Abstract
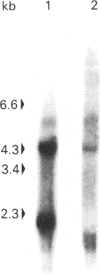
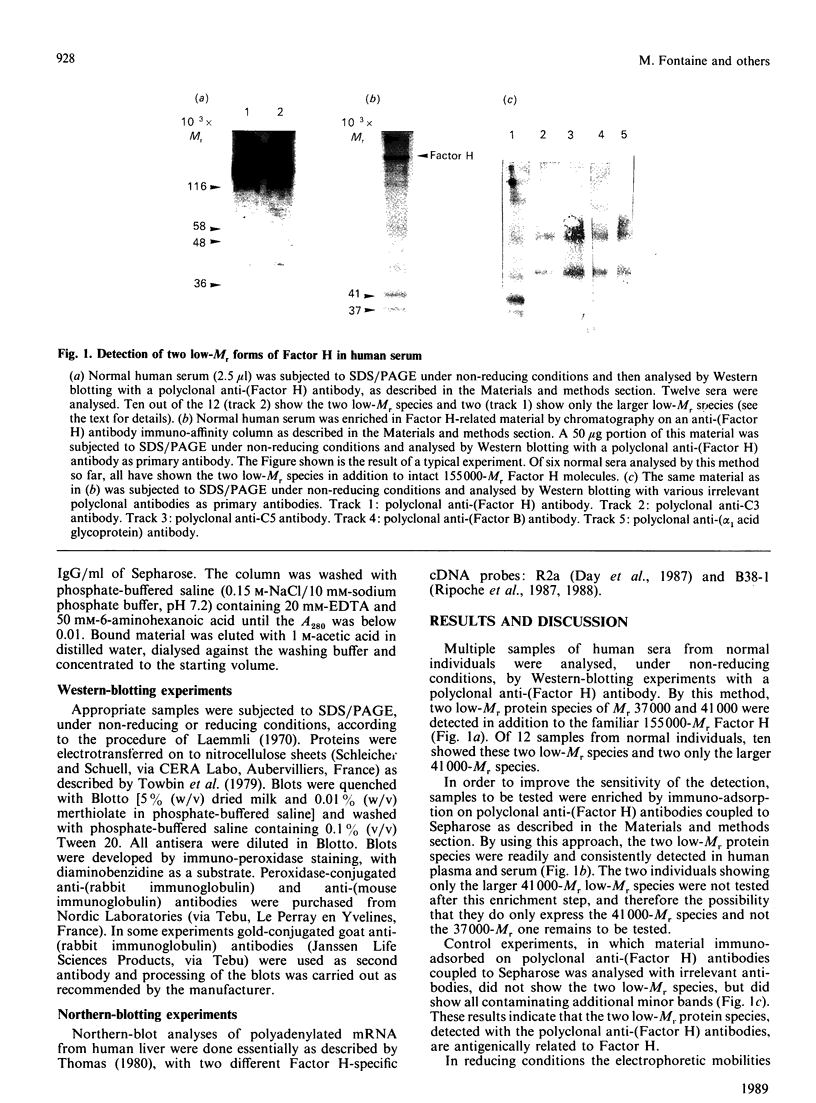
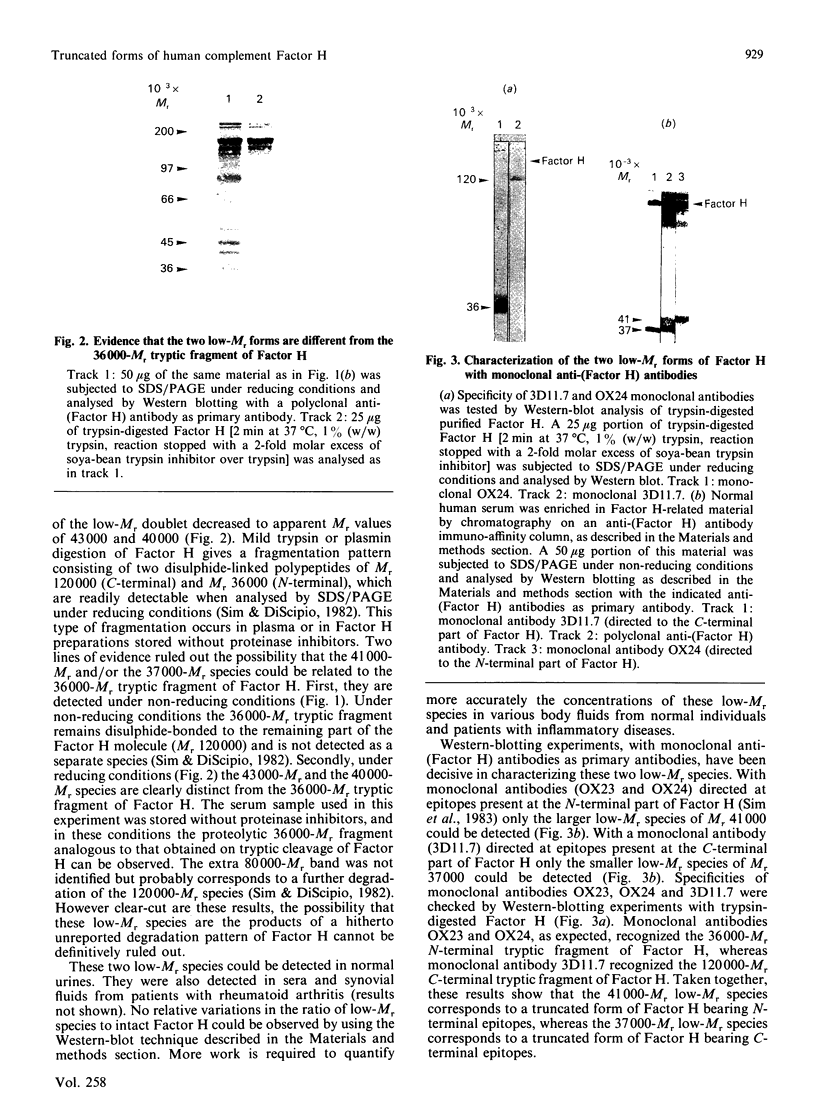
By the use of Western-blot analyses with polyclonal anti-(Factor H) antibodies, two low-Mr protein species of Mr 41,000 and 37,000 under non-reducing conditions and 43,000 and 40,000 under reducing conditions are consistently detected together with the well-known 155,000-Mr Factor H in human plasma and serum. These two additional species are also found in plasma, urine and synovial fluids. The 41,000-Mr species but not the 37,00-Mr species is detected by a monoclonal anti-(Factor H) antibody directed at the N-terminal part of Factor H. The 37,000-Mr species but not the 41,000-Mr species is detected by a monoclonal anti-(Factor H) antibody directed at the C-terminal part of Factor H. The 41,000-Mr and 37,000-Mr species are different from the well-characterized 36,000-Mr N-terminal tryptic fragment of Factor H. They are likely to represent translational products of the short Factor H mRNA species of 1.8 kb and 1.2-1.5 kb occurring in human liver that we have recently described.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsenz J., Schulz T. F., Lambris J. D., Sim R. B., Dierich M. P. Structural and functional analysis of the complement component factor H with the use of different enzymes and monoclonal antibodies to factor H. Biochem J. 1985 Dec 15;232(3):841–850. doi: 10.1042/bj2320841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. J., Ripoche J., Lyons A., McIntosh B., Harris T. J., Sim R. B. Sequence analysis of a cDNA clone encoding the C-terminal end of human complement factor H. Biosci Rep. 1987 Mar;7(3):201–207. doi: 10.1007/BF01124790. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Sim R. B. Expression of complement factor H on the cell surface of the human monocytic cell line U937. Eur J Immunol. 1985 Sep;15(9):935–941. doi: 10.1002/eji.1830150913. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche J., Al Salihi A., Rousseaux J., Fontaine M. Isolation of two molecular populations of human complement factor H by hydrophobic affinity chromatography. Biochem J. 1984 Jul 1;221(1):89–96. doi: 10.1042/bj2210089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche J., Day A. J., Harris T. J., Sim R. B. The complete amino acid sequence of human complement factor H. Biochem J. 1988 Jan 15;249(2):593–602. doi: 10.1042/bj2490593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Cordoba S., Lublin D. M., Rubinstein P., Atkinson J. P. Human genes for three complement components that regulate the activation of C3 are tightly linked. J Exp Med. 1985 May 1;161(5):1189–1195. doi: 10.1084/jem.161.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaeble W., Zwirner J., Schulz T. F., Linke R. P., Dierich M. P., Weiss E. H. Human complement factor H: expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 1987 Oct;17(10):1485–1489. doi: 10.1002/eji.1830171015. [DOI] [PubMed] [Google Scholar]
- Sim E., Palmer M. S., Puklavec M., Sim R. B. Monoclonal antibodies against the complement control protein factor H (beta 1 H). Biosci Rep. 1983 Dec;3(12):1119–1131. doi: 10.1007/BF01120205. [DOI] [PubMed] [Google Scholar]
- Sim R. B., DiScipio R. G. Purification and structural studies on the complement-system control protein beta 1H (Factor H). Biochem J. 1982 Aug 1;205(2):285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of C3b hemolytic activity by a plasma protein distinct from C3b inactivator. Science. 1976 Sep 10;193(4257):1011–1013. doi: 10.1126/science.948757. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]