Abstract
Background and Objectives
EEG and MRI features are independently associated with pediatric cardiac arrest (CA) outcomes, but it is unclear whether their combination improves outcome prediction. We aimed to assess the association of early EEG background category with MRI ischemia after pediatric CA and determine whether addition of MRI ischemia to EEG background features and clinical variables improves short-term outcome prediction.
Methods
This was a single-center retrospective cohort study of pediatric CA with EEG initiated ≤24 hours and MRI obtained ≤7 days of return of spontaneous circulation. Initial EEG background was categorized as normal, slow/disorganized, discontinuous/burst-suppression, or attenuated-featureless. MRI ischemia was defined as percentage of brain tissue with apparent diffusion coefficient (ADC) <650 × 10−6 mm2/s and categorized as high (≥10%) or low (<10%). Outcomes were mortality and unfavorable neurologic outcome (Pediatric Cerebral Performance Category increase ≥1 from baseline resulting in ICU discharge score ≥3). The Kruskal-Wallis test evaluated the association of EEG with MRI. Area under the receiver operating characteristic (AUROC) curve evaluated predictive accuracy. Logistic regression and likelihood ratio tests assessed multivariable outcome prediction.
Results
We evaluated 90 individuals. EEG background was normal in 16 (18%), slow/disorganized in 42 (47%), discontinuous/burst-suppressed in 12 (13%), and attenuated-featureless in 20 (22%) individuals. The median percentage of MRI ischemia was 5% (interquartile range 1–18); 32 (36%) individuals had high MRI ischemia burden. Twenty-eight (31%) individuals died, and 58 (64%) had unfavorable neurologic outcome. Worse EEG background category was associated with more MRI ischemia (p < 0.001). The combination of EEG background and MRI ischemia burden had higher predictive accuracy than EEG alone (AUROC: mortality: 0.92 vs 0.87, p = 0.03) or MRI alone (AUROC: mortality: 0.92 vs 0.84, p = 0.02; unfavorable: 0.83 vs 0.73, p < 0.01). Addition of percentage of MRI ischemia to clinical variables and EEG background category improved prediction for mortality (χ2 = 19.1, p < 0.001) and unfavorable neurologic outcome (χ2 = 4.8, p = 0.03) and achieved high predictive accuracy (AUROC: mortality: 0.97; unfavorable: 0.92).
Discussion
Early EEG background category was associated with MRI ischemia after pediatric CA. Combining EEG and MRI data yielded higher outcome predictive accuracy than either modality alone. The addition of MRI ischemia to clinical variables and EEG background improved short-term outcome prediction.
Introduction
Cardiac arrest (CA) occurs in more than 22,000 children each year in the United States.1,2 Survival rates have improved over time, with approximately 11% of children with out-of-hospital CA (OHCA) and 42% of children with in-hospital CA (IHCA) surviving to hospital discharge.1,3 However, due to hypoxic-ischemic brain injury (HIBI), up to 84%–91% of survivors have an unfavorable outcome at hospital discharge.1
EEG assesses brain activity, can be performed noninvasively at bedside, and is often available early after CA.4 Interrater agreement is high for key EEG patterns after CA.5 Severely abnormal EEG background patterns (e.g., attenuation, burst-suppression, and discontinuity) and lack of reactivity are associated with unfavorable neurologic outcome, while more normal EEG background patterns (e.g., slow and disorganized but continuous), presence of reactivity, and normal sleep spindles are associated with favorable neurologic outcome.6-8 EEG background features in the first day after CA are associated with outcome.7
Diffusion-weighted MRI can quantify HIBI after CA and is often performed 3–7 days after CA, before pseudo-normalization of apparent diffusion coefficient (ADC) signal. Lower whole-brain ADC, indicating more ischemia, is associated with worse outcomes in both adults and children.9-13 Children with high ischemia burden, defined as ADC <650 × 10−6 mm2/s in ≥10% of brain tissue, have significantly higher odds of unfavorable neurologic outcome compared with children with low ischemia burden, defined as ADC <650 × 10−6 mm2/s in <10% of brain tissue.14
A multimodal approach to neuroprognostication may be more accurate than reliance on a single modality. We previously demonstrated that combining EEG background patterns with clinical variables improved prognostic accuracy.15 However, limited studies have evaluated whether combining EEG and MRI improves outcome prediction after pediatric CA.16 This study had 3 aims. First, we aimed to assess the association of early EEG background with the percentage of MRI ischemia after pediatric CA. Second, we aimed to determine whether the combination of EEG background and MRI ischemia burden had higher predictive accuracy for outcomes than either modality alone. Third, we aimed to determine whether addition of percentage of MRI ischemia to EEG background and clinical factors had higher predictive accuracy for outcomes than EEG background and clinical factors alone.
Methods
This was a single-center retrospective observational cohort study (January 2005–December 2019) at the Children's Hospital of Philadelphia. We included all patients who were (1) younger than 18 years; (2) experienced IHCA or OHCA; (3) received post-CA care in the pediatric intensive care unit (PICU); and (4) had a clinical MRI obtained ≤7 days of return of spontaneous circulation (ROSC). We excluded patients (1) with concomitant traumatic brain injury, brain tumor, intracranial hemorrhage, meningoencephalitis, metabolic disease, and severe baseline brain atrophy; (2) with a baseline Pediatric Cerebral Performance Category (PCPC) score of 5 (vegetative state or coma), as in a previous analysis9; (3) who did not have continuous EEG (cEEG) monitoring initiated within 24 hours of ROSC; and (4) who did not have interpretable MRI scans.
Data were abstracted from the medical record and stored using Research Electronic Data Capture (Vanderbilt University, Nashville, TN).17,18 Data consisted of prospectively defined demographic, CA, post-CA care, EEG, MRI, and outcome variables. Post-CA care was determined by the clinical team, guided by an institutional post-CA clinical pathway.19 Some patients received therapeutic hypothermia to 33.0°C. Standard post-CA management did not include routine administration of prophylactic antiseizure medications, but convulsive and nonconvulsive seizures were generally treated. Benzodiazepine infusions were often administered for sedation.
cEEG was initiated per routine clinical care in patients who remained encephalopathic or did not return to their neurologic baseline after ROSC.20,21 cEEG was performed with portable Grass-Telefactor or Natus (Middleton, WI) video equipment with electrodes positioned according to the international 10-20 system. For this study, standardized EEG scoring was performed independently by electroencephalographers blinded to clinical data using EEG variable definitions previously published for this dataset4 and an EEG categorization system used in previous critical care EEG studies.7,15,22,23 EEG background was analyzed during initiation of EEG, within 24 hours of ROSC. The initial EEG background was categorized as normal, slow/disorganized, discontinuous/burst-suppression, or attenuated-featureless.24 The EEG was also assessed for seizures. Some of the EEG data from this cohort have been previously reported.4-6,15,25
Brain MRI scans were obtained on either a 1.5T or 3T Siemens scanner (Erlangen, Germany) within 7 days of ROSC per usual clinical care. All studies included T1, T2, FLAIR, and diffusion-weighted images. MRI analysis was performed using FMRIB software library.26 MRI processing was conducted by investigators blinded to clinical data including outcome assessment. We calculated whole-brain mean ADC and determined the percentage of voxels with ADC <650 × 10−6 mm2/s. Percentage of MRI ischemia was defined as the percentage of brain tissue with ADC <650 × 10−6 mm2/s and analyzed as a continuous variable. For some analyses, MRI ischemia burden was categorized as high (≥10% of brain tissue with ADC <650 × 10−6 mm2/s) vs low (<10% of brain tissue with ADC <650 × 10−6 mm2/s). The threshold of 10% was chosen based on adult11,12 and pediatric14 data that having MRI ADC below 650 × 10−6 mm2/s in >10% of brain tissue after CA was associated with unfavorable outcome. MRI data from this cohort have been previously reported.9,14
The primary outcome was mortality at PICU discharge, and the secondary outcome was unfavorable neurologic outcome assessed by PCPC score at PICU discharge. The PCPC is a validated 6-point scale of global neurologic function.27 The prearrest PCPC score was assigned based on information provided by guardians or based on review of prior medical visits included in the electronic medical record. The PCPC was scored independently by trained raters who were blinded to MRI, EEG, and outcome data. A favorable outcome was defined as discharge PCPC of 1, 2, or no change in PCPC score from the prearrest PCPC score. An unfavorable outcome was defined as a change in PCPC score of ≥1 that resulted in a PICU discharge PCPC score ≥3.9,15,28,29
Statistical Analysis
Descriptive statistics are reported as frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. We assessed differences in patients' demographic, CA, post-CA care, and outcome variables across EEG background category groups using ANOVA or Kruskal-Wallis tests for continuous variables and χ2 or Fisher exact tests for categorical variables where appropriate. Similarly, we assessed differences in these variables between the 2 MRI ischemia burden categories using 2-sample t tests or Wilcoxon rank sum tests for continuous variables and χ2 or Fisher exact tests for categorical variables.
The Kruskal-Wallis rank sum and pairwise Kruskal-Wallis tests assessed the association of EEG background category with percentage of MRI ischemia. Predictive accuracies of EEG background category and MRI ischemia burden for outcomes were determined by calculating the area under the receiver operating characteristic (AUROC) curve and compared with each other. Sensitivity, specificity, positive predictive value, and negative predictive value of EEG background category and MRI ischemia burden for outcomes were calculated. For the purposes of this calculation, EEG background patterns were dichotomized as severely abnormal (discontinuous/burst-suppression or attenuated-featureless) and normal to mildly abnormal (normal or slow/discontinuous).
Multivariable logistic regression was used to assess the association of EEG background category, percentage of MRI ischemia, and clinical variables with each outcome. Our base logistic regression model included EEG background category and the clinical variables previously identified in a clinically derived prediction model of outcome in this population: witnessed status of the arrest, number of epinephrine doses administered during resuscitation, and post-ROSC lactate.15 The likelihood ratio χ2 test was used to determine whether the addition of percentage of MRI ischemia to this base model improved model performance. Predictive accuracy was determined by calculating the AUROC. All statistical analyses were performed using R Statistical software (version 4.2.3; R Foundation, Vienna, Austria) and STATA software version 17 (StataCorp, College Station TX).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Children's Hospital of Philadelphia Institutional Review Board. Written informed patient consent was not required.
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
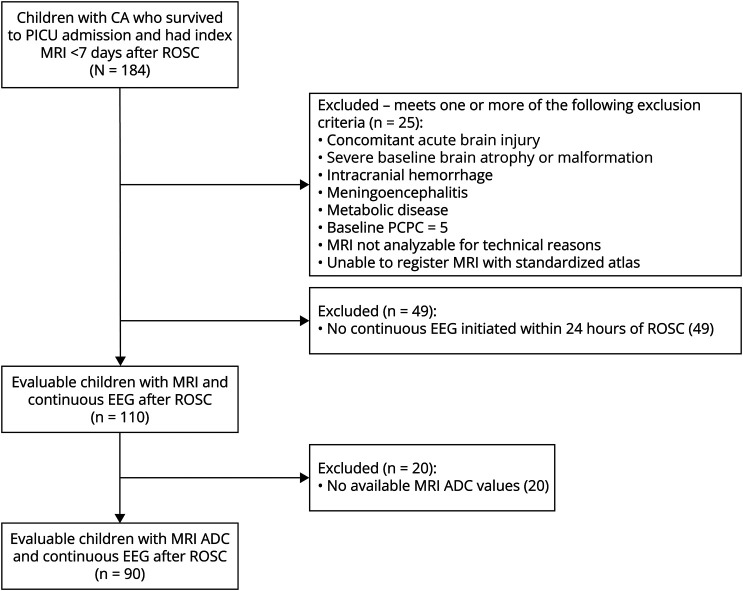
Ninety individuals were evaluated (Figure 1). The median age was 1.6 (IQR 0.6–5.8) years. Twenty-eight (31%) individuals were female. CA occurred out of hospital in 76 (84%) individuals of which 31 (41%) were witnessed. The median number of epinephrine doses received during cardiopulmonary resuscitation (CPR) was 2 (IQR 0–3), and the median initial lactate after ROSC was 4.2 mmol/L (IQR 2.2–8) (Table 1).
Figure 1. CONSORT Diagram.
ADC = apparent diffusion coefficient; CA = cardiac arrest; CONSORT = Consolidated Standards of Reporting Trials; PCPC = Pediatric Cerebral Performance Category; PICU = pediatric intensive care unit; ROSC = return of spontaneous circulation.
Table 1.
Patient Demographics, Cardiac Arrest, and Resuscitation Characteristics, EEG Characteristics, MRI Ischemia Burden, by Outcome (Mortality and Neurologic Outcome)
| Characteristic | Overall (N = 90) | Mortality | Neurologic outcome | ||||
| Alive (N = 62) | Dead (N = 28) | p Value | Favorable (N = 32) | Unfavorable (N = 58) | p Value | ||
| Patient demographics | |||||||
| Age, y | 1.6 (0.6–5.8) | 1.5 (0.5–4.9) | 2.6 (0.6–8.0) | 0.4 | 1.4 (0.6–5.3) | 2.0 (0.6–6) | 1.0 |
| Female | 28 (31) | 19 (31) | 9 (32) | 0.9 | 13 (41) | 15 (26) | 0.2 |
| Race | 0.1 | 0.3 | |||||
| White | 40 (44) | 30 (48) | 10 (36) | 16 (50) | 24 (41) | ||
| Black | 25 (28) | 18 (29) | 7 (25) | 11 (34) | 14 (24) | ||
| Asian | 2 (2) | 0 (0) | 2 (7) | 0 (0) | 2 (3) | ||
| Other | 23 (26) | 14 (23) | 9 (32) | 5 (16) | 18 (31) | ||
| Hispanic (N = 89) | 9 (10) | 5 (8) | 4 (14) | 0.5 | 1 (3) | 8 (14) | 0.2 |
| PCPC score prearrest | 0.3 | 0.7 | |||||
| 1 (normal) | 66 (73) | 44 (71) | 22 (79) | 24 (75) | 42 (72) | ||
| 2 (mild disability) | 7 (8) | 6 (10) | 1 (4) | 2 (6) | 5 (9) | ||
| 3 (moderate disability) | 9 (10) | 8 (13) | 1 (4) | 2 (6) | 7 (12) | ||
| 4 (severe disability) | 8 (9) | 4 (7) | 4 (14) | 4 (12) | 4 (7) | ||
| Any preexisting condition | 35 (39) | 25 (40) | 10 (36) | 0.7 | 14 (44) | 21 (36) | 0.5 |
| Former premature neonate | 16 (18) | 14 (23) | 2 (7) | 0.1 | 8 (25) | 8 (14) | 0.2 |
| Chronic lung disease | 13 (14) | 9 (15) | 4 (14) | 1.0 | 5 (16) | 8 (14) | 0.8 |
| Congenital heart disease | 4 (4) | 3 (5) | 1 (4) | 1.0 | 0 (0) | 4 (7) | 0.3 |
| Epilepsy | 8 (9) | 4 (7) | 4 (14) | 0.2 | 4 (12) | 4 (7) | 0.4 |
| Neuromuscular disease | 6 (7) | 3 (5) | 3 (11) | 0.4 | 3 (9) | 3 (5) | 0.7 |
| Cardiac arrest and resuscitation characteristics | |||||||
| Out-of-hospital arrest | 76 (84) | 51 (82) | 25 (89) | 0.4 | 25 (78) | 51 (88) | 0.2 |
| Witnessed arrest out-of-hospital arrest (N = 75) | 31 (41) | 26 (51) | 5 (21) | 0.01 | 13 (52) | 18 (36) | 0.2 |
| Bystander CPR for out-of-hospital arrest (N = 75) | 46 (61) | 31 (61) | 15 (62) | 0.9 | 18 (72) | 28 (56) | 0.2 |
| Arrest cause | |||||||
| ALTE/SIDS | 5 (6) | 2 (3) | 3 (11) | 0.2 | 0 (0) | 5 (9) | 0.2 |
| Drowning | 18 (20) | 11 (18) | 7 (25) | 0.4 | 4 (12) | 14 (24) | 0.2 |
| Respiratory failure | 31 (34) | 21 (34) | 10 (36) | 0.9 | 11 (34) | 20 (34) | 1.0 |
| Arrhythmia | 2 (2) | 2 (3) | 0 (0) | 1.0 | 2 (6) | 0 (0) | 0.1 |
| Hypotension/shock/sepsis | 6 (7) | 6 (10) | 0 (0) | 0.2 | 4 (12) | 2 (3) | 0.2 |
| Trauma | 11 (12) | 8 (13) | 3 (11) | 1.0 | 2 (6) | 9 (16) | 0.3 |
| Seizures | 4 (4) | 2 (3) | 2 (7) | 0.6 | 2 (6) | 2 (3) | 0.6 |
| Toxin ingestion | 8 (9) | 6 (10) | 2 (7) | 1.0 | 6 (19) | 2 (3) | 0.02 |
| Unknown | 10 (11) | 7 (11) | 3 (11) | 1.0 | 4 (12) | 6 (10) | 0.7 |
| First documented rhythm | 0.03 | <0.01 | |||||
| PEA/asystole | 46 (51) | 25 (40) | 21 (75) | 8 (25) | 38 (66) | ||
| Bradycardia | 11 (12) | 10 (16) | 1 (4) | 5 (16) | 6 (10) | ||
| Ventricular fibrillation | 4 (4) | 4 (7) | 0 (0) | 4 (12) | 0 (0) | ||
| Ventricular tachycardia | 1 (1) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | ||
| Unknown | 28 (31) | 22 (35) | 6 (21) | 14 (44) | 14 (24) | ||
| CPR duration in minutes (N = 75) | 14 (5–30) | 8 (3.5–20) | 30 (15–40) | <0.01 | 4.5 (2–6) | 20 (10–30) | <0.01 |
| No. of epinephrine doses (N = 87) | 2 (0–3) | 0 (0–3) | 4 (2–5) | <0.01 | 0 (0–0) | 3 (1–4) | <0.01 |
| Intubated (N = 87) | 63 (72) | 41 (66) | 22 (88) | 0.04 | 19 (59) | 44 (80) | 0.04 |
| Initial lactate (N = 87) | 4.2 (2.2–8) | 3.1 (1.6–6.5) | 6.6 (3.8–9.7) | <0.01 | 2.2 (1.2–3.4) | 5.8 (3.3–9.8) | <0.01 |
| Therapeutic hypothermia | 10 (11) | 6 (10) | 4 (14) | 0.5 | 3 (9) | 7 (12) | 1.0 |
| Benzodiazepine infusion (N = 63) | 44 (70) | 32 (74) | 12 (60) | 0.2 | 15 (68) | 29 (71) | 0.8 |
| EEG characteristics | |||||||
| EEG background category | <0.01 | <0.01 | |||||
| Normal | 16 (18) | 16 (26) | 0 (0) | 12 (38) | 4 (7) | ||
| Slow/disorganized | 42 (47) | 37 (60) | 5 (18) | 18 (56) | 24 (41) | ||
| Discontinuous/burst-suppression | 12 (13) | 4 (6) | 8 (29) | 1 (3) | 11 (19) | ||
| Attenuated/featureless | 20 (22) | 5 (8) | 15 (54) | 1 (3) | 19 (33) | ||
| EEG seizures | 25 (28) | 14 (23) | 11 (39) | 0.1 | 3 (9) | 22 (38) | <0.01 |
| MRI ischemia | |||||||
| Percentage of MRI ischemia | 5 (1–18) | 1.6 (0.3–6) | 33 (13–65) | <0.01 | 0.5 (0.1–3.7) | 11 (2.3–35) | <0.01 |
| High MRI ischemia burden | 32 (36) | 9 (15) | 23 (82) | <0.01 | 2 (6) | 30 (52) | <0.01 |
| Disposition | |||||||
| Disposition (N = 61) | <0.01 | ||||||
| Chronic care facility | 8 (13) | 8 (13) | 0 | 2 (6.2) | 6 (21) | ||
| Home | 33 (54) | 33 (54) | 0 | 26 (81) | 7 (24) | ||
| Rehabilitation | 20 (33) | 20 (33) | 0 | 4 (12) | 16 (55) | ||
Abbreviations: ADC = apparent diffusion coefficient; ALTE = apparent life-threatening event; CPR = cardiopulmonary resuscitation; IQR = interquartile range; PEA = pulseless electrical activity; ROSC = return of spontaneous circulation; SIDS = sudden infant death syndrome.
Data are presented as n (%) or median (IQR). High MRI ischemia burden defined as ≥10% of brain tissue with ADC <650 × 10−6 mm2/s.
The initial EEG background category was normal in 16 (18%), slow/disorganized in 42 (47%), discontinuous/burst-suppression in 12 (13%), and attenuated-featureless in 20 (22%). Electrographic seizures occurred in 25 (28%) (Table 1). Univariate analyses indicated that worse EEG background category was associated with mortality (odds ratio [OR] 5.5 [2.8–10.7]; p < 0.001) and unfavorable neurologic outcome (OR 4.4 [2.1–9.1]; p < 0.001).
Brain MRI was performed at a median of 3 (IQR 2–5) days after CA. The median percentage of MRI ischemia was 5% (IQR 1–18). Thirty-two individuals (36%) had high MRI ischemia burden (Table 1). On univariate analyses, high MRI ischemia burden was associated with mortality (OR 26.6 [8.0–88.0]; p < 0.001) and unfavorable neurologic outcome (OR 15.5 [3.4–71.2]; p < 0.001).
Twenty-eight (31%) individuals died. The cause of death was withdrawal of life-sustaining therapy (WLST) in 15 (54%), brain death in 12 (43%), and rearrest without ROSC in 1 (4%) (eTable 1, links.lww.com/WNL/D405). Mortality was associated with witnessed status, initial documented rhythm, CPR duration, number of epinephrine doses, intubation, and initial lactate after ROSC (Table 1). Fifty-eight (64%) individuals had an unfavorable neurologic outcome at ICU discharge. Unfavorable neurologic outcome was associated with first documented rhythm, CPR duration, number of epinephrine doses, intubation, initial lactate after ROSC, presence of seizures, and discharge disposition (Table 1).
Association of Early EEG Background Category With Brain MRI Ischemia
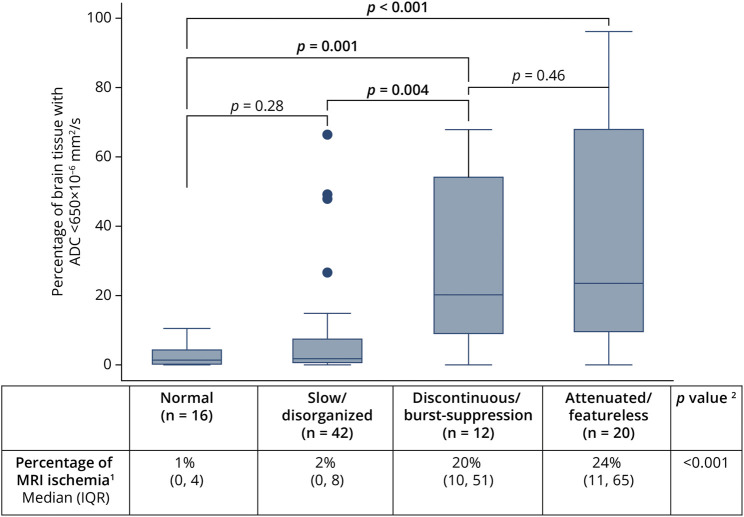
Worsening EEG background category was associated with higher percentage of MRI ischemia (p < 0.001) (Figure 2). Notably, the percentage of MRI ischemia was significantly higher among individuals with discontinuous/burst-suppressed and attenuated-featureless EEG background than among individuals with normal and slow/disorganized EEG background (Figure 2). Individuals with worse EEG background categories more frequently had high MRI ischemia burden: normal (1/16; 6.3%), slow/disorganized (7/42; 17%), discontinuous/burst suppression (9/12; 75%), and attenuated-featureless (15/20; 75%).
Figure 2. Association of EEG Background Category With Percentage of MRI Ischemia (Percentage of Brain Tissue With ADC <650 × 10−6 mm2/s).
1Percentage of brain tissue with ADC <650 × 10−6 mm2/s. 2Kruskal-Wallis rank sum test; pairwise Kruskal-Wallis tests used to compare EEG background category groups as depicted in graph. ADC = apparent diffusion coefficient; IQR = interquartile range.
EEG and MRI results were discordant in 16 of 90 (18%) individuals. Eight individuals had normal or slow/disorganized EEG background category but high MRI ischemia burden. Among those 8 individuals, 7 had unfavorable neurologic outcome, including death in 4 individuals. A different 8 individuals had discontinuous/burst-suppression or attenuated-featureless EEG background category but low MRI ischemia burden. Among those 8 individuals, 7 had unfavorable neurologic outcome, including death in 4 individuals (eTable 2, links.lww.com/WNL/D405).
Predictive Accuracy of Early EEG Background and MRI Ischemia Burden for Outcomes
The combination of EEG background category and MRI ischemia burden had higher predictive accuracy than EEG alone for mortality (AUROC: 0.92 vs 0.87, p = 0.03), but not for unfavorable neurologic outcome (AUROC: 0.83 vs 0.79, p = 0.07), and higher predictive accuracy than MRI ischemia burden alone for mortality (AUROC: 0.92 vs 0.84, p = 0.02) and unfavorable neurologic outcome (AUROC 0.83 vs 0.73, p < 0.01) (Table 2). There was no difference in predictive accuracy between EEG background category and MRI ischemia burden for mortality (AUROC EEG 0.87 vs MRI 0.84, p = 0.58) or unfavorable neurologic outcome (AUROC EEG 0.79 vs MRI 0.73, p = 0.19). EEG background category alone and MRI ischemia burden alone had equivalent sensitivity and specificity for mortality and unfavorable neurologic outcome (eTable 3, links.lww.com/WNL/D405).
Table 2.
Predictive Accuracies of Early EEG Background Category, MRI Ischemia Burden, and Their Combination for Outcomes
| AUROC | p Value | |
| Mortality | ||
| EEG background category | 0.87 | 0.03* |
| EEG background category and high vs low MRI ischemia | 0.92 | |
| High vs low MRI ischemia | 0.84 | 0.02* |
| EEG background category and high vs low MRI ischemia | 0.92 | |
| Unfavorable neurologic outcome | ||
| EEG background category | 0.79 | 0.07 |
| EEG background category and high vs low MRI ischemia | 0.83 | |
| High vs low MRI ischemia | 0.73 | <0.01* |
| EEG background category and high vs low MRI ischemia | 0.83 |
Abbreviations: ADC = apparent diffusion coefficient; AUROC = area under receiver operating characteristic.
EEG background category: normal, slow/disorganized, discontinuous/burst-suppression, or attenuated-featureless. High vs low MRI ischemia: ADC <650 × 10−6 mm2/s in ≥10% vs <10% of brain voxels.
* p Value achieves statistical significance (<0.05).
Impact of Adding MRI to a Predictive Model of Clinical Features and Early EEG Background on Outcome Prediction
The addition of percentage of MRI ischemia to our previously published model of clinical variables (witnessed status, number of epinephrine doses administered during resuscitation, and post-ROSC lactate) and EEG background category significantly improved prediction for mortality (χ2 = 19.1, p < 0.001) and unfavorable neurologic outcome (χ2 = 4.8, p = 0.03) (Table 3). With the inclusion of percentage of MRI ischemia, these predictive models achieved high predictive accuracies for mortality (AUROC: 0.97; sensitivity: 0.42, specificity 1.0) and unfavorable neurologic outcome (AUROC: 0.92; sensitivity: 0.85, specificity 0.83). Predictive accuracy for unfavorable neurologic outcome was lower when analyzed in only survivors (eTable 4, links.lww.com/WNL/D405).
Table 3.
Comparison of Predictive Models With and Without Percentage of MRI Ischemia for Outcomes
| OR | 95% CI | p Value | AUROC | Likelihood ratio (χ2) | p Value | |
| Unfavorable neurologic outcome | ||||||
| Clinical and EEG model (N = 83) | 0.902 | 4.82 | 0.028* | |||
| Covariates | ||||||
| Witnessed arrest | 0.29 | 0.07–1.15 | 0.078 | |||
| Lactate | 1.12 | 0.96–1.32 | 0.146 | |||
| No. of epi doses (continuous) | 2.19 | 1.29–3.73 | 0.004* | |||
| Initial EEG (continuous) | 2.29 | 0.81–6.46 | 0.117 | |||
| Clinical, EEG and MRI model (N = 83) | 0.919 | |||||
| Covariates | ||||||
| Witnessed arrest | 0.28 | 0.07–1.19 | 0.085 | |||
| Lactate | 1.11 | 0.97–1.29 | 0.139 | |||
| No. of epi doses (continuous) | 2.11 | 1.23–3.61 | 0.007* | |||
| Initial EEG (continuous) | 1.58 | 0.55–4.48 | 0.392 | |||
| Percentage of MRI ischemia (continuous) | 1.11 | 0.97–1.26 | 0.122 | |||
| Mortality | ||||||
| Clinical and EEG model (N = 83) | 0.924 | 19.09 | <0.001* | |||
| Covariates | ||||||
| Witnessed arrest | 0.12 | 0.02–0.61 | 0.010* | |||
| Lactate | 1.01 | 0.86–1.19 | 0.887 | |||
| No. of epi doses (continuous) | 1.34 | 0.93–1.93 | 0.121 | |||
| Initial EEG (continuous) | 6.20 | 2.26–17.03 | <0.001* | |||
| Clinical, EEG and MRI model (N = 83) | 0.971 | |||||
| Covariates | ||||||
| Witnessed arrest | 0.12 | 0.02–0.81 | 0.029* | |||
| Lactate | 1.00 | 0.81–1.24 | 0.981 | |||
| No. of epi doses (continuous) | 1.34 | 0.88–2.02 | 0.168 | |||
| Initial EEG (continuous) | 4.65 | 1.41–15.29 | 0.012* | |||
| Percentage of MRI ischemia (continuous) | 1.11 | 1.03–1.19 | 0.004* |
Abbreviations: AUROC = area under receiver operating characteristic; OR = odds ratio.
* p Value achieves statistical significance (<0.05).
Discussion
In this single-center, retrospective, observational study of children resuscitated from CA, more severe initial EEG background categories were strongly associated with a higher percentage of brain MRI ischemia. This reflects an association of early electrical brain dysfunction with cellular injury on later neuroimaging findings. Although EEG and MRI measures were correlated, each modality was observed to separately contribute to the outcome model. The combination of EEG background category and MRI ischemia burden yielded higher predictive accuracy for both mortality and unfavorable neurologic outcome than either modality alone. Finally, addition of percentage of MRI ischemia to a model of readily available clinical variables and EEG background category improved short-term outcome prediction, achieving high predictive accuracy of 0.97 for mortality and 0.92 for unfavorable neurologic outcome at PICU discharge. This finding supports improved accuracy of multimodal prediction of outcomes from pediatric CA.
Consistent with the adult literature, we found that early EEG background features were associated with the burden of diffusion restriction on MRI.30 Specifically, severely abnormal EEG background category (discontinuous/burst-suppressed or attenuated-featureless) was associated with higher percentage of MRI ischemia.30,31 The pathologic EEG and MRI findings are likely consequences of the same hypoxic-ischemic cellular injury, which initially leads to Na/K ion exchange pump dysfunction and abnormal electrical activity detected by EEG and subsequently produces cytotoxic edema detected by MRI.32 Clinically, their close association indicates early EEG may inform estimates of the amount of ischemia likely to be seen on subsequent MRI.
While we found a strong association of early EEG background category with MRI ischemia burden, 16 (18%) individuals had discordant EEG background category and MRI ischemia burden. Among children with only 1 modality reflecting severe abnormality, 50% died and 88% had unfavorable neurologic outcome. These findings highlight that these 2 modalities assess different aspects of brain injury; EEG detects cortical activity while MRI assesses the whole brain. Furthermore, they were assessed at different time points in care. EEG was obtained early after CA, and patients with initially presumed severe injury may have improved with targeted postarrest care such as targeted temperature management, prevention of hypotension, and seizure control. Similarly, patients with an early reassuring EEG may have sustained secondary brain ischemia from myocardial dysfunction or respiratory failure. Thus, the discrepancy in these patients may not only reflect differences in injury detection but also the evolution of brain injury after CA, which may be modified by post-CA care. Furthermore, patients may die from progression of underlying disease (e.g., cancer or refractory shock), regardless of EEG and MRI findings.
Clinically, severe abnormality of either EEG or MRI is typically perceived as being associated with an unfavorable outcome. Children with a high percentage of MRI ischemia had high rates of unfavorable outcome and death regardless of benign early EEG background findings. Our data suggest that patients with normal to mildly abnormal early EEG background do not necessarily have favorable outcome,7 and outcome prediction can be enhanced by combining EEG background with MRI ischemia burden. This finding aligns with a recent study of adults that found MRI to be complementary with EEG for prediction of post-CA outcome.33 Conversely, among children with severely abnormal early EEG background, the rate of death and unfavorable neurologic outcome was high regardless of MRI ischemia burden. Thus, when there is a mismatch between EEG background severity and MRI ischemia burden, concern for unfavorable neurologic outcome should remain, and clinicians should continue to rely on clinical examination and time for recovery to determine outcomes.
Previous studies have found that EEG background and MRI ischemia burden are independently associated with unfavorable outcome after CA.14,16,29,34 EEG is rapidly available at many institutions early after CA and provides clinicians with real-time assessment of cerebral dysfunction in patients with post-CA encephalopathy. cEEG or routine EEG may be used to assess initial EEG background within 24 hours of ROSC. Unlike EEG, MRI is not easily obtained and the full scope of HIBI may not be evident within the first 48 hours following CA due to ongoing secondary brain injury. MRI is usually obtained after the completion of post-CA care to delineate tissue injury and assist with prognostication. In our cohort, early EEG background category and MRI ischemia burden yielded similar predictive accuracies for outcomes, and combining modalities significantly increased predictive accuracy for outcomes. Our combined predictive accuracies differ slightly from those of a previous study,16 likely due to different definitions of MRI features and outcomes. Our finding supports that EEG and MRI detect different aspects of injury at different time points in post-CA treatment and are complementary tools in outcome prediction.
Finally, we found that the addition of the percentage of MRI ischemia to clinical variables and early EEG background category improved short-term neurologic outcome prediction. The most robust and parsimonious clinically derived prediction model of short-term neurologic outcome after pediatric CA was previously defined and includes clinical variables (witnessed status, number of epinephrine doses administered, and post-ROSC lactate) and early EEG background category.15 This model yielded a predictive accuracy of 0.90 for unfavorable neurologic outcome and 0.83 for death.15 In this study, with the addition of percentage of MRI ischemia, the model achieved higher predictive accuracy of 0.92 for short-term unfavorable neurologic outcome and 0.97 for death. The independent contributions of EEG and MRI to a multimodal model of outcome support that they measure different aspects of brain injury. The multimodal model achieves a sensitivity for unfavorable neurologic outcome of 0.85, exceeding the sensitivity of EEG background alone or MRI ischemia burden alone (both 0.52). Clinically, this allows for increased identification of children who will have an unfavorable neurologic outcome, informs counseling of families as information becomes sequentially available from multiple modalities, and supports that decisions made with multimodal data several days after ROSC are more accurate than with early modalities alone. Several studies have supported the association of MRI ischemia burden with outcomes after CA and advocated for its incorporation into multimodal prognostication.13,34,35 Our results build upon these studies and prior MRI ischemia definitions to support the added benefit of quantitative diffusion-weighted neuroimaging for multimodal prognostication after pediatric CA.14
The strength of this study is that it used multiple diagnostic modalities over the first week following CA to predict outcomes in a mixed cohort of patients with IHCA and OHCA. The timing of MRI after ROSC is important for assessment of ischemia burden, given the natural evolution of ADC values with time after hypoxic ischemic brain injury. The children in our cohort had imaging performed at a median of 3 (IQR 2–5) days post-ROSC, which is timed before pseudo-normalization of ADC signal. However, there are several limitations to this study. This was a single-center retrospective study in which outcomes were assessed using a gross measure of neurologic function at PICU discharge, rather than longer-term neuropsychological outcomes. Patients who did not have clinically indicated MRI were excluded, limiting our cohort to those who lived long enough to undergo brain MRI and likely excluding patients who were clinically well enough to not require imaging. Age influences MRI diffusion restriction14 but is not accounted for in this analysis due to limited sample size. Although the presence of MRI diffusion restriction in any part of the brain has been associated with poor outcome after CA,16,36 our use of whole-brain ADC instead of focusing on specific location of diffusion restriction may limit our ability to more precisely predict neurologic function in survivors. EEG background may be affected by sedative medications, including benzodiazepine infusions when administered at high doses. However, we did not have information regarding dosing and could not control for it in our model. We did not assess the EEG data over time to evaluate whether later EEG background assessments or inclusion of EEG background changes over time affect outcome prediction. The neurologic examination was not included in this analysis because reliable information about the examination at varying time points after CA was not consistently available.
Of importance, clinicians were not blinded to EEG and MRI results, which creates risk of a self-fulfilling prophecy bias; a clinician may offer or recommend WLST based on perceived poor prognosis from EEG and/or MRI results, thus inflating estimates of study specificity for poor outcomes.37,38 This risk of self-fulfilling prophecy bias skewing results must be considered when interpreting retrospective observational studies that evaluate the accuracy of neuroprognostication modalities that are already used in routine clinical practice. Many studies define unfavorable neurologic outcome as those who die or survive with unfavorable neurologic outcome. To begin to address this concern, we evaluated our models in those who survived with unfavorable neurologic outcomes and thus did not undergo withdrawal of technologic support. Of note, this model, while still accurate, was less so than when those who died were included. The high specificity of the multimodal model for mortality and predictive accuracy for any outcome that includes mortality must be interpreted with caution in any retrospective study of prognostication after CA.
In summary, early EEG background category was associated with percentage of MRI ischemia after pediatric CA. The combination of early EEG background category and MRI ischemia burden yielded higher predictive accuracy for outcomes (mortality and unfavorable neurologic outcome) than either modality alone. Furthermore, the addition of percentage of MRI ischemia to early EEG background and clinical factors improved short-term outcome prediction and achieved high predictive accuracy.
Glossary
- ADC
apparent diffusion coefficient
- AUROC
area under the receiver operating characteristic
- CA
cardiac arrest
- cEEG
continuous EEG
- CPR
cardiopulmonary resuscitation
- HIBI
hypoxic-ischemic brain injury
- IHCA
in-hospital CA
- IQR
interquartile range
- OHCA
out-of-hospital CA
- OR
odds ratio
- PCPC
Pediatric Cerebral Performance Category
- PICU
pediatric intensive care unit
- ROSC
return of spontaneous circulation
- WLST
withdrawal of life-sustaining therapy
Appendix. Authors
| Name | Location | Contribution |
| Ashley M. Bach, MD, MPH | Department of Neurology, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Matthew P. Kirschen, MD, PhD | Department of Neurology, Departments of Anesthesia and Critical Care Medicine, and Department of Pediatrics, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| France W. Fung, MD | Department of Neurology, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Nicholas S. Abend, MD, MSCE | Department of Neurology, Departments of Anesthesia and Critical Care Medicine, and Department of Pediatrics, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Steve Ampah, PhD | Department of Biomedical and Health Informatics, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Antara Mondal, MS | Department of Biomedical and Health Informatics, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Jimmy W. Huh, MD | Departments of Anesthesia and Critical Care Medicine, and Department of Pediatrics, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Shih-Shan L. Chen, MD | Department of Neurosurgery, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content |
| Ian Yuan, MD | Departments of Anesthesia and Critical Care Medicine, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content |
| Kathryn Graham, MLAS | Departments of Anesthesia and Critical Care Medicine, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Jeffrey I. Berman, PhD | Department of Radiology, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Arastoo Vossough, MD, PhD | Department of Radiology, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Alexis Topjian, MD, MSCE | Departments of Anesthesia and Critical Care Medicine, and Department of Pediatrics, Children's Hospital of Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Footnotes
Editorial, page e209254
Study Funding
No targeted funding reported.
Disclosure
A.M. Bach reports no disclosures relevant to the manuscript. The institution of M.P. Kirschen has received research support from NIH. F.W. Fung reports no disclosures relevant to the manuscript. N.S. Abend has received personal compensation in the range of $5,000–$9,999 for serving as a consultant for Epilepsy Foundation, the institution of N.S. Abend has received research support from NIH, the institution of N.S. Abend has received research support from PCORI, and N.S. Abend has received publishing royalties from a publication relating to health care. S. Ampah reports no disclosures relevant to the manuscript. A. Mondal reports no disclosures relevant to the manuscript. J. Huh reports no disclosures relevant to the manuscript. S.-S.L. Chen reports no disclosures relevant to the manuscript. The institution of Dr. Yuan has received research support from Masimo Corp. K. Graham reports no disclosures relevant to the manuscript. J.I. Berman has received personal compensation in the range of $10,000–$49,999 for serving as a Consultant for McGowan Associates. J.I. Berman has received personal compensation in the range of $10,000–$49,999 for serving as an Expert Witness for McGowan Associates. A. Vossough has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Syneos Health, has received personal compensation in the range of $0–$499 for serving on a Scientific Advisory or Data Safety Monitoring board for DeepSight, and has received publishing royalties from a publication relating to health care. A.A. Topjian has received personal compensation in the range of $5,000–$9,999 for serving as an Expert Witness for plantiff and defense. Go to Neurology.org/N for full disclosures.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93-e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Ross CE, Fitzmaurice G, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12(7):e005580. [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS. Survival trends in pediatric in-hospital cardiac arrests: an analysis from get with the guidelines-resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42-49. doi: 10.1161/CIRCOUTCOMES.112.967968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung FW, Topjian AA, Xiao R, Abend NS. Early EEG features for outcome prediction after cardiac arrest in children. J Clin Neurophysiol. 2019;36(5):349-357. doi: 10.1097/WNP.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abend NS, Massey SL, Fitzgerald M, et al. Interrater agreement of EEG interpretation after pediatric cardiac arrest utilizing standardized critical care EEG terminology. J Clin Neurophysiol. 2017;34(6):534-541. doi: 10.1097/WNP.0000000000000424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abend NS, Wiebe DJ, Xiao R, et al. EEG factors after pediatric cardiac arrest. J Clin Neurophysiol. 2018;35(3):251-255. doi: 10.1097/WNP.0000000000000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topjian AA, Sánchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early electroencephalographic background features predict outcomes in children resuscitated from cardiac arrest. Pediatr Crit Care Med J. 2016;17(6):547-557. doi: 10.1097/PCC.0000000000000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducharme-Crevier L, Press CA, Kurz JE, Mills MG, Goldstein JL, Wainwright MS. Early presence of sleep spindles on electroencephalography is associated with good outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2017;18(5):452-460. doi: 10.1097/PCC.0000000000001137 [DOI] [PubMed] [Google Scholar]
- 9.Kirschen MP, Licht DJ, Faerber J, et al. Association of MRI brain injury with outcome after pediatric out-of-hospital cardiac arrest. Neurology. 2021;96(5):e719-e731. doi: 10.1212/WNL.0000000000011217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch KG, Mlynash M, Jansen S, et al. Prognostic value of a qualitative brain MRI scoring system after cardiac arrest. J Neuroimaging. 2015;25(3):430-437. doi: 10.1111/jon.12143 [DOI] [PubMed] [Google Scholar]
- 11.Hirsch KG, Mlynash M, Eyngorn I, et al. Multi-center study of diffusion-weighted imaging in coma after cardiac arrest. Neurocrit Care. 2016;24(1):82-89. doi: 10.1007/s12028-015-0179-9 [DOI] [PubMed] [Google Scholar]
- 12.Hirsch KG, Fischbein N, Mlynash M, et al. Prognostic value of diffusion-weighted MRI for post-cardiac arrest coma. Neurology. 2020;94(16):e1684-e1692. doi: 10.1212/WNL.0000000000009289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yacoub M, Birchansky B, Mlynash M, et al. The prognostic value of quantitative diffusion-weighted MRI after pediatric cardiopulmonary arrest. Resuscitation. 2019;135:103-109. doi: 10.1016/j.resuscitation.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 14.Kirschen MP, Berman JI, Liu H, et al. Association between quantitative diffusion weighted magnetic resonance neuroimaging and outcome after pediatric cardiac arrest. Neurology. 2022;99(23):e2615-e2626. doi: 10.1212/WNL.0000000000201189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topjian AA, Zhang B, Xiao R, et al. Multimodal monitoring including early EEG improves stratification of brain injury severity after pediatric cardiac arrest. Resuscitation. 2021;167:282-288. doi: 10.1016/j.resuscitation.2021.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AE, Ganninger AP, Mian AY, Friess SH, Guerriero RM, Guilliams KP. Magnetic resonance imaging adds prognostic value to EEG after pediatric cardiac arrest. Resuscitation. 2022;173:91-100. doi: 10.1016/j.resuscitation.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topjian A, Hutchins L, Nadkarni V, et al. Post CPR Clinical Pathway: PICU and CICU. 2014. Accessed December 1, 2022. chop.edu/clinical-pathway/cardiac-arrest-post-cpr-clinical-pathway. [Google Scholar]
- 20.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3-23. doi: 10.1007/s12028-012-9695-z [DOI] [PubMed] [Google Scholar]
- 21.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95. doi: 10.1097/WNP.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81(4):383-391. doi: 10.1212/WNL.0b013e31829c5cfe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung FW, Parikh DS, Jacobwitz M, et al. Validation of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020;61(12):2754-2762. doi: 10.1111/epi.16724 [DOI] [PubMed] [Google Scholar]
- 24.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications and clinical practice. J Clin Neurophysiol. 2015;32(2):96-108. doi: 10.1097/WNP.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abend NS, Xiao R, Kessler SK, Topjian AA. Stability of early EEG background patterns after pediatric cardiac arrest. J Clin Neurophysiol. 2018;35(3):246-250. doi: 10.1097/WNP.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 27.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28(7):2616-2620. doi: 10.1097/00003246-200007000-00072 [DOI] [PubMed] [Google Scholar]
- 28.Topjian AA, French B, Sutton RM, et al. Early post-resuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42(6):1518-1523. doi: 10.1097/CCM.0000000000000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41(1):210-218. doi: 10.1097/CCM.0b013e3182668035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barth R, Zubler F, Weck A, et al. Topography of MR lesions correlates with standardized EEG pattern in early comatose survivors after cardiac arrest. Resuscitation. 2020;149:217-224. doi: 10.1016/j.resuscitation.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 31.Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78(11):796-802. doi: 10.1212/WNL.0b013e318249f6bb [DOI] [PubMed] [Google Scholar]
- 32.Perkins GD, Callaway CW, Haywood K, et al. Brain injury after cardiac arrest. Lancet. 2021;398(10307):1269-1278. doi: 10.1016/S0140-6736(21)00953-3 [DOI] [PubMed] [Google Scholar]
- 33.Keijzer HM, Verhulst MMLH, Meijer FJA, et al. Prognosis after cardiac arrest: the additional value of DWI and FLAIR to EEG. Neurocrit Care. 2022;37(1):302-313. doi: 10.1007/s12028-022-01498-z [DOI] [PubMed] [Google Scholar]
- 34.Wouters A, Scheldeman L, Plessers S, et al. Added value of quantitative apparent diffusion coefficient values for neuroprognostication after cardiac arrest. Neurology. 2021;96(21):e2611-e2618. doi: 10.1212/WNL.0000000000011991 [DOI] [PubMed] [Google Scholar]
- 35.Moon HK, Jang J, Park KN, et al. Quantitative analysis of relative volume of low apparent diffusion coefficient value can predict neurologic outcome after cardiac arrest. Resuscitation. 2018;126:36-42. doi: 10.1016/j.resuscitation.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 36.Lopez Soto C, Dragoi L, Heyn CC, et al. Imaging for neuroprognostication after cardiac arrest: systematic review and meta-analysis. Neurocrit Care. 2020;32(1):206-216. doi: 10.1007/s12028-019-00842-0 [DOI] [PubMed] [Google Scholar]
- 37.Rohaut B, Claassen J. Decision making in perceived devastating brain injury: a call to explore the impact of cognitive biases. Br J Anaesth. 2018;120(1):5-9. doi: 10.1016/j.bja.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 38.Hunfeld M, Muusers MAC, Catsman CE, Castillo JD, Tibboel D, Buysse CMP. The current practice regarding neuro-prognostication for comatose children after cardiac arrest differs between and within European PICUs: a survey. Eur J Paediatr Neurol. 2020;28:44-51. doi: 10.1016/j.ejpn.2020.06.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.