Abstract
Tylosin is an important macrolide antibiotic produced by Streptomyces fradiae. In the biosynthesis of tylosin, macrocin O-methyltransferase TylF catalyzes the conversion of the side-product tylosin C (macrocin) to the primary component tylosin A (C/A conversion). This conversion is the rate-limiting step in the biosynthesis of tylosin, and affects the quality of the end product. To find a high activity and environment-adapted TylF enzyme, a TylF variant pool has been constructed via protein evolution approach in our previous study (Fan et al., 2023 [41]). In this study, the TylF variants with higher C/A conversion rates were expressed in E. coli and purified. The variants TylFY139F, TylFQ138H, F232Y and TylFT36S, V54A were shown to have a higher C/A conversion rate at 30 °C than that of TylF at 38 °C. Moreover, they had a greater acid resistance and showed more adaptable to the pH change during fermentation. Further protein structural and substrate-binding affinity analyses revealed that the T36S, V54A, Q138H, Y139F, and F232Y mutations enlarged the volume of the substrate-binding pocket, thereby increasing the affinity of enzyme variants for their substrates of SAM and macrocin, and decreasing the inhibition of SAH. Three of the TylF variants were overexpressed in the industrial tylosin-producing S. fradiae strain, and the recombinant strains showed the highest C/A conversion at 30 °C without heating up to 38 °C during the last 24 h of fermentation. This is of great energy-saving significance for tylosin industrial production.
Keywords: Streptomyces fradiae, Macrocin O-methyltransferase, SAM, Tylosin
Graphical Abstract
1. Introduction
S-adenosyl-L-methionine (SAM)-dependent methyltransferases (MTs), the ubiquitous enzymes involved in methylation, play important roles in a variety of biological processes [1], [2], [3], including cellular signaling [4], membrane biogenesis [5], pigment production [6], and production and functional regulation of biomolecules such as proteins and DNA/RNAs [7], [8], [9]. A noteworthy example is the protein arginine MT 5 (PRMT5), which is involved in genome organization, transcriptional control, cell cycle, and spliceosome assembly [10], [11]. In addition, SAM-dependent MTs are also particularly common in secondary metabolism, methylating a wide range of biosynthetic scaffolds, which can affect the physicochemical and biological properties of the resulting natural products [12], [13], [14], [15], among which oxygen atoms-modified O-MTs (OMTs) are the largest class (54 %) of the EC subclass [16]. For most MTs, S-adenosyl-L-homocysteine (SAH), the demethylated hydrolystate of SAM, is an inhibitor of MTs that competes with SAM for SAM-dependent transmethylation [17]. Therefore, SAM-dependent MTs are potentially industrially relevant for the biosynthesis of active pharmaceutical ingredients [18], [19], [20], [21], [22].
Methylation of an antibiotic can have both indirect and direct effects on its potency. Tylosin, is a 16-membered macrolide antibiotic, composed of four bioactive forms, tylosin A, B, C and D, with tylosin A having the greatest share (80–90 %) and activity [23], [24]. In tylosin-producing strain Streptomyces fradiae (S. fradiae), class-I SAM-dependent OMTs TylF methylates tylosin C (macrocin) to tylosin A at 3′-OH (hereinafter referred to as “C/A conversion”), which is the final as well as the main rate-limiting step in tylosin biosynthesis [25], [26]. In the previously analyzed series of S. fradiae mutant strains, the increased tylosin production was accompanied with the increased levels of TylF expression [27], [28], [29]. It is noteworthy that the enzyme activity of TylF reaches its maximum at 72 h and then began to decline gradually during fermentation [27], [28], [29], and that rapid loss of enzyme specific activity of TylF in the late fermentation leads to the production of large amounts of by-product tylosin D without biologically activity, thereby reducing the quality (purity) of tylosin end product [27]. Commercial tylosin requires the tylosin A content to be above 95 %. Thus, at the late stage of industrial fermentation, the temperature needs to be raised from 30 °C to 38 °C for 24 h to promote the C/A conversion since the maximum enzyme activity of TylF is at 38 °C. This is an extremely energy-intensive process. Therefore, improving the C/A conversion efficiency of TylF at a lower temperature is crucial for ensuring the quality of final product of tylosin and reducing the energy consumption.
Currently, many strategies are explored to find the superior enzymes, such as directed protein evolution, structural-based rational design and computer-assisted design [30], [31], [32], [33], [34]. Directed evolution does not require a molecular understanding of the impact of mutation on the protein structure and provides a novel and more convenient way to modify enzymes [35]. At present, directed evolution methods mainly include error-prone PCR [36], DNA shuffling [37] and site-directed mutagenesis [38], [39]. In our previous work, a pool of TylF variants has been generated by using error-prone PCR, Several, S. fradiae strains expressing the TylF variants exhibited a higher yield of tylosin, among which the Y139F mutation was found to be able to improve the enzyme activity and heat resistance of TylF [40].
In this study, we further characterized the remaining seven TylF variants. Among them, TylFQ138H, F232Y and TylFT36S, V54A had been significantly improved in terms of acid resistance, catalytic activity, substrate affinity and minimizing product inhibition. Particularly, in contrast to the wild-type TylF which reaches its maximal enzymatic activity at 38 °C, the variants TylFQ138H, F232Y and TylFT36S, V54A showed optimal activity at 32 °C in vitro. Protein structure and substrate-binding affinity analyses indicated that the T36S, V54A, Q138H, Y139F, and F232Y mutations enlarged the volume of the substrate-binding pocket, thereby increasing the affinity of the enzyme variants for the substrates. The TylF variants TylFY139F and TylFT36S, V54A were expressed in the industrial tylosin-producing S. fradiae SF-3 strain, and the engineered strains displayed an almost complete C/A conversion at 30 °C without the need of heating up to 38 °C during the last 24 h of fermentation. It has a great potential for energy conservation in the tylosin industry.
2. Materials and methods
2.1. Bacterial strains, culture conditions, and plasmids
The bacterial strains and plasmids used in this work are shown in Table S1. S. fradiae SF-3, an industrial strain, donated by HVSEN Biotech Co., Ltd, Wuhan, China, was used as the parental strain for S. fradiae engineering and tylosin production. The expression tylF and its variants was achieved by introducing pSET152 plasmid in to S. fradiae SF-3. S. fradiae and its derived strains were cultured in tryptic soy broth (TSB) or on tryptic soy agar (TSA) (BD, Franklin Lakes, NJ, USA) and Gause’s No.1 medium (10 g KNO3, 1 g NaCl, 0.8 g K2HPO4·3H2O, 0.5 g MgSO4·7H2O, 0.01 g FeSO4·H2O, 20 g agar, and 20 g corn starch per liter (pH 7.2)) at 30 °C. Apramycin (50 µg/ml) was used to screen the transformants. E. coli DH5α was served as the host strain for regular cloning, and E. coli BL21(DE3) was used for prokaryotic expression of His-tag fusion proteins. E. coli ET12567 was used for E. coli-Streptomyces conjugal transfer, and was cultivated with chloramphenicol (25 µg/ml) and kanamycin (25 µg/ml). All E. coli strains were grown in lysogeny broth (LB) or on LB agar at 37 °C. The seed medium and the fermentation medium were used for the fermentation of the Streptomyces strains as previously described [41].
2.2. Protein expression and purification
To express the protein TylF and its variants, the DNA fragment encoding the tylF gene was cloned into pET28a to obtain recombinant plasmids. The resulting recombinant plasmids were used to transform E. coil BL21 (DE3) cells. Protein expression was then induced in the presence of IPTG, and purified in soluble form as an N-His6-tagged recombinant proteins. All purification steps were performed as previously described [40].
2.3. Measurement of TylF activity
TylF or its variant protein was added to 1 ml of the reaction buffer containing 50 mM Tris-HCl, 10 mM MgCl2, 0.4 mM SAM, 0.2 mM macrocin, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 6 mM 2-mercaptoethanol at pH 7.6 with/or without 0.2 mM SAH. The mixture was incubated at 30 °C for 1 h followed by incubation at 100 ℃ for 10 min to terminate the reaction. Then, the supernatant was collected for high-performance liquid chromatography (HPLC). Each set of experiments was repeated three times. SAH inhibition rate (%) was calculated according to the following formula, in which m stands for mass (μg/ml):
2.4. Reverse-phase HPLC (RP-HPLC)
To quantitatively determine tylosin production, the fermentation supernatant was harvested by centrifugation and the tylosin was quantified by RP-HPLC (Agilent 1260, Agilent Technologies, Palo Alto, CA, USA) using a C18 column (ODS-3, 4.6 × 250 mm, Φ 5 µm) as previously described [42]. The sample was eluted at a thermostatic temperature of 30 °C with 2 M sodium perchlorate (pH 2.5) and acetonitrile (60: 40, v/v). The tylosin and other intermediates UV absorbances were recorded at 280 nm.
2.5. Bioinformatics analysis, structural modelling, and molecular docking
The amino acid sequences of 3′-O-methyltransferase were aligned using MEGA 6 and were presented by the ESPript 3.0 server [43]. I-TASSER server (http://zhanglab.ccmb.med.umich.edu/I-TASSER) was used to predict the 3D structures of TylF and its variants [44], [45]. Molecular docking was performed using Autodock 4.2.6 software [46]. PyMOL software (PyMOL Molecular Graphics System, Version 2.5.7, Schrödinger, LLC.) was used for the visualization of protein-ligand interaction. The volumes of the substrate-binding pockets of TylF and its variants were calculated with the ProteinsPlus tool (https://proteins.plus/) [47].
2.6. The thermal stability analysis by nanoscale differential scanning fluorimetry (NanoDSF)
The thermal stability of TylF and its variant proteins was measured using NanoDSF. A mixture containing 8 μM protein in buffer containing 50 mM Tris-HCl, 10 mM MgCl2, 1 mM PMSF, and 6 mM 2-mercaptoethanol at pH 7.6 (or at different pH) with 0.4 mM SAM or/and 0.2 mM macrocin. The samples were loaded into standard grade glass capillary tubes (NanoTemper Technologies) and analyzed using the Prometheus NT.48 NanoDSF device (NanoTemper Technologies), which monitors the shift of intrinsic tryptophan fluorescence of proteins upon unfolding by detecting the fluorescence at emission wavelengths of 330 and 350 nm. The temperature was increased linearly from 20 °C to 65 °C at a rate of 1 °C/min with an excitation power of 20 %. The fluorescence intensity ratio and its first derivative were calculated with the manufacturer’s software (PR.ThermControl, version 2.1.2). All melting temperatures (Tm) are the mean of triplicates.
2.7. The binding affinity analysis of SAM, SAH and macrocin by NanoDSF
The thermal stability of TylF and its variant proteins was measured using NanoDSF. A mixture containing 8 μM protein in buffer containing 50 mM Tris-HCl, 10 mM MgCl2, 1 mM PMSF, and 6 mM 2-mercaptoethanol at pH 7.6 (or at different pH) with 0.4 mM SAM or/and 0.2 mM macrocin. Program followed described above. The binding affinity of ligands was evaluated by comparing the Tm of protein in the absence or presence of the compounds and calculating the shift in the Tm (ΔTm). All Tm are the mean of triplicates.
2.8. Construction of tylosin engineering strains
The sequences of the primers used in this study are listed in Table S2. To obtain the recombinant plasmids (pSET152::PtylF-tylF, pSET152::PtylF-tylFY139F, pSET152::PtylF-tylFT36S, V54A, pSET152::PtylF-tylFT36S, V54A, Y139F), the promoter and target module were inserted into the pSET152 vector through double enzymatic digestion and ligation, and the resulting plasmids were verified by DNA sequencing. The plasmids were introduced into S. fradiae SF-3 by conjugation transfer. To determine tylosin production, the strains were first cultured in conical flasks containing 50 ml seed medium at 30 °C with rotary shaking at 220 rpm for 2 days, and then the mycelium was sub-cultured in 30 ml of fermentation medium for another 6 days under the same conditions, then increased the temperature from 30 °C to 32 °C/38 °C or not change for 24 h. The samples after fermentation were used for analyzing the production of the tylosin components of the recombinant strains by HPLC.
3. Results
3.1. Purification of recombinant TylF and its variants
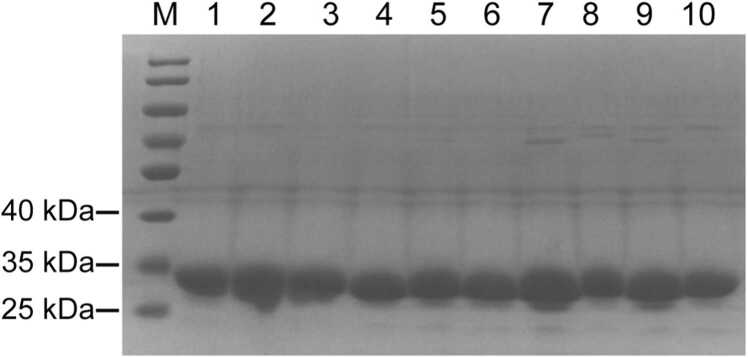
We have previously identified several mutations in TylF from a random mutagenesis library that can increase the yield of tylosin [40]. One of the variants, TylFY139F, was shown to be able to improve the enzyme activity and heat resistance of TylF [40]. To further characterize the effect of the other mutations on the TylF activity and tylosin production, all the nine TylF variants as well as the wild type TylF were expressed in E. coli and purified. It was shown that all variants exhibited a molecular weight similar to that of wild-type TylF, which was approximately 29 kDa, implying successful expression and purification (Fig. 1).
Fig. 1.
SDS-PAGE analysis of TylF and its variants. Lane 1–10 represents the purified recombinant TylF, TylFY25H, G256D, TylFQ138H, F232Y, TylFK62E, TylFY139F, TylFT36S, V54A, TylFL123P, N165S, V182G, TylFA197T and TylFV54D, respectively.
3.2. Enzymatic characterization of TylF and its variants
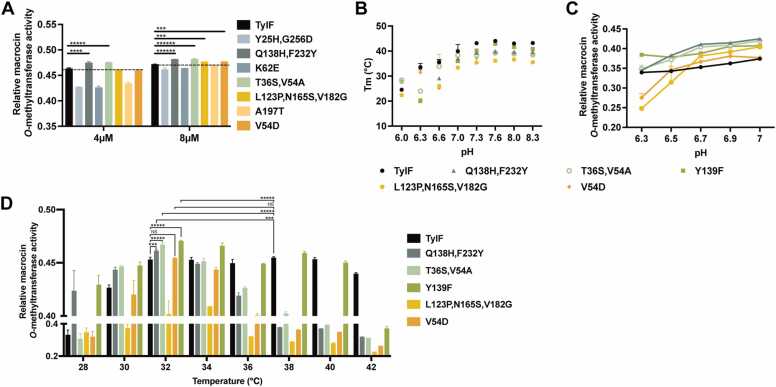
To investigate whether the mutations in the TylF variants influence its enzymatic activity, catalytic activity of the proteins were measured. As shown in Fig. 2A, four variants, TylFQ138H, F232Y, TylFT36S, V54A, TylFL123P, N165S, V182G, and TylFV54D, exhibited a significantly higher catalytic activity than that of the wild-type TylF. Due to reduced intracellular pH by fatty acid accumulation at the late fermentation, it is necessary to increase the response of TylF to acid stress. For this, we analyzed the thermal stability of TylF and the five variants using NanoDSF in buffers with different pH. As shown in Fig. 2B, the proteins were stable in buffers between pH 7.3 and pH 8.3 with the optimal stability at pH 7.6, but the stability decreased drastically when the pH was below 7.0 (the raw thermograms and first derivative were provided in Fig. S1). The Tm of TylFQ138H, F232Y and TylFV54D could not be detected at pH 6.0 (Fig. 2B). Thus, we measured catalytic activity of these proteins in pH 6.3 to pH 7.0. It was shown that TylFQ138H, F232Y, TylFT36S, V54A, and TylFY139F showed higher catalytic activity than TylF, especially under weak acid condition (pH 6.3–6.7) (Fig. 2C). During the late fermentation phase of tylosin fermentation, incubation at a higher temperature was usually conducted to promote the conversion of macrocin to tylosin A. Therefore, we measured the enzyme activity of TylF and its variants at different temperatures. TylF reached the optimal activity at 32–40 °C, with the highest activity at 38 °C (Fig. 2D). In contrast, its variants reached the optimal activity at 28–34 °C, peaking at 32 °C (Fig. 2D). Moreover, the enzyme activity of TylFQ138H, F232Y, TylFT36S, V54A, and TylFY139F at 32 °C was significantly higher than that of wild-type TylF at 32 °C, and even higher than that of TylF at 38 °C (Fig. 2C). These results suggest that TylFQ138H, F232Y, TylFT36S, V54A, and TylFY139F were the candidate targets for the further study.
Fig. 2.
Relative macrocinO-methyltransferase activity analysis of TylF and its variants TylFY25H, G256D, TylFQ138H, F232Y, TylFK62E, TylFY139F, TylFT36S, V54A, TylFL123P, N165S, V182G, TylFA197Tand TylFV54D. Enzyme activity assays were performed with different concentration of enzymes (A) or at different pH (C) and temperature (D). The reaction products were analyzed by HPLC. Statistical significances were determined by using the two-tailed student’s t-test. *** indicates p < 0.001, **** indicates p < 0.0001, ***** indicates p < 0.00001, ****** indicates p < 0.000001, and NS indicates no significance (the same hereinafter). (B) The NanoDSF analysis of the thermal stability of enzyme in different pH environments.
3.3. Structure analysis of TylF and its variants
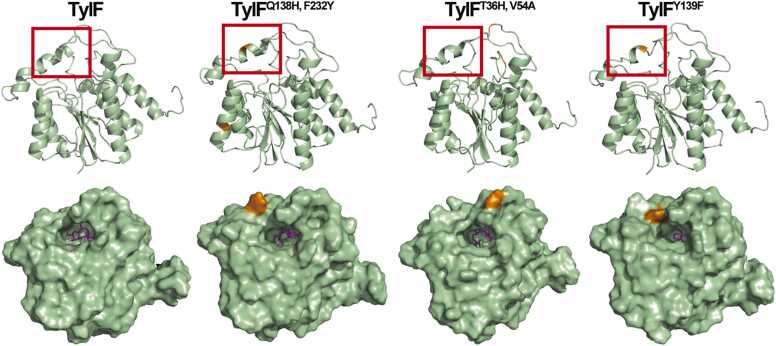
To investigate how these mutations influence the activity of TylF, the structures of TylF as well as the variant proteins were predicted using I-TASSER and visualized using PyMOL as shown in Fig. 3. By comparing the structures, it was shown in the red box (residues 136–143) that the α-helix at the top of TylFQ138H, F232Y, TylFT36S, V54A and TylFY139F was shorter than that of TylF (Fig. 3), which led to remarkably enlarged entrance of the cavity compared to TylF. Moreover, the binding pocket volume of TylFQ138H, F232Y, TylFT36S, V54A and TylFY139F was larger than that of TylF (Table 1). Therefore, these mutation sites enlarged the entrance and binding pocket volume, which may result in the improved catalytic activity.
Fig. 3.
Stereoviews showing the overall structure of TylF, TylFQ138H, F232Y, TylFT36S, V54Aand TylFY139F. Upper panel: Cartoon representation. Different regions between TylFQ138H, F232Y, TylFT36S, V54A, TylFY139F and TylF were shown in red box. Lower panel: Molecular surface representation. The SAM ligand is purple. Throughout, the mutation site is colored orange.
Table 1.
Calculated substrate-binding pocket surface and volume of TylF and its variants.
| Protein | Surface | Volume |
|---|---|---|
| TylF | 254.11 Ų | 121.34 ų |
| TylFQ138H, F232Y | 296.77 Ų | 212.99 ų |
| TylFT36S, V54A | 253.25 Ų | 161.79 ų |
| TylFY139F | 335.02 Ų | 236.54 ų |
3.4. Binding affinity of TylF and its variants to macrocin, SAM and SAH
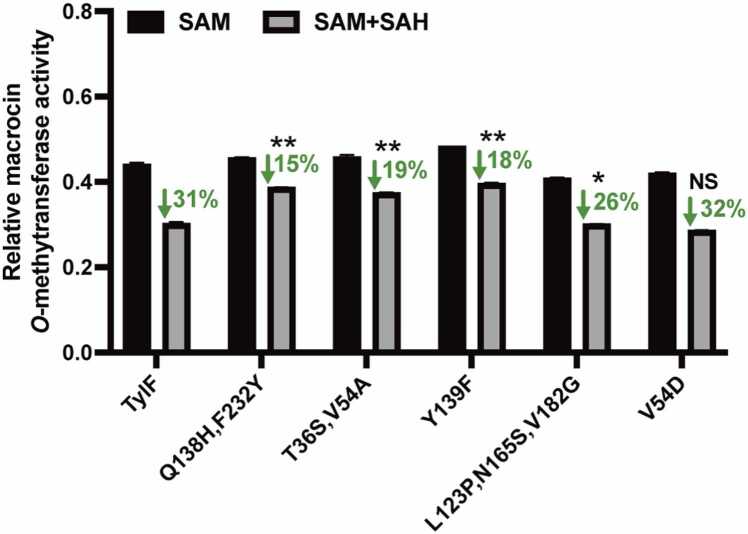
In order to further investigate whether the changes in the protein structures of TylFQ138H, F232Y, TylFT36S, V54A, and TylFY139F influence their binding to the substrates and products, the binding affinity of the proteins with SAM, SAH and tylosin C were analyzed by NanoDSF (the raw thermograms and first derivative see in Fig. S2). The NanoDSF results demonstrated that the affinity to the substrates (SAM and tylosin C) of TylFT36S, V54A and TylFY139F, but not TylFQ138H, F232Y, was higher than that of TylF (Table 2 and Fig S2). However, reduced binding to SAH was observed in varying degrees for TylFQ138H, F232Y, TylFT36S, V54A, and TylFY139F compared to the wild-type protein (Table 2 and Fig S2). After adding SAH to the enzyme activity reaction, it was found that the inhibition of SAH to the catalytic activities of TylFQ138H, F232Y, TylFT36S, V54A, and TylFY139F was significantly lower than that of the wild-type TylF (Fig. 4). It follows that these mutation sites reduced the inhibitory effect of product SAH, or/and increased the affinity of TylF to the substrates SAM and tylosin C.
Table 2.
Binding affinity of TylF and its variants to macrocin, SAM and SAH by NanoDSF.
| TylF | TylFQ138H, F232Y | TylFT36S, V54A | TylFY139F | TylFL123P, N165S, V182G | TylFV54D | |
|---|---|---|---|---|---|---|
| TmB (°C) | 38.358 | 36.74 | 29.609 | 35.071 | 37.549 | 34.616 |
| TmB+m (°C) | 41.493 | 38.799 | 37.072 | 36.022 | 37.862 | 39.297 |
| TmB+m+SAM | 46.774 | 42.903 | 43.118 | 44.978 | 39.642 | 42.419 |
| TmB+m+SAH (°C) | 46.778 | 44.057 | 41.909 | 41.05 | 45.453 | 45.09 |
| ΔTmm (°C) | 3.135 | 2.059 | 7.463 | 4.681 | 0.951 | 0.313 |
| ΔTmSAM (°C) | 5.281 | 4.104 | 6.046 | 5.681 | 3.62 | 4.557 |
| ΔTmSAH (°C) | 5.285 | 5.258 | 4.837 | 1.753 | 9.431 | 7.228 |
B, buffer; m, macrocin; TmB was calculated in the buffer system and so on, the average of the three duplications on show; ΔTm was calculated by the following formula: ΔTmm = TmB+m -TmB; ΔTmSAM = TmB+m+SAM - TmB+m; ΔTmSAH = TmB+m+SAH -TmB+m.
Fig. 4.
Enzyme activity assay to measure the inhibition of SAH to TylF, TylFQ138H, F232Y, TylFT36S, V54A, TylFY139F, TylFL123P, N165S, V182Gand TylFV54D.
3.5. Structure analysis of combined mutant TylFT36S, V54A, Y139F
To maximize the catalytic activity of TylF, we combined the three mutations (T36S, V54A, and Y139F) that we had identified as being optimal for catalytic activity, environment-adapted, and higher substrate affinity (Table 3). The structure of the combinatorial mutant TylFT36S, V54A, Y139F was predicted. As shown in Fig. 5A & 5B, T36S, V54A, and Y139F mutations resulted in the conversion of the grey-marked α-helix (residues 173–179) in the wild-type TylF to a random coil. Compare with TylF, the surface and volume of substrate-binding pocket (yellow) were enlarged (Fig. 5C & 5D). Thus, TylFT36S, V54A, Y139F might be a better target for improving the C/A conversion rate and therefore being a superior variant that can be further used for S. fradiae bioengineering for tylosin production.
Table 3.
Enzymatic properties of TylFQ138H, F232Y, TylFT36S, V54A and TylFY139F.
| TylFQ138H, F232Y | TylFT36S, V54A | TylFY139F | |
|---|---|---|---|
| Enzyme activity | + | + | + |
| Acid-resistant | + | + | + |
| High temperature-resistant | - | - | + |
| The surface and volume of Binding pocket | + | + | + |
| Binding affinity of macrocin | - | + | + |
| Binding affinity of SAM | - | + | + |
| Binding affinity of SAH | - | - | - |
| Inhibition of SAH | - | - | - |
Fig. 5.
Overall structure of TylF and TylFT36S, V54A, Y139F. The overall structure of TylF (A) and TylFT36S, V54A, Y139F (B) in cartoon representation, and their different region was shown in grey. The overall structure of TylF (C) and TylFT36S, V54A, Y139F (D) in molecular surface representation.
3.6. Tylosin production of TylF variant strains
In order to further verify the impact of TylFT36S, V54A, TylFY139F, and TylFT36S, V54A, Y139F on the production of tylosin A and its derivatives in S. fradiae, tylF and the variants were overexpressed in S. fradiae SF-3 strain. The fermentation products were analyzed by HPLC to quantitatively determine the production of tylosin A and tylosin C. As shown in Fig. 6, the yield of tylosin A reached its maximum at 38 °C during the last 12 h of fermentation in SF-3 strain, while 30 °C or 32 °C was already enough for the variant strains SF-3::PtylF-tylFT36S, V54A (30 °C), SF-3::PtylF-tylFY139F (30 °C) and SF-3::PtylF-tylFT36S,V54A,Y139F (32 °C) (Fig. 6A). Notably, to some extent, the engineered strains containing the mutations eliminated the side effects of overexpression of the wild-type TylF protein on tylosin A production (Fig. 6A). All engineered strains had lower production of the by-product tylsoin C and higher C/A conversion efficiency (Fig. 6B & 6C). Therefore, these results suggest that SF-3::PtylF-tylFT36S, V54A, SF-3::PtylF-tylFY139F and SF-3::PtylF-tylFT36S,V54A,Y139F had a lower optimal catalytic temperature (30–32 °C) and higher C/A conversion efficiency.
Fig. 6.
HPLC analysis of tylosin production of SF-3 and engineering strains at different temperatures at the later stages of fermentation. (A) HPLC analysis of tylosin A production in SF-3 and engineering strains. (B) HPLC analysis of tylosin C production in SF-3 and engineering strains. (C) The conversion of tylosin C to tylosin A of SF-3 and engineering strains.
3.7. Enzymatic characterization of TylFT36S, V54A, Y139F
To further characterize the combinatory variant TylFT36S, V54A, Y139F, the protein was purified TylFT36S, V54A, Y139F and its catalytic activity was measured. It was shown that with the acidity and temperature increased, the catalytic activity of TylFT36S, V54A, Y139F gradually decreased compared with TylF (Fig. 7A & 7B). NanoDSF assay revealed that the thermal stability of TylFT36S, V54A, Y139 decreased more drastically than that of TylF between pH 6.3 to pH 7.0 (Fig. 7C & Fig. S3). The denaturation temperature of TylFT36S, V54A, Y139F was at least 3.7 °C lower than that of TylF, after adding alternative substrates to the same buffer system (Fig. 7D). Further protein thermostability analysis results showed that the denaturation temperature of the mutant protein was lower by at least about 2 °C than that of TylF (Fig. 7D, Table 2 and Table 4), indicating that these mutations reduced the protein thermostability of TylF. These results suggest that combining mutations (T36S, V54A, and Y139F) might amplify this side-effect, thereby reducing the catalytic activity of TylFT36S, V54A, Y139F.
Fig. 7.
Relative macrocinO-methyltransferase activity of TylF and TylFT36S, V54A, Y139F. Enzyme activity assays were performed at different pH (A) and temperature (B). Measured stability of TylF, TylFQ138H, F232Y, TylFT36S, V54A, TylFY139F and TylFT36S, V54A, Y139F in different pH environments (C) and the presence of different substrates (D) by NanoDSF.
Table 4.
Thermal stability of TylF and its variants by NanoDSF.
| ΔTmB+m+SAM | |
|---|---|
| TylF | - |
| TylFQ138H, F232Y | − 3.871 |
| TylFT36S, V54A | − 3.656 |
| TylFY139F | − 1.796 |
| TylFL123P, N165S, V182G | − 7.132 |
| TylFV54D | − 4.355 |
| TylFT36S, V54A, Y139F | − 4.404 |
4. Discussion
In the biosynthetic pathways of natural products, insufficient amounts or ineffective activity of certain rate-limiting enzymes often leads to the accumulation of nonspecific intermediates. During the biosynthesis of tylosin by S. fradiae, conversion of tylosin C to tylosin A (C/A conversion) is a rate-limiting step and incomplete C/A conversion results in an increase of the by-products tylosin C and D, which is adverse to product quality [27]. The conversion is catalyzed by the O-methyltransferase TylF. Therefore, increasing the activity of TylF is of great importance for tylosin production. Three primary directions of TylF modification are to: 1) preserve or promote the methyl transfer activity of TylF during the regular fermentation process; 2) improve the catalytic activity of TylF at low temperatures; and 3) increase various environment-adapted ability of TylF. To this end, we have previously identified nine TylF mutation that could improve tylosin A production by TylF mutagenesis study [40]. In this study, eight of the TylF mutations were further characterized, and our results suggest that three TylF variants were shown to exhibit a higher enzyme activity at 32 °C and be acid-resistant in vitro. Overexpressing two of them in S. fradiae was able to perform efficient C/A conversion at 30 °C without heating up to 38 °C during the last 24 h of fermentation. We have further provided insights underlying these phenotypes of these mutations from the prospective of protein structures. Our findings provide valuable guidance for bioengineering of S. fradiae to optimize tylosin industrial production.
The influence of the enzymatic reaction rate is mainly limited by the concentration of enzyme under the greater proportional of the substrate concentration [48], [49]. The kinetic characterization of another TylF superfamily protein NovP reveals its low catalytic ability to 4′-O-methylation of desmethyldescarbamoylnovobiocin in vitro with Kcat = 0.400 ± 0.006 min−1 (Kcat, catalytic constant) and KM = 9.5 ± 3 μM (KM, Michaelis constant) [50]. Therefore, within a certain reaction time, an enzyme concentration of 8 µM exhibited a higher catalytic performance than 4 µM. As for the activity of enzyme, it is closely related to its protein structure, which is particularly evident in its substrate affinity, product inhibition, substrate/product exchange efficiency and other characteristics. In some TylF homologous protein-related studies, the substrate-binding pocket was suggested to consist of an N-terminal 'lid loop' domain (residues 27–53) and α-helical 'lid domain' (residues 118–146) [51], [52], [53]. This is consistent with our Autodock-4 molecular docking results. In this study, we found five superior mutations surround the substrate-binding pocket of TylF enzyme, that could affect the entrance and volume of the pocket. Among them, the mutation V54A located close to the T36S that was situated in the 'lid loop' domain, the Q138H and Y139F mutations located in the α-helical 'lid domain' while the F232Y was in strictly conserved residue. Following amino acid substitution at these locations (except residues 232), the steric hindrance of substrate-binding pocket was reduced, and there were differences in hydrophobic interaction (Fig. 3 & 5). The larger pocket facilitates substrate binding to the active site [54]. It is consistent with the affinity of macrocin and SAM results obtained for the TylF variants by NanoDSF assay (Fig. 4 and Table 3). In addition, the amino acid substitutions also led to the enzyme binding preference for SAM rather than SAH, reducing the inhibition of SAH and thus improving its enzyme activity. Thus, beneficial mutation sites might affect enzymatic characterization by altering the hydrophobic environment and binding pocket volume.
Based on the above positive results, combined targeted mutagenesis was the next rational step for further improving the catalyze activity. Therefore, we constructed three modified strains harboring the variant TylFT36S, V54A, Y139F with combined mutations tested above, and analyzed the yield of tylosin A and C. Surprisingly, additive or synergistic effects were not obtained from this strategy. Most helical structures affect enzyme stability and activity [55]. The disappearance of the α-helix at residues 173–179 in the three-dimensional structure of TylFT36S, V54A, Y139F might be the main reason for the sharp decrease in its protein stability. Enzyme stability and activity are usually negatively correlated [56]. Wang et al. improved the Tm of Streptomyces sp. strain S9 GH10 xylanase by 7.0 °C but lowered the catalytic activity by 75 % [57]. In addition, we also observed that the optimal temperature of TylFT36S, V54A and TylFY139F was 32 °C in vitro but 30 °C in Streptomyces, in contrast to TylFT36S, V54A, Y139F. During fermentation, the substrate is synthesized, consumed, and regenerated, while the product is synthesized and gradually accumulates (although part of the product is exocytosed outside the cell). Because of the substrate's polarity, changing the molar ratio has a direct effect on the enzyme's activity and mass transfer [58]. The substrate molar ratio is another key factor driving enzyme activity [59]. Trimethylation of MTs wild type NRMT1variants NRMT1N209I and NRMT1P211S commences with a 1:1 substrate molar ratio but does not reach wild type levels [60]. Therefore, multiple factors mediate influence on enzyme activity.
The fermentation products of tylsoin have four main components, in which tylosin A is the primary one and the presence of the other components negatively affects quality of the end product of tylsoin. The purity of tylosin A was 95.2 % for the original strain SF-3, and in our study greater than 98 % of tylosin A purity was achieved in the modified strains SF-3::PtylF-tylFT36S, V54A, SF-3::PtylF-tylFY139F and SF-3::PtylF-tylFT36S, V54A, Y139F. This significantly increased the quality of the end product. Moreover, energy cost is also an important factor that needs to be taken into consideration for tylosin production. For normal tylosin fermentation, temperature must be increased from 30 °C up to 38 °C during the last 24 h of fermentation to increase the C/A conversion. In our study, it was shown that SF-3::PtylF-tylFT36S, V54A and SF-3::PtylF-tylFY139F could achieve efficient C/A conversion at 30 °C without heating up, which as a consequence significantly decreased the energy cost for industrial production of tylosin.
5. Conclusions
In this work, we generated and further characterized the enzymatic properties of four TylF variants that can improve the yield of tylosin. Three of them TylFQ138H, F232Y, TylFT36S, V54A and TylFY139F have a higher C/A conversion rate at 32 °C. The modified S. fradiae strains SF-3::PtylF-tylFT36S, V54A and SF-3::PtylF-tylFY139F could achieve a much higher C/A conversion at 30 °C without heating up to 38 °C during the last 24 h of fermentation. This is the first study that report and characterize the mutations in the rate-limiting enzyme TylF which provides valuable guidance for productivity improvement and energy conservation in tylosin industry.
CRediT authorship contribution statement
Yujun Tao: Methodology, Investigation, Formal analysis. Chaoyue Yan: Writing – original draft, Methodology, Investigation, Formal analysis. Jun Dai: Resources. Jingyan Fan: Methodology, Investigation. Shuo Li: Resources. Rui Zhou: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Data curation, Conceptualization. Qi Huang: Writing – review & editing, Resources, Project administration, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Hubei Provincial Bioengineering Technology Research Center for Animal Health Products for their technical support. This work was funded by the National Key R & D Plans of China (No. 2021YFD1800401 to R. Z.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.csbj.2024.08.020.
Contributor Information
Qi Huang, Email: qhuang@mail.hzau.edu.cn.
Rui Zhou, Email: rzhou@mail.hzau.edu.cn.
Appendix A. Supplementary material
Supplementary material
References
- 1.Cantoni G.L. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- 2.Richter M. Functional diversity of organic molecule enzyme cofactors. Nat Prod Rep. 2013;30(10):1324–1345. doi: 10.1039/c3np70045c. Oct 11. [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Wang Z., Cai H., Zhou C. Progress in the microbial production of S-adenosyl-L-methionine. World J Microbiol Biotechnol. 2016;32(9):153. doi: 10.1007/s11274-016-2102-8. [DOI] [PubMed] [Google Scholar]
- 4.Zubieta C., Ross J.R., Koscheski P., Yang Y., Pichersky E., Noel J.P. Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell. 2003;15(8):1704–1716. doi: 10.1105/tpc.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C.C., Smith C.V., Glickman M.S., Jacobs W.R., Jr, Sacchettini J.C. Crystal structures of mycolic acid cyclopropane synthases from Mycobacterium tuberculosis. J Biol Chem. 2002;277(13):11559–11569. doi: 10.1074/jbc.M111698200. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Z., Sattler S., Maeda H., Sakuragi Y., Bryant D.A., DellaPenna D. Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell. 2003;15(10):2343–2356. doi: 10.1105/tpc.013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol. 2010;6(12):863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 8.Arrowsmith C.H., Bountra C., Fish P.V., Lee K., Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 9.Qazi T.J., Quan Z., Mir A., Qing H. Epigenetics in Alzheimer's disease: perspective of DNA methylation. Mol Neurobiol. 2018;55(2):1026–1044. doi: 10.1007/s12035-016-0357-6. [DOI] [PubMed] [Google Scholar]
- 10.Stopa N., Krebs J.E., Shechter D. The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci. 2015;72(11):2041–2059. doi: 10.1007/s00018-015-1847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L., Ning J., Tian X., Wang C., Zhang L., Ma X., et al. Fluorescent probes for bioactive detection and imaging of phase II metabolic enzymes. Coord Chem Rev. 2019;399:213026.1–213026.15. [Google Scholar]
- 12.Law B.J.C., Struck A.W., Bennett M.R., Wilkinson B., Micklefield J. Site-specific bioalkylation of rapamycin by the RapM 16-O-methyltransferase. Chem Sci. 2015;6(5):2885–2892. doi: 10.1039/c5sc00164a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter J.M., Chiou G., Bothwell I.R., Xu W., Garg N.K., Luo M., et al. Expanding the structural diversity of polyketides by exploring the cofactor tolerance of an inline methyltransferase domain. Org Lett. 2013;15(14):3774–3777. doi: 10.1021/ol401723h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S., Zhang J., Huber T.D., Sunkara M., Hurley K., Goff R.D., et al. Facile chemoenzymatic strategies for the synthesis and utilization of S-adenosyl-(l)-methionine analogues. Angew Chem Int Ed Engl. 2014;53(15):3965–3969. doi: 10.1002/anie.201308272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelraheem E., Thair B., Varela R.F., Jockmann E., Popadić D., Hailes H.C., et al. Methyltransferases: functions and applications. Chembiochem. 2022;23(18) doi: 10.1002/cbic.202200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wlodarski T., Kutner J., Towpik J., Knizewski L., Rychlewski L., Kudlicki A., et al. Comprehensive structural and substrate specificity classification of the Saccharomyces cerevisiae methyltransferome. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De La Haba G., Cantoni G.L. The enzymatic synthesis of S-adenosyl-l-homocysteine from adenosine and homocysteine. J Biol Chem. 1959;234(3):603–608. [PubMed] [Google Scholar]
- 18.Nelson J.T., Lee J., Sims J.W., Schmidt E.W. Characterization of SafC, a catechol 4-O-methyltransferase involved in saframycin biosynthesis. Appl Environ Microbiol. 2007;73(11):3575–3580. doi: 10.1128/AEM.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constable D.J.C., Dunn P.J., Hayler J.D., Humphrey G.R., Leazer J.L., Linderman R.J., et al. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 2007;9(5):411–420. [Google Scholar]
- 20.Malla S., Koffas M.A., Kazlauskas R.J., Kim B.G. Production of 7-O-methyl aromadendrin, a medicinally valuable flavonoid, in Escherichia coli. Appl Environ Microbiol. 2012;78(3):684–694. doi: 10.1128/AEM.06274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaaban M.R., Mayhoub A.S., Farag A.M. Recent advances in the therapeutic applications of pyrazolines. Expert Opin Ther Pat. 2012;22(3):253–291. doi: 10.1517/13543776.2012.667403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett M.R., Shepherd S.A., Cronin V.A., Micklefield J. Recent advances in methyltransferase biocatalysis. Curr Opin Chem Biol. 2017;37:97–106. doi: 10.1016/j.cbpa.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Teeter J.S., Meyerhoff R.D. Aerobic degradation of tylosin in cattle, chicken, and swine excreta. Environ Res. 2003;93(1):45–51. doi: 10.1016/s0013-9351(02)00086-5. [DOI] [PubMed] [Google Scholar]
- 24.Loke M.L., Ingerslev F., Halling-Sørensen B., Tjørnelund J. Stability of Tylosin A in manure containing test systems determined by high performance liquid chromatography. Chemosphere. 2000;40(7):759–765. doi: 10.1016/s0045-6535(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 25.Seno E.T., Pieper R.L., Huber F.M. Terminal stages in the biosynthesis of tylosin. Antimicrob Agents Chemother. 1977;11(3):455–461. doi: 10.1128/aac.11.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltz R.H., Seno E.T., Stonesifer J., Wild G.M. Biosynthesis of the macrolide antibiotic tylosin. A preferred pathway from tylactone to tylosin. J Antibiot. 1983;36(2):131–141. doi: 10.7164/antibiotics.36.131. [DOI] [PubMed] [Google Scholar]
- 27.Seno E.T., Baltz R.H. S-Adenosyl-L-methionine: macrocin O-methyltransferase activities in a series of Streptomyces fradiae mutants that produce different levels of the macrolide antibiotic tylosin. Antimicrob Agents Chemother. 1982;21(5):758–763. doi: 10.1128/aac.21.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seno E.T., Baltz R.H. Properties of S-adenosyl-L-methionine:macrocin O-methyltransferase in extracts of Streptomyces fradiae strains which produce normal or elevated levels of tylosin and in mutants blocked in specific O-methylations. Antimicrob Agents Chemother. 1981;20(3):370–377. doi: 10.1128/aac.20.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer N.J., Kreuzman A.J., Dotzlaf J.E., Yeh W.K. Purification, characterization, and kinetic mechanism of S-adenosyl-L-methionine:macrocin O-methyltransferase from Streptomyces fradiae. J Biol Chem. 1988;263(30):15619–15625. [PubMed] [Google Scholar]
- 30.Choi J.M., Han S.S., Kim H.S. Industrial applications of enzyme biocatalysis: current status and future aspects. Biotechnol Adv. 2015;33(7):1443–1454. doi: 10.1016/j.biotechadv.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A., Shvetsov A., Soboleva E., Kil Y., Sergeev V., Surzhik M. Thermostability improvement of Aspergillus awamori glucoamylase via directed evolution of its gene located on episomal expression vector in Pichia pastoris cells. Protein Eng Des Sel. 2019;32(6):251–259. doi: 10.1093/protein/gzz048. [DOI] [PubMed] [Google Scholar]
- 32.Sharma A., Gupta G., Ahmad T., Mansoor S., Kaur B. Enzyme engineering: current trends and future perspectives. Food Rev Int. 2019;37(2):121–154. [Google Scholar]
- 33.Lim S.J., Oslan S.N. Native to designed: microbial-amylases for industrial applications. PeerJ. 2021;9 doi: 10.7717/peerj.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong L., Zheng J., Wang X., Wang X., Huang H., Yang H., et al. Improvement of thermostability and catalytic efficiency of glucoamylase from Talaromyces leycettanus JCM12802 via site-directed mutagenesis to enhance industrial saccharification applications. Biotechnol Biofuels. 2021;14(1):202. doi: 10.1186/s13068-021-02052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng F., Zhu L., Schwaneberg U. Directed evolution 2.0: improving and deciphering enzyme properties. Chem Commun. 2015;51(48):9760–9772. doi: 10.1039/c5cc01594d. [DOI] [PubMed] [Google Scholar]
- 36.Li S., Chen D., Liu Z., Tao S., Zhang T., Chen Y., et al. Directed evolution of TetR for constructing sensitive and broad-spectrum tetracycline antibiotics whole-cell biosensor. J Hazard Mater. 2023;460 doi: 10.1016/j.jhazmat.2023.132311. [DOI] [PubMed] [Google Scholar]
- 37.Yu H., Ma S., Li Y., Dalby P.A. Hot spots-making directed evolution easier. Biotechnol Adv. 2022;56 doi: 10.1016/j.biotechadv.2022.107926. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L., Baumann U., Reymond J.L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004;32(14) doi: 10.1093/nar/gnh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu G., Li A., Acevedo-Rocha C.G., Sun Z., Reetz M.T. The crucial role of methodology development in directed evolution of selective enzymes. Angew Chem Int Ed Engl. 2020;59(32):13204–13231. doi: 10.1002/anie.201901491. [DOI] [PubMed] [Google Scholar]
- 40.Fan J., Yao Z., Yan C., Hao M., Dai J., Zou W., et al. Discovery of a highly efficient TylF methyltransferase via random mutagenesis for improving tylosin production. Comput Struct Biotechnol J. 2023;21:2759–2766. doi: 10.1016/j.csbj.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Z., Fan J., Dai J., Yu C., Zeng H., Li Q., et al. A high-throughput method based on microculture technology for screening of high-yield strains of tylosin-producing Streptomyces fradiae. J Microbiol Biotechnol. 2023;33(6):831–839. doi: 10.4014/jmb.2210.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamidian K., Amini M., Samadi N. Consistency evaluation between matrix components ratio and microbiological potency of tylosin major components. Daru. 2018;26(2):155–164. doi: 10.1007/s40199-018-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert X., Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42(Web Server issue):W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat Methods. 2015;12(1):7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy A., Kucukural A., Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5(4):725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen J.B., Felby C., Jørgensen H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol Biofuels. 2009;2(1):11. doi: 10.1186/1754-6834-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta R., Kumar S., Gomes J., Kuhad R.C. Kinetic study of batch and fed-batch enzymatic saccharification of pretreated substrate and subsequent fermentation to ethanol. Biotechnol Biofuels. 2012;5:16. doi: 10.1186/1754-6834-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freel Meyers C.L., Oberthür M., Xu H., Heide L., Kahne D., Walsh C.T. Characterization of NovP and NovN: completion of novobiocin biosynthesis by sequential tailoring of the noviosyl ring. Angew Chem Int Ed Engl. 2004;43(1):67–70. doi: 10.1002/anie.200352626. [DOI] [PubMed] [Google Scholar]
- 51.Bernard S.M., Akey D.L., Tripathi A., Park S.R., Konwerski J.R., Anzai Y., et al. Structural basis of substrate specificity and regiochemistry in the MycF/TylF family of sugar O-methyltransferases. ACS Chem Biol. 2015;10(5):1340–1351. doi: 10.1021/cb5009348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin J.L., McMillan F.M. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr Opin Struct Biol. 2002;12(6):783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 53.Gómez García I., Stevenson C.E., Usón I., Freel Meyers C.L., Walsh C.T., Lawson D.M. The crystal structure of the novobiocin biosynthetic enzyme NovP: the first representative structure for the TylF O-methyltransferase superfamily. J Mol Biol. 2010;395(2):390–407. doi: 10.1016/j.jmb.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J., Li Y., Wang J., Yu Z., Liu Y., Tong Y., et al. Adaptive steered molecular dynamics combined with protein structure networks revealing the mechanism of Y68I/G109P mutations that enhance the catalytic activity of d-psicose 3-epimerase from Clostridium bolteae. Front Chem. 2018;6:437. doi: 10.3389/fchem.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang X., Carey J. Role of heme in structural organization of cytochrome c probed by semisynthesis. Biochemistry. 1999;38(48):15944–15951. doi: 10.1021/bi9919089. [DOI] [PubMed] [Google Scholar]
- 56.Siddiqui K.S. Some like it hot, some like it cold: temperature dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnol Adv. 2015;33(8):1912–1922. doi: 10.1016/j.biotechadv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Wang K., Luo H., Tian J., Turunen O., Huang H., Shi P., et al. Thermostability improvement of a streptomyces xylanase by introducing proline and glutamic acid residues. Appl Environ Microbiol. 2014;80(7):2158–2165. doi: 10.1128/AEM.03458-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szczęsna Antczak M., Kubiak A,Antczak T., Bielecki S. Enzymatic biodiesel synthesis – key factors affecting efficiency of the process. Renew Energy. 2008;34(5):1185–1194. [Google Scholar]
- 59.Liu W., Luo X., Tao Y., Huang Y., Zhao M., Yu J., et al. Ultrasound enhanced butyric acid-lauric acid designer lipid synthesis: based on artificial neural network and changes in enzymatic structure. Ultrason Sonochem. 2022;88 doi: 10.1016/j.ultsonch.2022.106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shields K.M., Tooley J.G., Petkowski J.J., Wilkey D.W., Garbett N.C., Merchant M.L., et al. Select human cancer mutants of NRMT1 alter its catalytic activity and decrease N-terminal trimethylation. Protein Sci. 2017;26(8):1639–1652. doi: 10.1002/pro.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material