While aortic valve calcification (AVC) is a key component in aortic stenosis (AS), the pathophysiology underlying of AVC remains incompletely understood. Lipoprotein (a) [Lp(a)], an atherogenic and proinflammatory lipoprotein and high-sensitivity C-reactive protein (hsCRP), a measure of inflammation, both contribute to AVC. Lp(a) and hsCRP have a significant interaction in the context of atherosclerotic cardiovascular disease (ASCVD), but whether these risk factors have an interaction with regard to AVC is unknown 1,2
6,676 participants aged 45–84 years without overt cardiovascular disease had cardiac CT preformed at Visit 1 (2000–02) of the Multi-Ethnic Study of Atherosclerosis (MESA). The MESA protocol was approved by the Institutional Review Boards at the participating institutions and written informed consent was obtained from all participants.
Non-contrast cardiac compted tomography (CT) scans were used to measure AVC with quantification using the Agatston method, which has a low intra-reader (4.4%) and inter-scan (9.7%) variability.3 We examined 1) incident AVC among the 5,133 participants with AVC=0 at baseline and 2) progression of AVC among the 488 with baseline AVC >0 who had a repeat CT scan, which was performed at Visit 2 (2002–04) among half of the cohort, Visit 3 (2004–05) among the other half, or at Visit 5 (2010–12) where half were randomized to repeat cardiac CT.
Since hsCRP and Lp(a) are not normally distributed variables, they were logarithmically transformed when modeled as continuous variables. Multivariable adjusted logisitic regression models were used to calculate prevalence ratios (PR) and adjusted Cox Proportional were used to calculate hazard ratios (HR) for incident AVC. Adjusted linear regression modeling was performed to examine annualized progression of AVC among participants with baseline AVC >0. Statistical significance was defined as a p-value <0.05, except for interaction testing where a more conservative value of 0.10 was used.
There were 6,676 participants (mean age 62.1 ± 10.2 years, 53% women) and AVC was present at baseline among 13.7% (n=893), while 423 participants developed incident AVC over 10-years follow-up. The median hsCRP value was 1.91 (10th percentile 0.42, 90th percentile 9.0) and for AVC was 0 (10% percentile 0, 90th percentile 21).
Higher Lp(a) levels were associated with a higher prevalence of AVC (unadjusted PR 1.17 [95% CI 1.09–1.26], asdjusted PR: 1.12 [95% CI: 1.05–1.20]). When assessed as a categorical variable, both Lp(a) 50–99.9 mg/dL (unadjusted PR 1.46 [95% CI 1.25–1.70], adjusted PR: 1.36 [95% CI: 1.17–1.58]) and Lp(a) ≥100 mg/dL (unadjusted PR 1.85 [95% CI 1.49–2.29], adjusted PR: 1.59 [95% CI: 1.31–1.94]) were associated with a higher prevalence of AVC. hsCRP was not associated with prevalent AVC either as a continuous variable (unadjusted PR 1.05 [95% CI 1.00–1.20], asjusted PR 1.04 [95% CI 0.99–1.11]) or as a categorical variable (unadjusted PR 1.11 [95% CI 0.98–1.25], adjusted PR 1.13 [95% CI 0.99–1.28]).
When assessed as a continuous variable, Lp(a) was associated with a higher risk of incident AVC (unadjusted HR 1.16 [95% CI 1.05–1.27], adjusted HR 1.17 [95% CI: 1.05–1.29]) and as a cateogorical variable, both Lp(a) 50–99.9 mg/dL (unadjusted HR 1.50 [95% CI 1.18–1.92], adjusted HR 1.54 [95% CI: 1.19–1.99]) and Lp(a) ≥100 mg/dL (unadjusted HR 1.59 [95% CI 1.06–2.37], adjusted HR 1.54 [95% CI: 1.01–2.33]) were associated with a higher risk of incident AVC. hsCRP was not associated with an increased risk of incident AVC when assessed as a continuous (unadjusted HR 1.05 [95% CI 0.97–1.14], adjusted HR 1.01 [95% CI 0.92–1.11]) or categorical variable (unadjusted HR 1.13 [95% CI 0.93–1.37), adjusted HR 1.08 [95% CI 0.88–1.32]).
When assessed as a continuous variable, Lp(a) was associated with an increased risk of incident AVC when hsCRP ≥2 mg/L (unadjusted HR 1.21 [95% CI 1.06–1.38, adjusted HR 1.24 [95% CI: 1.06–1.43]), but not when hsCRP <2 mg/L (unadjusted HR 1.10 [95% CI 0.97–1.25), adjusted HR 1.12 [95% CI 0.97–1.29]). There was a stepwise increase in the hazard of incident AVC with higher Lp(a) levels when hsCRP ≥2 mg/L, but not when hsCRP <2 mg/L (Figure). Compared to participants with hsCRP <2 mg/dL and Lp(a) <50 mg/dL, those with both elevated Lp(a) ≥50 mg/dL and elevated hsCRP ≥2 mg/L had a nearly two-fold increased risk for incident AVC (unadjusted HR 1.74 [95% CI 1.43–2.10], adjusted HR 1.83 [95% CI: 1.32–2.54]). The addition of Lp(a) to a model with tradition risk factors and hsCRP resulted in a non-significant 0.0046 change in the ROC for predicting incident AVC (p=0.15). Interaction testing between hsCRP and Lp(a) as continuous variables for incident AVC was not significant (p=0.29), but categorical interaction testing between hsCRP and Lp(a) (<100 mg/L or ≥100 mg/dL) was significant (p = 0.064). The overall results showed similar findings when examined by race/ethnicity.
Figure:
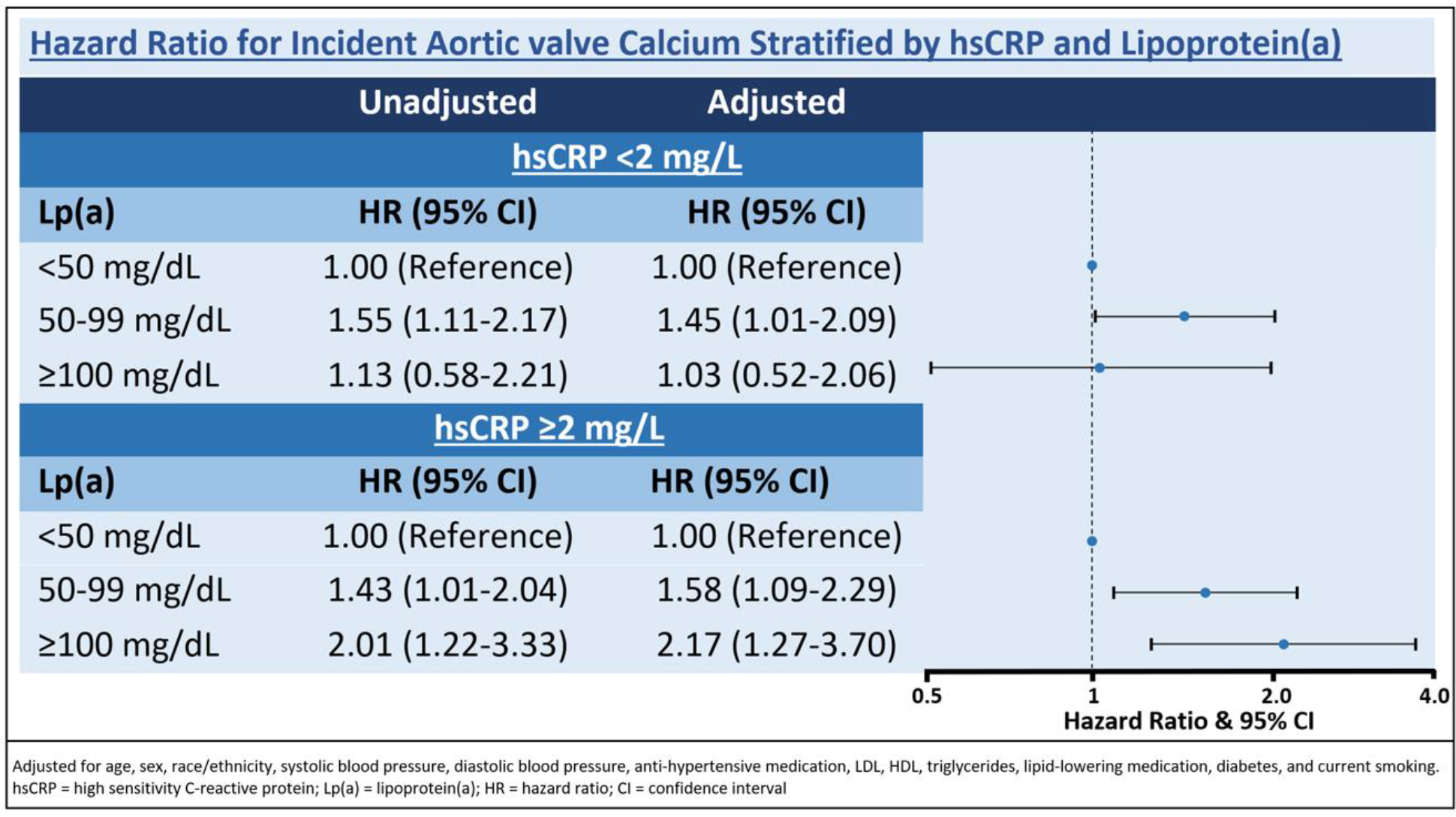
Hazard ratio for incident aortic valve calcium stratified by hsCRP and lipoprotein(a)
The β coefficient for annualized AVC progression was lowest for participants with hsCRP <2 mg/dL and Lp(a) <50mg/dL and highest when hsCRP ≥2 mg/L and Lp(a) >50mg/dL, but the results were not statistically significant for either group (p=0.60 and p=0.27).
Our finding that participants with both elevated hsCRP and elevated Lp(a) have a nearly two-fold increased risk of developing AVC is in keeping with a prior MESA analysis that found a significant interaction between eleavted Lp(a) and hsCRP with an associated 1.6-fold increased risk of incident ASCVD.1
While there is no specific screening or risk stratification algorithm to identify the individuals most likely to develop AVC or AS, our findings may be helpful in identifying persons at high risk for AS who may be of potential interest for future trials examining novel Lp(a) reduction therapies to slow or prevent AS.
One limitation of this study is that we did not examine changes in hsCRP and Lp(a) levels over time, although it has been reported that Lp(a) levels remain stable time 4.
In conclusion, there was no significant association of hsCRP with prevalent or incident AVC. Higher Lp(a) levels were independently associated with AVC and participants with both an elevated hsCRP and elevated Lp(a) had the greatest risk for incident AVC. Further research is needed to determine whether this phenotype may be useful to identify persons at increased risk of AVC who may benefit from novel treatments and/or early reduction of their atherogenic lipid burden.
Acknowledgments
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, and R01 HL071739 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding:
Seamus Whelton was supported by a NIH R21 grant HL150458-01A1.
Footnotes
Conflict of Interest Statement: The authors have no relevant conflicts of interests and relationships with industry to disclose.
References
- 1.Zhang W, Speiser JL, Ye F, et al. High-sensitivity C-reactive protein modifies the cardiovascular risk of lipoprotein(a): Multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2021;78(11):1083–1094. doi: S0735-1097(21)05693-X [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz Gregory G, Michael S, Andreas Z, et al. Elevated C-reactive protein amplifies association of lipoprotein(a) with cardiovascular risk and clinical benefit of alirocumab. J Am Coll Cardiol. 2022;80(24):2356–2359. 10.1016/j.jacc.2022.09.035. doi: 10.1016/j.jacc.2022.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Takasu J, Katz R, et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13(2):166–172. doi: S1076-6332(05)00812-3 [pii]. [DOI] [PubMed] [Google Scholar]
- 4.Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): Impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016;57(7):1111–1125. https://pubmed.ncbi.nlm.nih.gov/26637279 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4918859/. doi: 10.1194/jlr.R051904. [DOI] [PMC free article] [PubMed] [Google Scholar]


