapy combined regimen was applied in only four patients in total as the first-line therapy, and no patients received immunotherapy after disease progression. The median number of chemotherapy cycles was consistent at six for both groups [interquartile range (IQR), 4–6, P=0.56]. Relative dose intensity per cycle, calculated for etoposide and irinotecan, was largely analogous, with both groups having a median intensity of 98% (P=0.98). Concurrent chemoradiotherapy and prophylactic cranial irradiation use did not significantly differ between groups (P=0.59 and P=0.37, respectively). The discontinuation of early treatment was observed identically in 21.1% of the patients in both YAP1 expression groups (P>0.99). The YAP1-negative group reported a 4.0% treatment-related mortality rate, with no cases in the YAP1-positive group (P=0.60).
Association of YAP1 expression with treatment response and DoR
The YAP1-positive group presented with a higher incidence of CR (18.4%, n=7) compared with the YAP1-negative group (9.2%, n=14) (Table 2). This difference did not reach statistical significance (P=0.18). This tendency was more pronounced in the LD subgroup, where the YAP1-positive group showed a CR rate of 46.2% (n=6), which nearly doubled the 22.4% (n=13) in the YAP1-negative group. A direct comparison was difficult in the ED subgroup because the number of CRs is very low, with only one patient achieving CR in each group. Despite these variances in CR, the objective response rate (ORR) remained relatively consistent across both YAP1-positive and YAP1-negative groups regardless of tumor stage.
Table 2. Treatment response by YAP1 expression.
| Treatment response | YAP1-negative | YAP1-positive | P |
|---|---|---|---|
| Total | n=152 | n=38 | |
| CR | 14 (9.2) | 7 (18.4) | 0.18 |
| PR | 112 (73.7) | 23 (60.5) | |
| SD, PD, or not evaluated | 26 (17.1) | 8 (21.1) | |
| Objective response rate (CR + PR) | 126 (82.9) | 30 (78.9) | 0.57 |
| Limited-stage | n=58 | n=13 | |
| CR | 13 (22.4) | 6 (46.2) | 0.09 |
| PR | 40 (69.0) | 5 (38.5) | |
| SD, PD, or not evaluated | 5 (8.6) | 2 (15.4) | |
| Objective response rate (CR + PR) | 53 (91.4) | 11 (84.6) | 0.60 |
| Extensive-stage | n=94 | n=25 | |
| CR | 1 (1.1) | 1 (4.0) | 0.48 |
| PR | 72 (76.6) | 18 (72.0) | |
| SD, PD, or not evaluated | 21 (22.3) | 6 (24.0) | |
| Objective response rate (CR + PR) | 73 (77.7) | 19 (76.0) | 0.86 |
The variables are presented as number (%). CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Regarding the expression status of ASCL1 and NeuroD1 in patients who achieved CR within the YAP1-positive group, our analysis showed no significant differences according to CR status (Table S4). The distribution of ASCL1 (P=0.97) and NeuroD1 (P=0.32) expression levels was not statistically different between patients who achieved CR and those who did not. These findings suggest that the expression levels of ASCL1 and NeuroD1 may not significantly impact CR status in the YAP1-positive group.
The median DoR for the YAP1-positive and YAP1-negative groups was 5.2 (IQR, 3.7–12.9) and 5.8 (IQR, 4.3–9.2) months, respectively (P=0.75). Notably, when focusing on patients who achieved CR in the LD subgroup, the YAP1-positive group exhibited a substantially longer median DoR compared to the YAP1-negative group [64.8 (IQR, 46.1–74.8) vs. 36.4 (IQR, 15.0–41.3) months], although the difference was not statistically significant (P=0.06) (Figure S3).
Prognostic implications of YAP1 expression on survival outcomes
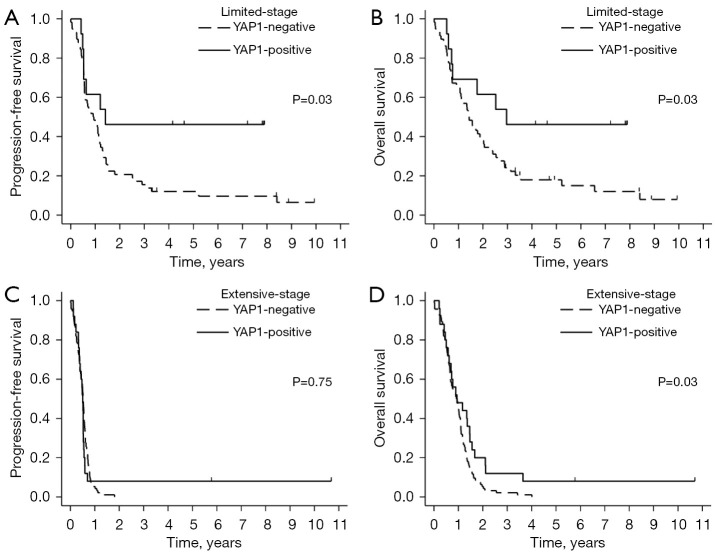
The study had a median follow-up duration of 93 months. Figure 4 displays the OS and PFS rates, stratified by YAP1 expression and tumor stage. Among patients with LD, those in the YAP1-positive group had significantly better PFS compared to the YAP1-negative group (median PFS: 17 vs. 11 months; 95% CI: 6.3 to not reached vs. 6.8 to 14.2; P=0.03). This distinction was not observed in patients with ED, where median PFS was 6.1 vs. 6.3 months in the YAP1-positive and YAP1-negative groups, respectively (95% CI: 4.5 to 6.4 vs. 5.4 to 6.8; P=0.75) (Figure 4A,4C). Moreover, the YAP1-positive group demonstrated a significantly superior OS compared to the YAP1-negative group in both LD (median OS: 35.6 vs. 16.9 months; 95% CI: 8.6 to not reached vs. 12.9 to 24.3; P=0.03) and ED patients (median OS: 11.3 vs. 11 months; 95% CI: 7.5 to 17.6 vs. 8.1 to 13; P=0.03) (Figure 4B,4D). Similar patterns were observed in the analyses according to the TNM stage system, with TNM stage III showing results comparable to LD, and TNM stage IV comparable to ED (Figure S4). Figure S5 further elaborates on the prognostic value of YAP1 among a subset of 175 patients with neuroendocrine subtypes, specifically in those with either ASCL1- or NeuroD1-dominant subtypes. Consistent with the overall patient population, the YAP1-positive group showed superior OS across all tumor stages and longer PFS, specifically in LD.
Figure 4.
Kaplan-Meier survival curves by YAP1 expression. Progression-free survival (A,C) and overall survival (B,D) in limited- and extensive-stage, respectively.
On multivariate analysis (Table S5), several factors were identified as independent favorable prognostic indicators for OS, including the age less than 70 years, an ECOG PS of 0–1, LD, absence of bone metastasis, and a YAP1-positive expression (hazard ratio: 0.597; 95% CI: 0.387 to 0.921; P=0.02).
Discussion
In this study, we assessed the clinical implications of YAP1 expression in SCLC. The YAP1-positive group showed better PFS and OS in the LD subgroup and improved OS in the ED subgroup. This finding remained consistent even in patients with neuroendocrine subtypes. Furthermore, positive YAP1 expression was associated with a higher rate of durable CR, particularly in the LD subgroup. Differences in clinical outcomes were not attributed to the imbalance in baseline and treatment-related characteristics between the groups.
The prognostic role of YAP1 expression in SCLC has not been established, showing both favorable and unfavorable survival outcomes in patients with positive YAP1 expression or YAP1-dominant subtype. On the favorable side, the SCLC-Y subtype was associated with a T-cell inflamed gene expression profile, correlating with longer-term survival in SCLC (12). Qi et al. suggested that the YAP1-dominant subtype, determined by IHC, correlated with reduced infiltration of CTLA4+ T-cell and favorable survival outcome in stage I to III SCLC (9). Sun et al. also reported that YAP1 expression was positively correlated with CD8+ T-cell, macrophage, neutrophil, and dendritic cell infiltration and was associated with improved survival in lung cancer (13). Because immunotherapy was rarely performed in our cohort, it may be difficult to explain our finding in terms of immune response. However, the enhanced treatment response to cytotoxic chemotherapy observed in the YAP1-positive group might be related to the property of an immunologically active tumor microenvironment. Cytotoxic chemotherapy can serve as immune stimuli by inducing T-cell priming and recruitment at the tumor site (14). Additionally, cytotoxic chemotherapy may selectively target immunosuppressive cells and potentially increase effector immune cell function (15). These considerations suggest that YAP1 expression may indicate a more robust immune response, a favorable clinical feature.
Because no functional study was performed, our study cannot fully understand a mechanism that explains the immunologically active property of YAP1-positive tumors and their variation across different stages. Nevertheless, observations of durable CR in the LD subgroup with YAP1-positive tumors may suggest a robust immune response in this population. This inference is supported by that immune environment is conducive to long-term treatment response (16). Moreover, the increased treatment response observed in the YAP1-positive group with lower tumor burden (LD in our cohort) might be explained by immunoediting, where the immune system can more effectively control tumor growth when the overall tumor volume is smaller (17). Additionally, the enhanced treatment outcomes observed in the LD subgroup with YAP1-positive tumors might be explained by the abscopal effect, where local treatments like radiotherapy not only affect the treated area but also have a systemic impact on distant tumor sites. This phenomenon is thought to be immune-mediated, with a more pronounced effect in immunologically active tumors (18). To further understand and validate these hypotheses, it’s crucial to examine the infiltration of immune cells in both YAP1-positive and -negative tumors and determine their clinical significance.
In contrast, several studies have reported that YAP1-positive expression or YAP1-dominant subtype may be a negative prognostic factor in SCLC. Chen et al. suggested that the SCLC-Y subtype exhibited high expression of programmed death ligand-1 (PD-L1), impaired T-cell functionality, and poor prognosis compared to other subtypes (10). This indicates that positivity for YAP1 may be associated with immune evasion, resulting in poor treatment outcomes. Furthermore, Hwang et al. reported that YAP1 expression was associated with a higher locoregional recurrence rate (6). In particular, YAP1 expression was related to worse OS only in certain SCLC subtypes. Specifically, in combined SCLC, but not in pure SCLC, YAP1 expression was significantly higher and served as an unfavorable predictor for OS (19). YAP1 also plays a crucial role in epithelial-to-mesenchymal transition, leading to increased metastatic potential and worse clinical outcomes in various cancers (20-22).
It is unclear why such varied results exist among studies, including our cohort. The heterogeneity of the SCLC disease entity should be entertained. SCLC has various molecular and phenotypic variations, which may significantly influence study outcomes. Ding et al. showed significant ASCL1 expression on POU2F3-dominant SCLC (11). Gay et al. found that a single SCLC tumor contained substantial proportions of multiple subtypes, with ASCL1 and NeuroD1 expressing cells located in distinct spatial regions (8). Recently, Ng et al. showed that SMARCA4 mutations are commonly observed in SCLC-Y cell lines, leading to reduced SMARCA4 expression and characteristics of SMARCA4-deficient undifferentiated tumors, rather than SCLC. This finding suggests that YAP1 may not be a subtype-determining transcription factor in SCLC (23). Furthermore, in our study, the coexpression of non-neuroendocrine markers POU2F3 and YAP1 in the neuroendocrine-dominant subtype makes subtype classifications difficult, suggesting a complex interplay of transcription factors (Figure 2C). This is further supported by the presence of the neuroendocrine markers ASCL1 and NeuroD1 in non-neuroendocrine-dominant subtypes (Figure 2B). These findings suggest an incomplete distinction among SCLC subtypes and indicate the need for a revised classification. Moreover, methodological issues may affect the results, particularly when determining dominant subtypes based on transcription factors. There is a lack of standardization in assessment methods, including RNA sequencing and IHC staining. The cut-off values defining ‘positive’ or ‘high’ expression of molecular markers or transcription factors are also not universal among the studies (6,11,24). Furthermore, while high YAP1 expression is associated with an inflamed gene expression profile and potentially favorable prognosis (12), it also correlates with immune evasion mechanisms, such as increased PD-L1 expression and T-cell impairment (10). This dual role may explain why studies for YAP1 expression resulted in different clinical outcomes. Lastly, the clinical variables and endpoints used to assess outcomes, such as PFS, OS, or DoR, vary between studies, possibly leading to different interpretations of the prognostic role of YAP1.
The main limitation of this study is the retrospective design, which introduces selection bias and a lack of control over confounding variables. Small subgroup sizes may limit the statistical power to detect differences in outcomes. For example, the absence of statistical differences in OS between negative and high YAP1 expression (P=0.20), as well as between low and high YAP1 expression (P=0.72), raises the question of whether YAP1 truly serves as a prognostic biomarker (Figure 3H). The number of patients with high YAP1 expression was only 5 in the LD subgroup and 8 in the ED subgroup. The small subgroup size also made it difficult to statistically confirm the difference in DoR in the LD subgroup that achieved CR (Figure S3B) and to perform subgroup analyses according to significant clinical variables such as age and ECOG PS. The single-center study design questions the generalizability of the results. Another significant limitation pertains to the temporal scope of our sample collection [2006–2020], which precedes the widespread adoption of PD-L1 inhibitors in first-line treatment for ED-SCLC. Indeed, the majority of the patients in this study did not receive immunotherapy. This study’s timeframe limits the applicability of our findings in the current treatment landscape. Moreover, our study does not fully address the mechanism connecting YAP1 expression to immune activity within the tumor microenvironment. This gap should be addressed by functional studies and research focused on patients undergoing immunotherapy. While we have recognized the importance of considering other biomarker expressions such as CD8, PD-L1, and SMARCA4, we were constrained by the availability of our specimens. We had only partial unstained slides remaining after the initial test staining, and many small biopsy specimens had limited material left in the paraffin blocks. Therefore, it was challenging to perform additional IHC on this cohort.
Conclusions
Our study suggests the potential prognostic and predictive role of YAP1 expression in SCLC, particularly in the LD subgroup. However, the complex relationship between YAP1 expression and immune activity needs further investigation. Integrating additional biomarkers may enhance our understanding of SCLC’s immune landscape and neuroendocrine characteristics, potentially providing a more detailed prognostic framework.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (No. RS-2023-00219399).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Gyeongsang National University Hospital (GNUH 2023-12-002) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-317/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-317/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-317/dss
References
- 1.Cohen S, Brennan B, Banerjee M, et al. Temporal trends in small cell lung cancer: Analysis of the U.S. Surveillance, Epidemiology and End Results (SEER) database. J Clin Oncol 2023;41:e20641. 10.1200/JCO.2023.41.16_suppl.e20641 [DOI] [Google Scholar]
- 2.Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012;13:115-22. 10.1016/j.cllc.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 3.Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. 10.1016/S1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 5.Rudin CM, Poirier JT, Byers LA, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019;19:289-97. 10.1038/s41568-019-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang S, Hong TH, Kim HK, et al. Whole-Section Landscape Analysis of Molecular Subtypes in Curatively Resected Small Cell Lung Cancer: Clinicopathologic Features and Prognostic Significance. Mod Pathol 2023;36:100184. 10.1016/j.modpat.2023.100184 [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Li S, Wang H, et al. Molecular subtypes, predictive markers and prognosis in small-cell lung carcinoma. J Clin Pathol 2023. [Epub ahead of print]. doi: . 10.1136/jcp-2023-209109 [DOI] [PubMed] [Google Scholar]
- 8.Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021;39:346-360.e7. 10.1016/j.ccell.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi J, Zhang J, Liu N, et al. Prognostic Implications of Molecular Subtypes in Primary Small Cell Lung Cancer and Their Correlation With Cancer Immunity. Front Oncol 2022;12:779276. 10.3389/fonc.2022.779276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Sun C, Wang H, et al. YAP1 expression is associated with survival and immunosuppression in small cell lung cancer. Cell Death Dis 2023;14:636. 10.1038/s41419-023-06053-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding XL, Su YG, Yu L, et al. Clinical characteristics and patient outcomes of molecular subtypes of small cell lung cancer (SCLC). World J Surg Oncol 2022;20:54. 10.1186/s12957-022-02528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owonikoko TK, Dwivedi B, Chen Z, et al. YAP1 Expression in SCLC Defines a Distinct Subtype With T-cell-Inflamed Phenotype. J Thorac Oncol 2021;16:464-76. 10.1016/j.jtho.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun K, Zhang XD, Liu XY, et al. YAP1 is a Prognostic Biomarker and Correlated with Immune Cell Infiltration in Pancreatic Cancer. Front Mol Biosci 2021;8:625731. 10.3389/fmolb.2021.625731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opzoomer JW, Sosnowska D, Anstee JE, et al. Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer. Front Immunol 2019;10:1654. 10.3389/fimmu.2019.01654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 2013;62:203-16. 10.1007/s00262-012-1388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541-50. 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Li L, Wu S, et al. Abscopal effect of radiation therapy and nivolumab in a patient with combined small-cell lung cancer: a case report. Immunotherapy 2022;14:909-14. 10.2217/imt-2021-0050 [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Guo Y, Liu L, et al. YAP1 protein expression has variant prognostic significance in small cell lung cancer (SCLC) stratified by histological subtypes. Lung Cancer 2021;160:166-74. 10.1016/j.lungcan.2021.06.026 [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Zhang X, Jiang Y, et al. YAP1 activation promotes epithelial-mesenchymal transition and cell survival of renal cell carcinoma cells under shear stress. Carcinogenesis 2022;43:301-10. 10.1093/carcin/bgac014 [DOI] [PubMed] [Google Scholar]
- 21.Si M, Song Y, Wang X, et al. CXCL12/CXCR7/β-arrestin1 biased signal promotes epithelial-to-mesenchymal transition of colorectal cancer by repressing miRNAs through YAP1 nuclear translocation. Cell Biosci 2022;12:171. 10.1186/s13578-022-00908-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Jang G, Sim SH, et al. SMARCA4 Depletion Induces Cisplatin Resistance by Activating YAP1-Mediated Epithelial-to-Mesenchymal Transition in Triple-Negative Breast Cancer. Cancers (Basel) 2021;13:5474. 10.3390/cancers13215474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng J, Cai L, Girard L, et al. Molecular and Pathologic Characterization of YAP1-Expressing Small Cell Lung Cancer Cell Lines Leads to Reclassification as SMARCA4-Deficient Malignancies. Clin Cancer Res 2024;30:1846-58. 10.1158/1078-0432.CCR-23-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baine MK, Hsieh MS, Lai WV, et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J Thorac Oncol 2020;15:1823-35. 10.1016/j.jtho.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]