Abstract
Ukraine faced significant fluctuations in COVID-19 morbidity and mortality, alongside an escalating HIV epidemic. This mixed-methods study, conducted between February and August 2022, employed a sequential explanatory design combining a quantitative analysis of national data and qualitative interviews to investigate the pandemic's effects on HIV services in Ukraine. The observed trends confirmed that the pandemic significantly disrupted facility-based HIV testing due to logistical challenges, an increased burden on healthcare workers, and supply shortages. Meanwhile, community-based testing showed resilience, largely attributed to programmatic adjustments rather than the pandemic itself. The initiation of antiretroviral therapy declined, especially during initial lockdowns, reflecting diminished treatment capacities. Despite these challenges, telemedicine and home medication delivery innovations supported antiretroviral therapy adherence. Furthermore, improvements in viral load testing and suppression rates showed healthcare resilience. The study highlights the critical need for adaptable, sustainable healthcare strategies in crises, emphasized during the war with Russia.
Keywords: antiretroviral therapy, access to treatment, prevention programs, viral load monitoring, healthcare infrastructure
Plain Language Summary
How COVID-19 Changed HIV Care in Ukraine: Challenges, Adaptations, and Innovations
In recent times, Ukraine, like many other countries, has been dealing with two big health problems: the COVID-19 pandemic and the ongoing HIV epidemic. With over 104 million cases of COVID-19 reported in Europe by early 2022, Ukraine faced the coronavirus as well as an increasing HIV crisis, especially among older adults and through various ways of spreading. This study, done between February and August 2022, aimed to understand how the COVID-19 pandemic affected the HIV services in Ukraine. By using numbers and in-depth interviews with health officials, service providers, and community members, we looked into the state of HIV care during this challenging period. Our findings show that the effects of the pandemic on HIV services were mixed. While HIV testing done in the community managed to adjust and keep going despite the changes, services in healthcare facilities ran into many problems. Lockdowns and restrictions made it hard for people to get to these places, leading to a big drop in HIV testing and the start of antiretroviral therapy, a key treatment for managing HIV. Despite these challenges, there were important changes and new ideas. Services such as telemedicine and delivering medication were started to make sure patients could continue their antiretroviral therapy without any breaks. The testing for viral load, which is important for checking how well HIV treatment is working, slowly went up, showing a system that could adapt to the pressures of the pandemic. The ability to adjust and keep going shown by some HIV services in Ukraine during the COVID-19 pandemic highlights the need for healthcare delivery methods that can change as needed and last over time. This study points out the importance of ongoing efforts to support people living with HIV, especially when facing big challenges, and gives valuable lessons for managing healthcare services during difficult times like the conflict with Russia.
Introduction
As of January 1, 2022, more than 104 million confirmed COVID-19 cases, the disease caused by the SARS-CoV-2 virus, have been reported in Europe. 1 Ukraine recorded its first COVID-19 case on March 3, 2020, followed by the implementation of its first lockdown on March 12, 2020.2,3 Throughout 2020–2021, Ukraine experienced substantial fluctuations in COVID-19 morbidity, hospitalization, and mortality, characterized by three prominent waves that peaked on November 15, 2020, with 819.3 cases per 100,000 population; April 11, 2021, with 829.8 per 100,000; and November 4, 2021, reaching 1684.9 cases per 100,000 population, indicating significant shifts in the pandemic's effects over time. 4
The Eastern Europe and Central Asia (EECA) region is experiencing the world's highest increase in HIV disease. 5 Ukraine emerged as one of the most heavily impacted countries in Europe, with adult HIV prevalence reaching 1.0%. 6 Initially, the epidemic was driven by transmission among people who inject drugs (PWID). The proportion of new HIV cases attributed to heterosexual intercourse has steadily increased, reaching 73.6% in 2019 7 ; however, the misclassification of cases due to injecting drug use and male-to-male sex remains significant. 8 Models estimate 245,000 people living with HIV (PLWH) in Ukraine by the end of 2021. 6 Despite significant efforts to scale-up antiretroviral therapy (ART), Ukraine did not meet the 2020 Joint United Nations Programme on HIV/AIDS (UNAIDS) targets. 6
As a result of the “20–50–80” Plan implementation initiated in 2018, 9 the Ukrainian government assumed the majority of the funding of HIV prevention, care and support programs, which previously were fully funded by international donors. Additional funding from the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GF) and the United States President's Emergency Plan for AIDS Relief (PEPFAR), is awarded to three principal recipients: the Public Health Center of the Ministry of Health of Ukraine (PHC), the “100% Life” organization (formerly the All-Ukrainian Network of PLWH), and the Alliance for Public Health (APH), to serve primarily catalytic purposes, with a focus on technical assistance and innovative interventions for key populations (KPs).10,11 Community-based prevention for KPs, including harm reduction and HIV testing, as well as a range of linkage-to-care interventions are provided by a strong network of non-governmental organizations (NGOs).12-14 Confirmatory HIV testing, treatment, and laboratory monitoring are provided at specialized healthcare facilities free of charge as part of the guaranteed healthcare package.15,16
The COVID-19 pandemic has dealt unprecedented effects on national health systems and undermined global efforts to address major health challenges, including the HIV epidemic.17-19 The disruptions in HIV services were primarily due to quarantine measures; transportation lockdowns; antiretroviral drug (ARV) shortages from manufacturer shutdowns; the reallocation of healthcare workers from PLWH care to COVID-19 patient support; and a decrease or total discontinuation of face-to-face consultations.19-23
On April 6, 2020, Ukraine implemented more stringent COVID-19 restrictions, including the closure of educational institutions, shops, and fitness centers; significant reduction in public transportation; and the mandatory use of face masks in all public spaces. 3 Beginning in September 2020, every oblast (administrative unit) in Ukraine was assigned one of four COVID-19 risk categories, determined by two indicators: COVID-19 incidence and hospital bed occupancy rates. 24 Public health measures were then applied in cities and regions within these oblasts based on their respective COVID-19 risk assessments, ie, “adaptive lockdown.”2,24 Nationwide, HIV clinics encouraged patients to avoid transportation while continuing their HIV treatment and following the guidance of public health authorities. 25
We conducted this study to systematically assess the nation-wide effects of the COVID-19 pandemic and its associated restrictions on the provision of key HIV services in Ukraine. The second aim was to explore the mechanisms underlying these effects and the programmatic measures implemented. The results of this assessment offer insights into the health system issues, which may continue to affect the continuum of care for PLWH after the COVID-19 pandemic is over, particularly in the context of full-scale Russian war on Ukraine.
Methods
Study Design Overview
Between February and August 2022, we conducted a mixed-methods study with a sequential explanatory design26,27 which encompassed two phases. In the first phase, we collected and analyzed quantitative data on key HIV service indicators, focusing on their trends and potential breakpoints. This quantitative component included a statistical analysis of aggregated national data on key HIV service indicators from January 2019 to December 2021. We collected data on the number of HIV tests conducted, the yield of positive tests, the number of individuals initiating ART, and viral load suppression rates. In the second phase, we employed qualitative methods to interpret the trends, explore the program response, and identify gaps in health program planning and administration. The mixed-method analysis enabled us to integrate findings from both phases during the analysis.
Data Collection
COVID-19 epidemiologic data and the timeline of restrictive measures were obtained from public sources.28-30 National-level aggregated data on key HIV service indicators from January 2019 to December 2021 were collected from public sources7,31 and direct communication with service providers. Some indicators were available for monthly reporting periods, others for quarterly reporting periods. The selected indicators’ definitions are provided in Supplementary Table 1, and raw indicator data in Supplementary Table 2.
Qualitative data were collected using in-depth interviews with 14 key informants (KIs), including service providers, program managers of the GF and PEPFAR principal recipients, national policymakers, and civil society. The KIs were identified and sampled through collaboration with HIV program implementers and professional networks (directly by phone or email), ensuring a comprehensive representation of stakeholders involved in HIV service provision. The interviews were conducted in Ukrainian by two female qualitative research experts (staff of Ukrainian Institute on Public Health Policy, with MD degrees and >10 years of qualitative experience). Each interview lasted between 45 and 60 min each and took place between February and August 2022. The participants did not have personal knowledge of the interviewers. To accommodate participants’ schedules and the ongoing COVID-19 pandemic-related restrictions, interviews were conducted in person (at workplace) or remotely. All interviews were audio-recorded and then transcribed. The transcripts were not returned to the participants for correction.
There were no standardized interview guides that addressed the research questions (impact of COVID-19 on HIV service provision in Ukraine) or examined the specific trends observed in the quantitative indicators (eg gaps in HIV testing in NGOs). Therefore, following the sequential-explanatory design, the guides were developed by investigators based on the findings of the quantitative phase. The interview guides focused on the HIV testing and treatment cascade and COVID-19 effects, addressing the individual, interpersonal, community, and health system levels of the Social Ecological Model. 32 The interviews explored service provision adaptation, policy barriers, and pandemic-induced changes in provider workload and client satisfaction. All interviews were transcribed verbatim for analysis. Since the number of participants was fixed, and their professional qualifications were sufficient to understand all questions in the interview, piloting of the interview guides was not deemed necessary.
Analytic Approach
To analyze trends in selected indicators and assess significant breakpoints in the trends, we used Joinpoint Trend Analysis Software version 5.0.2, developed by the National Cancer Institute for trend analysis in population-based disease surveillance systems. 33 By using aggregated monthly or quarterly indicators as inputs, this method identifies the time point(s) when a trend change occurs, calculates the monthly/quarterly percentage change (MPC/QPC) in rates between trend breakpoints, and estimates the average monthly/quarterly percentage change (AMPC/AQPC) in the entire period studied. The software fits a model for each possible number of breakpoints, from 0 to k, where k is determined by (i) the number of available data points, and (ii) pre-set minimum number of data points from the start or end of a series to breakpoints. In our study, k was six for monthly indicators and three for quarterly indicators. In the next step, the models were compared using the data-driven Weighted Bayesian Information Criterion,34,35 and the model with best fit was selected. (Model selection criteria and results are provided in Supplementary Table 3 and model graphs in Supplementary Figures 4–11.) Based on the selected model, MPC/QPC was calculated for each time segment and AMPC/AQPC for the entire period. Confidence intervals (CIs) for MPC/QPC were calculated using the empirical quantile method, 33 and if the 95% CI did not include 0, the trend was considered significant.
A thematic analysis of the qualitative data 36 was conducted to identify key concepts and factors relevant to understanding how the COVID-19 pandemic and other phenomena have influenced HIV treatment cascade indicators. This analysis used the MAXQDA software package to scrutinize the interview transcripts. The research team, consisting of two experienced qualitative researchers, developed and coded domains, constructed core ideas, and created categories for cross-case analysis. To ensure that insights emerged proximally to the data, the team followed a rigorous coding process, repeatedly comparing the themes to the data in the transcripts. Consistency and saturation of themes were assessed through continuous comparison and verification until no new themes emerged, ensuring the findings were sufficiently explanatory. This method enabled the identification of consistent themes across different cases.
Results
Participant Characteristics
The participants of the in-depth interviews were 14 KIs, including policymakers, representatives of international and donor organizations, healthcare providers, researchers, representatives of civil society and of KPs. The median age was 43 years, 3/14 were men.
COVID-19 Pandemic
From 2020 to 2021, the COVID-19 pandemic in Ukraine exhibited three infection surges: 349,683 cases in November 2020, 386,349 in April 2021, and a peak of 514,103 in November 2021 (Supplementary Figure 1). Cumulatively, 1,064,479 cases were recorded in 2020 and 2,635,184 in 2021, totaling 3,699,663 cases. Mortality rates peaked at 6750 deaths in December 2020, 12,545 in April 2021, and 21,867 in November 2021. Overall, 21,284 deaths occurred in 2020 and 87,571 in 2021, summing up to 108,855 deaths from March 2020 through December 2021.
HIV Testing in Community Settings
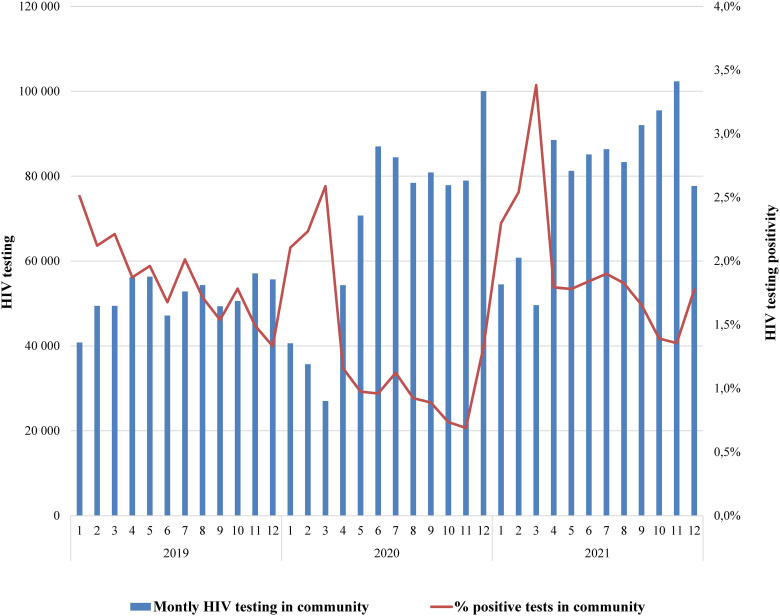
As Figure 1 illustrates, in 2019, the number of HIV tests conducted by NGOs in community settings ranged between 41,000 and 57,000 per month, with a non-significant MPC of 1.2 [95% CI: −1.3 to 5.8] (Table 1). After the first breakpoint in December’19, the number of tests dropped to 27,000 (MPC = −17.8 [−24.6–−2.7]) by March’20, and then steeply increased to 54,000 in April 2020 and then to 100,000 in December 2020. Another three-month decline was observed in January–March 2021, with a significant gradual increase by the end of the year (MPC = 4.1 [1.0–13.4]). There was no apparent correlation with the COVID-19 epidemic, and the three-year AMPC (2.1 [1.4–2.9]) confirmed the overall increasing trend. The percentage of positive HIV test results (yield) at NGOs demonstrated a corresponding pattern. In 2019 and 2020 there was a gradual decrease from 2.5% to 0.7% (MPC = −4.6 [−6.1 to −3.5] for 2019), with a sharp yield increases in January–March of 2020 and 2021, when the number of tests was lowest. After March 2021, yield was higher than in previous years but also showed a steady decline (MPC = −6.3 [−13.7 to −2.6]).
Figure 1.
Number of HIV tests and proportion of positive results in community settings, 2019–2021.
Table 1.
Monthly and quarterly percent changes based on joinpoint regression models in selected HIV service indicators in Ukraine, 2019–2021.
| No. of Joinpoints in the Final Model | Time Segment | Segment Dates | MPC/QPC | MPC/QPC 95% CI |
Significance of Trend | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| A. Number of HIV tests at NGOs (all combined) by month | ||||||
| 4 | 0 | Jan 2019–Dec 2019 | 1.2 | −1.3 | 5.8 | |
| 4 | 1 | Dec 2019–Mar 2020 | −17.8 | −24.6 | −2.7 | * |
| 4 | 2 | Mar 2020–Jun 2020 | 45.6 | 16.9 | 60.4 | * |
| 4 | 3 | Jun 2020–Feb 2021 | −4.3 | −15.6 | 0.0 | * |
| 4 | 4 | Feb 2021–Dec 2021 | 4.1 | 1.0 | 13.4 | * |
| 4 | Full range | Jan 2019–Dec 2021 | 2.1 | 1.4 | 2.9 | * |
| B. Proportion of HIV positive tests at NGOs | ||||||
| 2 | 0 | Jan 2019–Nov 2020 | −4.6 | −6.1 | −3.5 | * |
| 2 | 1 | Nov 2020–Feb 2021 | 43.7 | 13.9 | 57.3 | * |
| 2 | 2 | Feb 2021–Dec 2021 | −6.3 | −13.7 | −2.6 | * |
| 2 | Full range | Jan 2019–Dec’20 21 | −1.7 | −2.6 | −0.9 | * |
| C. Number of HIV tests at health facilities by quarter | ||||||
| 0 | Full range | Q1 2019–Q4 2021 | −1.1 | −1.8 | −0.3 | * |
| D. Proportion of HIV-positive tests at health facilities | ||||||
| 2 | 0 | Q1 2019–Q3 2019 | −3.2 | −6.7 | 1.7 | |
| 2 | 1 | Q3 2019–Q3 2020 | 3.4 | 1.1 | 6.6 | * |
| 2 | 2 | Q3 2020–Q4 2021 | −1.5 | −4.1 | −0.3 | * |
| 2 | Full range | Q1 2019–Q4 2021 | −0.1 | −0.7 | 0.7 | |
| E. Number of PLWH initiating ART by month | ||||||
| 0 | Full range | Jan 2019–Dec 2021 | −1.0 | −1.3 | −0.6 | * |
| F. Total number of PLWH on ART by month | ||||||
| 6 | 0 | Jan 2019–Mar 2019 | 1.0 | 0.8 | 1.2 | * |
| 6 | 1 | Mar 2019–Oct 2019 | 0.7 | 0.6 | 1.1 | * |
| 6 | 2 | Oct 2019–Feb 2020 | 1.1 | 0.7 | 1.2 | * |
| 6 | 3 | Feb 2020–Sep 2020 | 0.7 | 0.3 | 0.8 | * |
| 6 | 4 | Sep 2020–Jan 2021 | 0.3 | 0.1 | 0.6 | * |
| 6 | 5 | Jan 2021–Oct 2021 | 0.7 | 0.7 | 0.8 | * |
| 6 | 6 | Oct 2021–Dec 2021 | 0.4 | 0.2 | 0.5 | * |
| 6 | Full range | Jan 2019–Dec 2021 | 0.68 | 0.67 | 0.69 | * |
| G. Number of VL tests by quarter | ||||||
| 3 | 0 | Q1 2019–Q4 2019 | 1.9 | 1.6 | 2.1 | * |
| 3 | 1 | Q4 2019–Q3 2020 | −0.5 | −0.8 | −0.3 | * |
| 3 | 2 | Q3 2020–Q2 2021 | 1.9 | 1.7 | 2.2 | * |
| 3 | 3 | Q2 2021–Q4 2021 | −1.1 | −1.5 | −0.7 | * |
| 3 | Full range | Q1 2019–Q4 2021 | 0.68 | 0.6 | 0.8 | * |
| H. Proportion of VL tests <1000 copies/mL | ||||||
| 2 | 0 | Q1 2019–Q4 2019 | 0.08 | 0.06 | 0.11 | * |
| 2 | 1 | Q4 2019–Q4 2020 | 0.00 | −0.02 | 0.02 | |
| 2 | 2 | Q4 2020–Q4 2021 | 0.10 | 0.08 | 0.12 | * |
| 2 | Full range | Q1 2019–Q4 2021 | 0.06 | 0.05 | 0.06 | * |
MPC, monthly percent change; QPC, quarterly percent change; CI, confidence intervals; LL, lower limit; UL, upper limit; ART, antiretroviral therapy; NGOs, non-governmental organizations; PLWH, people living with HIV; VL, HIV-1 viral load; * indicates a significant trend (MPC/QPC CI does not include 0).
The in-depth interviews revealed that testing declines in January–March and corresponding yield increases result from program organization and the shift to government funding. Before 2019, HIV testing in community settings was funded by donors and managed by APH and 100% Life and primarily targeted KPs like PWID, men who have sex with men, and sex workers. The transition starting in 2019 was challenged by unpreparedness and inflexible procedures, causing early year contract delays with local service providers, reducing testing rates. The remaining donor-funded programs sustained testing levels, with their focused approach yielding higher results. In 2021, a PEPFAR-funded program scaled up index testing and optimized case finding, leading to increasing testing volume and yield.
[After the transition] in the first months of the year organizations are slowly gearing up, and begin to catch up [with testing] in March. This may also be connected to signing finding agreements. [Representative of civil society, female, 38 yo]
Unfortunately, changes in the government system are slow. [representative of civil society, female, 59 yo]
With regard to COVID-19, participants described a swift adaptation of services that sustained the overall level of HIV testing in community settings. Interventions for additional client motivation (such as financial incentives, improved service accessibility and flexibility, outreach services) were implemented in PEPFAR projects.
In the program activities, a significant amount of work has been done to reorganize service provision: changes in NGO working hours, client outreach points were modified to limit the number of people in indoor spaces without affecting overall testing accessibility. We communicated with clients by phone and delivered self-testing kits. [Representative of civil society, female, 38 yo]
According to several respondents, the decrease in HIV testing yield, especially in 2020, could be partially attributed to a shift in testing targets. During the lockdowns, NGOs required additional resources to sustain their operations, but without additional funding, they were limited in their ability to continue intensive outreach and to access high-risk groups.
In the context of the pandemic, when we lack sufficient resources, we work with those with whom we can. [Representatives of civil society, female, 38 yo]
HIV Testing in Healthcare Facilities
In 2020, there was a substantial 22.5% decline in the number of HIV tests conducted in healthcare facilities, from on average 630,000 to 490,000 per quarter (Supplementary Figure 2)). This decline was particularly noticeable in the second quarter of 2020, dropping to 407,000 tests. Although the further decrease in 2021 was only marginal (to 480,000 per quarter), the best-fit model did not identify any significant breakpoints, with an overall AQPC of −1.1 [−2.6 to −0.9]. The testing yield declined to a minimum of 0.7% in Q3 2019, followed by a significant increase to 1.2 in Q3 2020 (QPC = 3.4 [1.1–6.6]), and then again, a decrease to 0.9% in Q4 2021 (QPC = −1.5 [−4.1 to −0.3]).
The respondents primarily attributed the decrease in HIV testing at healthcare facilities to the ongoing COVID-19 pandemic. The main reasons for the decline were transportation restrictions, re-orientation of infectious disease facilities to serve COVID patients, and reduced working hours for other ambulatory patients.
Severe quarantine measures were implemented, which hampered the clients’ ability to access testing sites. In 2021, the volume of testing in healthcare facilities declined specifically during the periods of peak pressure on the healthcare system because it was overloaded with COVID. [Representative of donor organization, male, 36 yo]
Another important reason was the shortage of testing kits supplied by the Ministry of Health of Ukraine in a range of regions, which exacerbated during the pandemic due to logistical challenges (complex supply routes in certain regions, complicated regulations for redistribution between the regions). Additionally, as mentioned by some participants, the testing volume in public healthcare institutions, mainly using enzyme-linked immunosorbent assay, significantly declined even in the pre-pandemic period due to the closure of several large facilities.
At the end of 2019, during the session of the regional administration, a decision was made to close several healthcare institutions. [Healthcare provider, female, 62 yo]
Another potential factor for the decrease in HIV testing in healthcare facilities could be the roll-out of national healthcare reform, the core of which was the transition to national health insurance and a pay-per-service approach, causing institutions to perform only those procedures for which they are paid. However, not all health insurance packages include HIV testing.
Previously, all institutions were supposed to conduct testing, but now they operate based on [insurance] packages. If HIV testing is not included in a package, such as surgical assistance, then they do not conduct the testing. [Healthcare provider, male, 45 yo]
Respondents emphasized the role of community-based interventions that assist clients to reach healthcare facilities for diagnostics, including compensations for transportation expenses, as one of the contributors to the increased HIV testing yield in healthcare settings.
ART Initiation and Retention
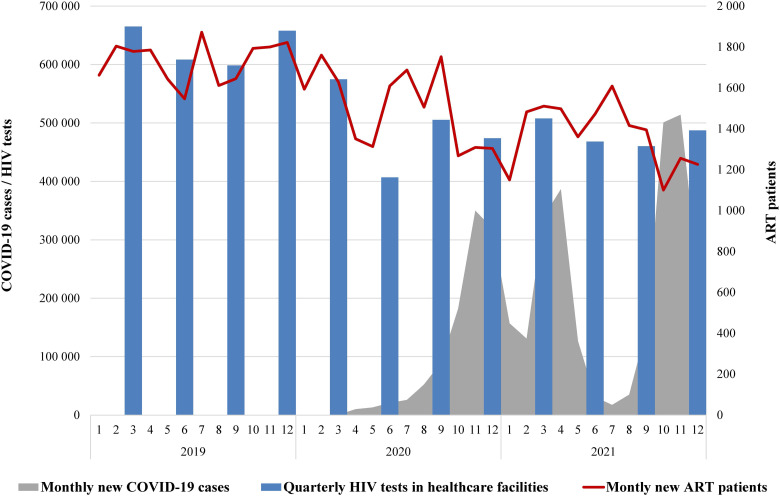
The number of patients initiating ART each month fluctuated with a gradual but consistent decline throughout the study period, from the peak of 1900 new patients in July 2019 to a minimum of 1100 in October 2021, as illustrated in Figure 2. The best-fit model did not demonstrate any significant trend breakpoints, and the overall AMPC was −1.0 [−1.3 to −0.6]. At the same time, the total number of patients on ART continued to increase gradually, from 103,000 in January 2019 to 130,000 in December 2021. The growth rate, however, had six significant breakpoints with MPC reaching maximum of 1.1 [0.7–1.2] during the pre-COVID period between October 2019 and February 2020, and with the lowest rates of 0.3 [0.1–0.6] between September 2020 and January 2021, and 0.4 [0.2–0.5] between October 2021 and December 2021, which coincided with the first and the third waves of COVID-19, respectively.
Figure 2.
HIV testing in healthcare facilities, number of new ART patients (2019–2021), and new COVID-19 cases (2020–2021).
The KIs acknowledged a consistent decline in ART initiation from 2019 to 2021, attributing it primarily to the effect of Ukraine's 2016 “treat all” policy. This policy led to intensified ART enrollment efforts by donors, program implementers, and service providers. Strategies included forming new multidisciplinary teams, incentivizing patients and staff, and implementing community-based linkage-to-care and retention interventions. These efforts resulted in significant progress toward the 90–90–90 goals, enrolling most easy-to-reach patients.
Everyone we could attract has already been attracted; new ART initiations now represent mostly newly identified individuals living with HIV. [Healthcare provider, male, 45 yo]
KIs concurred that the COVID-19 pandemic was not a major factor in this decline. Among the additional major challenges, respondents highlighted issues related to the central supply of medications, similar to those for testing kits, which persisted during the COVID-19 pandemic. The risk of running out of ARV medications forced HIV clinics to request re-distribution of remaining stock among the regions and reduce the dispensing amount for patients.
We were unable to provide stable patients with medications for 6 months; instead, we dispensed them monthly, at most for three months. In a manual mode, we managed the medication inventory by distributing it among regions. [Healthcare provider, female, 46 yo]
Participants provided a detailed description of adaptation mechanisms applied during the pandemic to enroll and retain patients. A broad range of innovative approaches encompassed the delivery of ARV medications by mail, the provision of ART via mobile clinics, and online consultations. A shift to service delivery via tele-health solutions was maximized in projects funded by the GF. Telemedicine played a significant role in maintaining ART adherence and improving viral load (VL) suppression rates. The implementation of telehealth approaches, flexible service hours, and prompt information exchange on service availability and demand was pivotal in addressing the challenges posed by the pandemic.
We can't even imagine now why we tolerated long queues when we could provide the same services remotely, without exposing ourselves and others to danger. Appropriate algorithms had to be developed for this. [Representative of civil society, female, 59 yo]
VL Testing and Viral Suppression
The logistical challenges that contributed to the reduction in HIV testing and ART initiation did not seem to affect VL testing. Concomitant with the expansion of patients on ART, the total annual number of VL tests increased from 318,000 tests in 2019 to 337,000 tests in 2020, and to 375,000 tests in 2021 (Supplementary Figure 3). The overall upward trend was significant (AQPC = 0.7 [0.6–0.7]), but the joinpoint model identified three significant breakpoints and intermittent trends, with the first decreasing segment in Q4 2019–Q3 2020 (QPC = −0.5 [−0.8 to −0.3]), which corresponded to the initial lockdown period, and the second one in Q2 2021–Q4 2021 (QPC = −1.1 [−1.5 to −0.7]), which started earlier than the final wave of COVID-19.
Without patient-level data, it was not possible to accurately calculate the VL coverage of ART patients, but if we assume that all tests within one quarter were for unique patients, and attrition was minimal, the approximate quarterly coverage fluctuated without a stable trend, between a minimum of 68% in Q3 2020 and a maximum of 77% in Q2 2021 (Supplementary Table 2).
The proportion of VL tests indicating viral suppression (<1000 copies/mL) increased overall from 94% to 96% (AQPC = 0.06 [0.05–0.06]), with two increasing trend segments in Q1 2019–Q4 2019 and Q4 2020–Q4 2021, and a plateau between, which corresponded to the initial COVID-19 period.
According to the KIs, the supply issues that impacted HIV testing and antiretroviral distribution also affected VL testing, particularly starting from Q2 2021. This led to the temporary unavailability of VL testing in certain regions and resulted in an overall decrease by the end of the study period. At that time, the PHC had to issue a guideline on prioritization of patients for VL testing.
Unfortunately, it's not the case that when a patient needs the VL test, they automatically get it. It all depends on 1) the capacity of the laboratory; 2) the availability of test kits. [Healthcare provider, male, 45 yo]
Despite shortages, VL testing coverage kept pace with the growing number of patients on ART. Prioritization of VL testing, just before the COVID-19 pandemic, intensified efforts from international donors, principal recipients, regional health authorities, and service providers to maintain its coverage. Notably, a PEPFAR project by APH incentivized patients, social workers, and medical providers for timely biannual VL testing.
Respondents discussed the dynamics of achieving an undetectable VL level among patients. All experts considered the observed viral suppression level to be entirely acceptable.
It's not just good, but excellent. Since 2012, our VL always ranged between 95–98%. [Representative of donor organization, male, 36 yo]
The experts noted that, due to the introduction of modern regimens, such as with dolutegravir, the impact of suboptimal adherence was mitigated, resulting in improved viral suppression. Additionally, strategies to address patient disengagement and adherence among patients in PEPFAR and GF projects resulted in improved VL suppression rates.
Crosscutting Issues and Challenges
Respondents highlighted the pandemic's early stage negative effects on service access and quality, focusing on lockdowns and restrictions impairing patient mobility and reduced operating hours of medical facilities and NGOs.
Lockdown – people couldn't access services; both patients and service providers fell ill. There was a significant burden on the healthcare system. [Representative of donor organization, male, 36 yo]
Respondents observed that during COVID-19, medical institutions and NGOs adapted services to maintain stability and client accessibility. Donor-funded projects, especially GF and PEPFAR, helped sustain testing and treatment levels, with these changes now standard practice.
The pandemic forced us to review and adapt the existing service packages to the new working conditions. These creative solutions helped overcome the barriers created by COVID. [Representative of civil society, female, 38 yo]
Experts from various sectors acknowledged the country's suboptimal crisis response mechanisms and the heavy reliance of program sustainability on donor support, advocating for more flexible and robust contingency planning in HIV program stabilization.
For sustainability, first – there must be stable funding, second – the presence of people, third – the presence of flexible algorithms and a departure from something rigid. [Representative of civil society, female, 59 yo]
Discussion
In this study, we analyzed trends in HIV testing and treatment in Ukraine from 2019 to 2021, assessing the effects of COVID-19. Using quantitative and qualitative methods, we found that the pandemic presented significant challenges, exerting considerable strain on HIV service providers and causing disruptions for clients. These disruptions, however, were moderate and did not correlate with the most severe surges in COVID-19 infection. This can be partially attributed to the concerted efforts of program funders and implementers.
Despite the COVID-related restrictions, HIV testing in community settings showed resilience and an overall increase. The yield of positive tests, however, showed mixed trends, not clearly linked to the pandemic. This declining yield may result from the continuous shrinkage of the undiagnosed PLWH population. In the context of low HIV incidence, this implies that even with optimized case-finding strategies, the yield is likely to continue decreasing.
Contrary to our findings, a survey of 71 community-based HIV testing services in 28 countries revealed a substantial (>50%) drop in HIV testing from March to May 2020 versus 2019, lacking recovery after confinement. 37 Quarantine measures in Europe greatly affected community-based HIV testing services; many closed as non-essential during initial lockdowns, potentially hindering HIV testing and prevention access for KPs.37,38 Our study underscores the adaptability of Ukrainian NGOs in responding to the challenges posed by the pandemic. The notable adjustments included the scale-up of self-testing, changes in working hours, and rapid expansion of tele-health technologies. Other studies confirm that HIV self-testing is a feasible and effective alternative to traditional in-person approaches.39-41
In contrast to NGO settings, healthcare facilities in Ukraine experienced a marked decline in HIV testing, showing a 22.5% decrease in 2020 compared to 2019. This downturn persisted into 2021, reflecting the pandemic's considerable effects on institutional testing capabilities. Importantly, our study found that this decline was primarily related to policy and program management issues and changes in government funding mechanisms, rather than COVID-19-related factors. The systemic healthcare issues in Ukraine and lack of preparedness for handling public health crises were also noted in a UNAIDS report. 42 Similar to Ukraine, a retrospective cohort study of secondary HIV testing data from 44 countries in Asia, Latin America, the Caribbean, Europe, and Africa also revealed a marked decline in HIV testing, despite mitigation efforts. 40
Our KIs highlighted the important role of community workers in facilitating client access to HIV diagnostics, as a key contributor to increasing testing yields in healthcare settings. The rise in HIV detections in some countries, coinciding with the COVID-19 pandemic, is attributed to expanded community, home-based, and index testing, focusing on higher-risk groups and symptomatic individuals.40,43
Our study documented a 12.9% reduction in the initiation of ART in 2020 relative to 2019, with a continued decline in 2021, indicating the pandemic's adverse effect on ART enrollment, despite a gradual increase in the overall number of patients on ART. The lowest monthly ART initiation rates coincided with the major waves of COVID-19 morbidity, suggesting a strain on the capacity of healthcare facilities. At the same time, a more significant reason for the decline in monthly ART enrollment, according to the KIs, was the reduction of the pool of eligible individuals due to the intensive linkage to care in the previous years.
Similar to Ukraine, in other countries, the COVID-19 pandemic accelerated the adoption of alternative healthcare approaches, including telemedicine, scheduled and home-based appointments, extended ART refills, postal drug delivery, and satellite clinics.19,40,44
Despite the decreased capacity of health facilities and laboratories during the peak periods of COVID-19 pandemic, VL testing in Ukraine was relatively less affected. The overall increase in VL testing followed the increase of patients on ART, facilitated by test kit availability, strengthened facility-level monitoring, and highly motivated patients and providers. The effectiveness of ART, reflected by the level of viral suppression, improved over the study period, likely attributed to the scale-up of modern regimens 45 and adherence interventions. 46 Early in the COVID-19 pandemic other PEPFAR-supported countries experienced declines in VL testing; but over time, they adapted laboratory services to meet SARS-CoV-2 demands.43,47 In many cases these countries regained and even increased VL testing and suppression by focusing on high-viremia PLWH, addressing stockouts and backlogs, and synchronizing testing with medication pickups. 43
The study confirms the pandemic's negative effects on access to services and their quality, particularly in the early stages. Lockdowns and other restrictions posed additional significant challenges for highly HIV-vulnerable groups, 48 as their impact extended beyond the disease and service access, amplifying broader health equity issues that initially contributed to their heightened susceptibility to HIV. 24 Our study highlights the importance of flexibility and adaptability in sustaining HIV programs, particularly during crises. Programs funded by the GF and PEPFAR played a pivotal role in upholding the HIV services in Ukraine during the COVID-19 pandemic. Their notable effects included securing drug supplies, modifying sub-grants, decentralizing services, facilitating cooperation across sectors, and expanding innovative patient engagement strategies such as self-testing and mail-based medication distribution.
Systemic shocks such as conflicts, economic crises, and natural disasters exacerbate health disparities, disproportionately affecting vulnerable groups and influencing HIV trends.11,49,50 Since the onset of the Russian invasion of Ukraine in 2014, Ukrainian healthcare and public health programs, including those related to HIV, have operated under immense stress, facing significant risks of service disruption due to physical threats in areas close to combat, overall funding instability, and health workforce deficit. 51 HIV transmission, among other factors, has also been affected by the internal displacement of PLWH. 49 In the current context of full-scale war since 2022, the risks for HIV programs have multiplied significantly. The lessons learned during the COVID-19 pandemic and subsequent adaptations in healthcare service delivery have been instrumental to prevent HIV program interruption in the current crisis.51-53
The study has several limitations. In the quantitative component, we analyzed aggregated national-level data, which did not permit us to examine trends at the regional level. These trends could significantly vary due to regional specifics. The HIV testing data represents the number of tests conducted, rather than the number of individuals tested, which may lead to a slight overestimation of HIV testing coverage. Since no validated instruments addressing the research questions existed, the interview guides were developed by investigators. The qualitative phase, though insightful, was limited to selected key informants and missed perspectives from patients and frontline healthcare workers. This may not fully capture the pandemic's impact on HIV services in Ukraine.
Conclusions
Collaborative efforts of funders, managers, and service providers in Ukraine mitigated the effects of the COVID-19 pandemic on HIV services. Community-based HIV testing showed resilience and growth. However, facility-based services, including HIV testing and ART initiation, declined during the initial lockdown period, and did not recover to pre-pandemic levels. While factors related to COVID-19, such as transportation restrictions and reduced healthcare facility capacity, contributed to the decline, epidemiological trends and program management challenges played a more significant role. VL testing remained less affected, affirming the continued increase in ART effectiveness. The pandemic underscores the need for flexible, sustainable healthcare approaches, particularly crucial in the ongoing context of Russia's war against Ukraine.
Supplemental Material
Supplemental material, sj-pdf-1-jia-10.1177_23259582241277649 for The Effects of COVID-19 Pandemic on HIV Service Provision in Ukraine by Oleksandr Neduzhko, Tetiana Kiriazova, Oleksandr Zeziulin, Liudmyla Legkostup, Serhii Riabokon, Jack A DeHovitz and Kostyantyn Dumchev in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Supplemental material, sj-docx-2-jia-10.1177_23259582241277649 for The Effects of COVID-19 Pandemic on HIV Service Provision in Ukraine by Oleksandr Neduzhko, Tetiana Kiriazova, Oleksandr Zeziulin, Liudmyla Legkostup, Serhii Riabokon, Jack A DeHovitz and Kostyantyn Dumchev in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Acknowledgments
We are grateful to all key informants and staff involved in the study.
Footnotes
Author Contributions: KD conceptualized the study, and ON, TK, JD, and OZ contributed to the study design. TK, OZ, LL, and SR collected the data. ON, TK, OZ, and KD analyzed and interpreted the data. ON wrote the first draft of the manuscript and subsequent drafts after revisions. All authors reviewed, edited, and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval and Informed Consent: The study protocol was approved by the Institutional Review Board of the Ukrainian Institute on Public Health Policy in Kyiv, Ukraine (Approval # 06-22, February 18, 2022). The quantitative analysis was based on aggregated national data; therefore, the individual informed consent was not required. In-depth interview participants provided verbal informed consent, having received detailed explanations of the study's confidentiality measures, risks, and benefits. The verbal consent process was documented by the interviewer, who recorded the date, and participant's verbal agreement to participate in the study. Verbal consent was selected over written consent because no personal information from participants was collected and the name and the signature on the consent form would be the only document linking interview data with the participant's identity. No personal identifiers were used in analysis or reporting. Participants received compensation of 300 Ukrainian Hryvnas (approximately 8 US dollars) for their time. The authors confirm that verbal informed consent was obtained from every in-depth interview participant as required by the study protocol.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the Fogarty International Center and the National Institute on Drug Abuse of the National Institutes of Health under Award Number D43 TW010562. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability Statement: The authors confirm that all data underlying the findings are provided in the Supplemental Materials.
ORCID iD: Oleksandr Neduzhko https://orcid.org/0000-0002-9539-246X
Supplemental Material: Supplemental material for this article is available online.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19), 2022, https://data.who.int/dashboards/covid19/cases?n=c (accessed December 24, 2023).
- 2.Mehta NK, Honchar I, Doroshenko O, et al. Impact of the COVID-19 pandemic on Ukrainian mortality, 2020-2021. PLoS One. 2023;18(5):e0285950. DOI: 10.1371/journal.pone.0285950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyrychko YN, Blyuss KB, Brovchenko I. Mathematical modelling of the dynamics and containment of COVID-19 in Ukraine. Sci Rep. 2020;10(1):19662. DOI: 10.1038/s41598-020-76710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podavalenko A, Malysh N, Zadorozhna V, et al. Analysis of the epidemic situation of the COVID-19 coronavirus infection in Ukraine. Folia Med Cracov. 2023;63(1):109-120. DOI: 10.24425/fmc.2023.145434 [DOI] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/ AIDS. In danger: UNAIDS Global AIDS update, 2022, https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update_en.pdf (accessed January 04, 2024). [PubMed]
- 6.Public Health Center of the Ministry of Health of Ukraine of Ukraine. HIV infection in Ukraine: information bulletin #53, 2022, https://phc.org.ua/sites/default/files/users/user90/HIV_in_UA_53_2022_EN.pdf (accessed January 03, 2024).
- 7.Public Health Center of the Ministry of Health of Ukraine of Ukraine. HIV infection in Ukraine: information bulletin #51, 2020, https://phc.org.ua/sites/default/files/users/user90/HIV_in_UA_51_2020.pdf (accessed January 03, 2024).
- 8.Dumchev K, Kornilova M, Kulchynska R, et al. Improved ascertainment of modes of HIV transmission in Ukraine indicates importance of drug injecting and homosexual risk. BMC Public Health. 2020;20(1):1288. DOI: 10.1186/s12889-020-09373-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joint United Nations Programme on HIV/AIDS. 20–50–80 to reach 100 in Ukraine, 2020, https://www.unaids.org/en/resources/presscentre/featurestories/2020/november/20201106_ukraine-20-50-80 (accessed January 24, 2024). [PubMed]
- 10.The Global Fund to Fight AIDS Tuberculosis and Malaria. Datasets: Ukraine., https://data.theglobalfund.org/location/UKR/documents (accessed December 27, 2023).
- 11.Friedman SR, Smyrnov P, Vasylyeva TI. Will the Russian war in Ukraine unleash larger epidemics of HIV, TB and associated conditions and diseases in Ukraine? Harm Reduct J. 2023;20(1):119. DOI: 10.1186/s12954-023-00855-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neduzhko O, Saliuk T, Kovtun O, et al. Community-based HIV prevention services for transgender people in Ukraine: current situation and potential for improvement. BMC Health Serv Res. 2023;23(1):631. DOI: 10.1186/s12913-023-09656-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trickey A, Semchuk N, Saliuk T, et al. Has resourcing of non-governmental harm-reduction organizations in Ukraine improved HIV prevention and treatment outcomes for people who inject drugs? Findings from multiple bio-behavioural surveys. J Int AIDS Soc. 2020;23(8):e25608. DOI: 10.1002/jia2.25608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuznetsova J, Sereda Y, Denisiuk O, et al. Linking intravenous drug users to treatment through non-governmental organizations in Ukraine: how well is it working? J Infect Dev Ctries. 2019;13(07.1):95s-102 s. DOI: 10.3855/jidc.11291 [DOI] [PubMed] [Google Scholar]
- 15.Deshko L, Bysaga Y, Kalyniuk S, et al. State obligations in provision of the primary physician's right to medical practice as entrepreneurship in light of transformation of the health care system in Ukraine. Georgian Med News. 2020;(303):194-199. [PubMed] [Google Scholar]
- 16.Kyselyova G, Martsynovska V, Volokha A, et al. Young people in HIV care in Ukraine: a national survey on characteristics and service provision. F1000Res. 2019;8:323. DOI: 10.12688/f1000research.18573.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFall AM, Menezes NP, Srikrishnan AK, et al. Impact of the COVID-19 pandemic on HIV prevention and care services among key populations across 15 cities in India: a longitudinal assessment of clinic-based data. J Int AIDS Soc. 2022;25(7):e25960. DOI: 10.1002/jia2.25960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132-e1141. DOI: 10.1016/S2214-109X(20)30288-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatechompol S, Avihingsanon A, Putcharoen O, et al. COVID-19 and HIV infection co-pandemics and their impact: a review of the literature. AIDS Res Ther. 2021;18(1):28. DOI: 10.1186/s12981-021-00335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Disruption in HIV, Hepatitis and STI services due to COVID-19, 2020, https://www.who.int/docs/default-source/hiv-hq/disruption-hiv-hepatitis-sti-services-due-to-covid19.pdf?sfvrsn=5f78b742_8 (accessed December 25, 2023).
- 21.Shiau S, Krause KD, Valera P, et al. The burden of COVID-19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020;24(8):2244-2249. DOI: 10.1007/s10461-020-02871-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalska JD, Skrzat-Klapaczynska A, Bursa D, et al. HIV care in times of the COVID-19 crisis - where are we now in Central and Eastern Europe? Int J Infect Dis. 2020;96:311-314. DOI: 10.1016/j.ijid.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Zhang C, Wang FS. COVID-19 in people with HIV. Lancet HIV. 2020;7(8):e524-e526. DOI: 10.1016/S2352-3018(20)30163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClarty L, Lazarus L, Pavlova D, et al. Socioeconomic burdens of the COVID-19 pandemic on LMIC populations with increased HIV vulnerabilities. Curr HIV/AIDS Rep. 2022;19(1):76-85. DOI: 10.1007/s11904-021-00591-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozanova J, Rich KM, Altice FL, et al. The initial response to COVID-19 disruptions for older people with HIV in Ukraine. Geriatrics (Basel). 2022;7(6):138. DOI: 10.3390/geriatrics7060138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tashakkori A, Teddlie C. SAGE Handbook of Mixed Methods in Social & Behavioral Research. 2 ed. SAGE Publications, Inc.; 2010. DOI: 10.4135/9781506335193. [DOI] [Google Scholar]
- 27.Ivankova NV, Creswell JW, Stick SL. Using mixed-methods sequential explanatory design: from theory to practice. Field Methods. 2006;18(1):3-20. DOI: 10.1177/1525822X05282260 [DOI] [Google Scholar]
- 28.European Centre for Disease Prevention and Control. Data on 14-day notification rate of new COVID-19 cases and deaths, https://www.ecdc.europa.eu/en/publications-data/data-national-14-day-notification-rate-covid-19 (accessed December 20, 2023).
- 29.European Centre for Disease Prevention and Control. Data on the daily number of new reported COVID-19 cases and deaths by EU/EEA country, https://www.ecdc.europa.eu/en/publications-data/data-daily-new-cases-covid-19-eueea-country (accessed January 24, 2024).
- 30.The Ministry of Health of Ukraine. COVID-19 pandemic in Ukraine. Current information about coronavirus and quarantine, 2022, https://covid19.gov.ua/en/ (accessed January 24, 2024).
- 31.Public Health Center of the Ministry of Health of Ukraine. HIV/AIDS Statistics, https://phc.org.ua/kontrol-zakhvoryuvan/vilsnid/statistika-z-vilsnidu (accessed January 24, 2024).
- 32.Ma PHX, Chan ZCY, Loke AY. The socio-ecological model approach to understanding barriers and facilitators to the accessing of health services by sex workers: a systematic review. AIDS Behav. 2017;21(8):2412-2438. DOI: 10.1007/s10461-017-1818-2 [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Chen HS, Byrne J, et al. Twenty years since Joinpoint 1.0: two major enhancements, their justification, and impact. Stat Med. 2022;41(16):3102-3130. DOI: 10.1002/sim.9407 [DOI] [PubMed] [Google Scholar]
- 34.National Cancer Institute. Joinpoint Trend Analysis Software, 2023, https://surveillance.cancer.gov/joinpoint/ (accessed December 12, 2023).
- 35.National Cancer Institute. How Joinpoint Selects the Final Model, 2023, https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/model-selection-method (accessed December 18, 2023).
- 36.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. DOI: 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 37.Fernandez-Lopez L, Simoes D, Casabona J, et al. Impact of the COVID-19 pandemic on community-based testing for HIV, viral hepatitis and sexually transmitted infections in the WHO European region, march to August 2020. Eur J Public Health. 2023;33(3):528-535. DOI: 10.1093/eurpub/ckad010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European AIDS Treatment Group. EATG Rapid Assessment. COVID-19 crisis’ impact on PLHIV and on communities most affected by HIV, 2020, https://www.politico.eu/wp-content/uploads/2020/04/EATG-Rapid-Assessment-COVID19-1-.pdf (accessed December 25, 2023).
- 39.Chenneville T, Gabbidon K, Hanson P, et al. The impact of COVID-19 on HIV treatment and research: a call to action. Int J Environ Res Public Health. 2020;17(12):4548. DOI: 10.3390/ijerph17124548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rick F, Odoke W, van den Hombergh J, et al. Impact of coronavirus disease (COVID-19) on HIV testing and care provision across four continents. HIV Med. 2022;23(2):169-177. DOI: 10.1111/hiv.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simoes D, Stengaard AR, Combs L, et al. Impact of the COVID-19 pandemic on testing services for HIV, viral hepatitis and sexually transmitted infections in the WHO European Region, March to August 2020. Euro Surveill. 2020;25(47):2001943. DOI: 10.2807/1560-7917.ES.2020.25.47.2001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.United Nations Ukraine. Assessment of the Socio-Economic Impact of COVID-19 in Ukraine. Kyiv; 2020. [Google Scholar]
- 43.Fisher KA, Patel SV, Mehta N, et al. Lessons learned from programmatic gains in HIV service delivery during the COVID-19 pandemic - 41 PEPFAR-supported countries, 2020. MMWR Morb Mortal Wkly Rep. 2022;71(12):447-452. DOI: 10.15585/mmwr.mm7112a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. DOI: 10.37765/ajmc.2020.42784 [DOI] [PubMed] [Google Scholar]
- 45.Dumchev K, Kiriazova T, Riabokon S, et al. Comparative clinical outcomes with scale-up of dolutegravir as first-line antiretroviral therapy in Ukraine. J Acquir Immune Defic Syndr. 2022;91(2):197-209. DOI: 10.1097/QAI.0000000000003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alliance for Public Health. 2020 Annual Report, https://aph.org.ua/wp-content/uploads/2022/01/ar2020en.pdf (accessed June 19, 2023).
- 47.Lecher SL, Naluguza M, Mwangi C, et al. Notes from the field: impact of the COVID-19 response on scale-up of HIV viral load testing - PEPFAR-supported countries, January-June 2020. MMWR Morb Mortal Wkly Rep. 2021;70(21):794-795. DOI: 10.15585/mmwr.mm7021a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nomah DK, Reyes-Uruena J, Llibre JM, et al. HIV and SARS-CoV-2 co-infection: epidemiological, clinical features, and future implications for clinical care and public health for people living with HIV (PLWH) and HIV most-at-risk groups. Curr HIV/AIDS Rep. 2022;19(1):17-25. DOI: 10.1007/s11904-021-00596-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasylyeva TI, Liulchuk M, Friedman SR, et al. Molecular epidemiology reveals the role of war in the spread of HIV in Ukraine. Proc Natl Acad Sci U S A. 2018;115(5):1051-1056. DOI: 10.1073/pnas.1701447115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alam K, Mahal A. Economic impacts of health shocks on households in low and middle income countries: a review of the literature. Global Health. 2014;10(1):21. DOI: 10.1186/1744-8603-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsagkaris C, Matiashova L, Vladychuk V, et al. Public health considerations over HIV amidst war and COVID-19 in Ukraine: harnessing contemporary history to address the syndemic. Ethics Med Public Health. 2022;22:100795. DOI: 10.1016/j.jemep.2022.100795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramirez C, Duron RM. The Russia-Ukraine war could bring catastrophic public-health challenges beyond COVID-19. Int J Infect Dis. 2022;120:44-45. DOI: 10.1016/j.ijid.2022.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uwishema O, Sujanamulk B, Abbass M, et al. Russia-Ukraine conflict and COVID-19: a double burden for Ukraine’s healthcare system and a concern for global citizens. Postgrad Med J. 2022;98(1162):569-571. DOI: 10.1136/postgradmedj-2022-141895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jia-10.1177_23259582241277649 for The Effects of COVID-19 Pandemic on HIV Service Provision in Ukraine by Oleksandr Neduzhko, Tetiana Kiriazova, Oleksandr Zeziulin, Liudmyla Legkostup, Serhii Riabokon, Jack A DeHovitz and Kostyantyn Dumchev in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Supplemental material, sj-docx-2-jia-10.1177_23259582241277649 for The Effects of COVID-19 Pandemic on HIV Service Provision in Ukraine by Oleksandr Neduzhko, Tetiana Kiriazova, Oleksandr Zeziulin, Liudmyla Legkostup, Serhii Riabokon, Jack A DeHovitz and Kostyantyn Dumchev in Journal of the International Association of Providers of AIDS Care (JIAPAC)